Abstract
Objectives:
To determine the rate of Gleason Grade Group (GG) upgrading in AA men with a prior diagnosis of low-grade PCa (GG 1 or GG 2) on 12-core systematic biopsy (SB) after multiparametric magnetic resonance imaging (mpMRI) and fusion biopsy (FB); and whether AA men who continued active surveillance (AS) after mpMRI and FB fared differently than a predominantly Caucasian (non-AA) population.
Patients and Methods:
A database of men who had undergone mpMRI and FB was queried to determine rates of upgrading by FB among men deemed to be AS candidates based on SB prior to referral. After FB, Kaplan-Meier curves were generated for AA men and non-AA men who then elected AS. The time to GG upgrading and time continuing AS were compared using the logrank test.
Results:
AA men referred with GG 1 disease on previous SB were upgraded to GG ≥ 3 by FB more often than non-AA men, 22.2% vs. 12.7%, p=0.01. A total of 32 AA men and 258 non-AA men then continued AS with a median follow-up of 39.19 months (IQR 24.24–56.41). Median time to progression was 59.7 and 60.5 months, p=0.26, respectively. The median time continuing AS was 61.9 months and not reached, p=0.80, respectively.
Conclusions:
AA men were more likely to be upgraded from GG 1 on SB to GG ≥ 3 on initial FB; however, AA and non-AA men on AS subsequently progressed at similar rates following mpMRI and FB. A greater tendency for SB to underestimate tumor grade in AA men may explain prior studies that have shown AA men to be at higher risk for progression during AS.
Keywords: Active Surveillance, African-American, Prostate Cancer, multi-parametric MRI, fusion biopsy
Introduction
Active Surveillance (AS) is increasingly utilized for men with NCCN low-risk (LR) or very-LR (VLR) prostate cancer (PCa) but has not been universally adopted, especially among African-American (AA) men.(1, 2) Reasons for this include the worry that AA men may harbor “hidden” or undetected high grade PCa or that AA men with LR disease may have a more aggressive phenotype not appropriate for AS.(3–5) These factors contribute to the observation that AA men are placed on AS only half as frequently as Caucasian men.(2)
The efficacy and safety of AS have been demonstrated in numerous studies.(6) However, these studies have been mainly confined to Caucasian populations limiting their generalizability. Studies examining VLR and LR AA men on AS, although with limited enrollment, have shown these men to have a three to nearly fourfold higher rate of progression as compared to non-AA men.(7–10)
Another plausible explanation for this disparity is that delays in diagnosis, possibly due to tumor location, could lead to more advanced or higher-grade disease when PCa is finally detected. An examination of RP specimens showed AA men to more often have dominant anterior tumors as compared to Caucasian men.(3, 11) Anterior tumors are more difficult to sample with a 12-core systematic biopsy (SB) alone, but with advancements in multi-parametric magnetic resonance imaging (mpMRI) and MRI-TRUS fusion biopsy (FB) techniques, 15% more anterior tumors and 30% more high risk tumors can be discovered.(12–14)
This study first examined the rate of FB upgrading of AA men referred to our institution with a diagnosis of Gleason Grade Group (GG) 1 (Gleason Score 3+3) or GG 2 (Gleason 3+4) PCa, based on SB prior to referral who were potential AS candidates. We then reviewed our cohort of AA men placed on AS after FB to see if they fared differently than our predominantly Caucasian (non-AA) population regarding rates of progression and time continuing AS.
Patients and Methods
Study Cohort:
A prospectively maintained database was queried for all patients referred to the National Cancer Institute (NCI) with a SB diagnosis of GG 1 or GG 2 PCa in whom a mpMRI and then confirmatory MRI-TRUS FB were subsequently performed. FB upgrading rates among AA men and non-AA men were recorded.
We then examined our cohort of AA and non-AA men who enrolled on AS at our institution after this initial FB. Patients were considered eligible for AS with any volume GG 1 disease and no thresholds for PSA. Patients with GG 2 disease were eligible for AS if they had refused definitive therapy and mpMRI had no features of stage T3 or greater disease. Patients were excluded if they did not have a subsequent follow-up mpMRI with FB at our institution to document GG. All patients on AS were then followed per our institution’s AS protocol including yearly PSA, mpMRI, and digital rectal exam (DRE). Repeat biopsy was performed if clinically warranted due to change in PSA, mpMRI, DRE or based on physician discretion. GG progression was defined as any upgrade in GG from prior biopsies and not based on overall tumor volume as seen on mpMRI or biopsy. The time the patient continued AS was determined from the last known physician encounter. GG progression and time continuing AS were both calculated from initial FB at our institution regardless of prior biopsy results. MR images were reviewed for tumor location information and pathology was reviewed for all patients who underwent RP.
Imaging and Biopsy Protocol:
Each patient referred for PCa management underwent mpMRI of the prostate at 3 Tesla (Achieva; Phillips Healthcare) with endorectal coil (BPX-30; Medrad, Pittsburgh, PA) and 16-channel cardiac surface coil (SENSE; Phillips Healthcare), as previously described (15). mpMRI sequences consisted of triplanar T2-weighted, diffusion weighted imaging (DWI), apparent diffusion coefficient (ADC) mapping, and dynamic contrast enhancement (DCE). Two radiologists identified prostatic lesions and assigned suspicion levels for PCa based on a previously validated in-house scoring system (SS) and, when available, the prostate imaging reporting and data system (PIRADS) version 2 scoring system. Each FB session consisted of a SB and targeted biopsy of any detectable lesions (at least two targeted cores per lesion) as described previously.(16) All biopsies were performed by a single urologist or interventional radiologist with more than 10 years’ experience.
Statistical Analysis:
Rates of upgrading were compared using chi-square analysis between different groups. Baseline characteristics of our AA and non-AA AS cohorts were compared utilizing student’s T-test and Fisher’s exact test for continuous and categorical variables, respectively. Kaplan-Meier survival graphs were created to determine median GG progression-free survival (PFS) and time continuing AS. These times were then compared using the logrank test. A two-tailed p-value <0.05 was considered statistically significant. Analyses were completed using STATA v15.0 (StataCorp LLC, College Station, TX, USA)
Results
From 2007 to 2017, a total of 84 AA men were referred to the NCI with a prior SB diagnosis of GG 1 (72) or GG 2 disease (12) compared to 431 Caucasian men, GG 1 (373) and GG 2 (58), and 27 men who identified with other ethnicities, 21 GG 1 and 6 GG 2 (Table 1).
Table 1.
Patients referred with low-grade (GG1 and GG2) PCa and resulting confirmatory fusion biopsy.
| Race | Outside SB | Confirmatory FB | ||||
|---|---|---|---|---|---|---|
| Gleason Group (n) | No Cancer, n (%) | GG 1, n (%) | GG 2, n (%) | ≥ GG 3, n (%) | ||
| AA men | African-American (84) | 1 (72) | 14 (19.4) | 20 (27.8) | 22 (30.6) | 16 (22.2) |
| 2 (12) | 2 (16.7) | 1 (8.3) | 4 (33.3) | 5 (41.7) | ||
| Non-AA men | Caucasian (431) | 1 (373) | 92 (24.7) | 124 (33.2) | 112 (30.0) | 45 (12.1) |
| 2 (58) | 8 (13.7) | 7 (12.1) | 30 (51.7) | 13 (22.4) | ||
| Other (27) | 1 (21) | 4 (19.0) | 7 (33.3) | 5 (23.8) | 5 (23.8) | |
| 2 (6) | 0 (0.0) | 2 (33.3) | 3 (50.0) | 1 (16.7) | ||
AA = African-American, GG = Gleason Grade Group, SB = Systematic Biopsy, FB = Fusion Biopsy
A total of 21/82 (25.6%) AA men and 64/458 (14.0%) non-AA men were upgraded to GG ≥ 3 at the time of initial FB, p=0.01. When analyzed by individual GG, 16/72 (22.2%) AA and 50/394 (12.7%) non-AA men referred with GG 1 were upgraded to GG ≥ 3 by FB, p=0.01. Additionally, 5/12 (41.2%) AA and 14/64 (21.9%) non-AA men referred with GG 2 tumors were upgraded to GG ≥ 3 by FB, p=0.15.
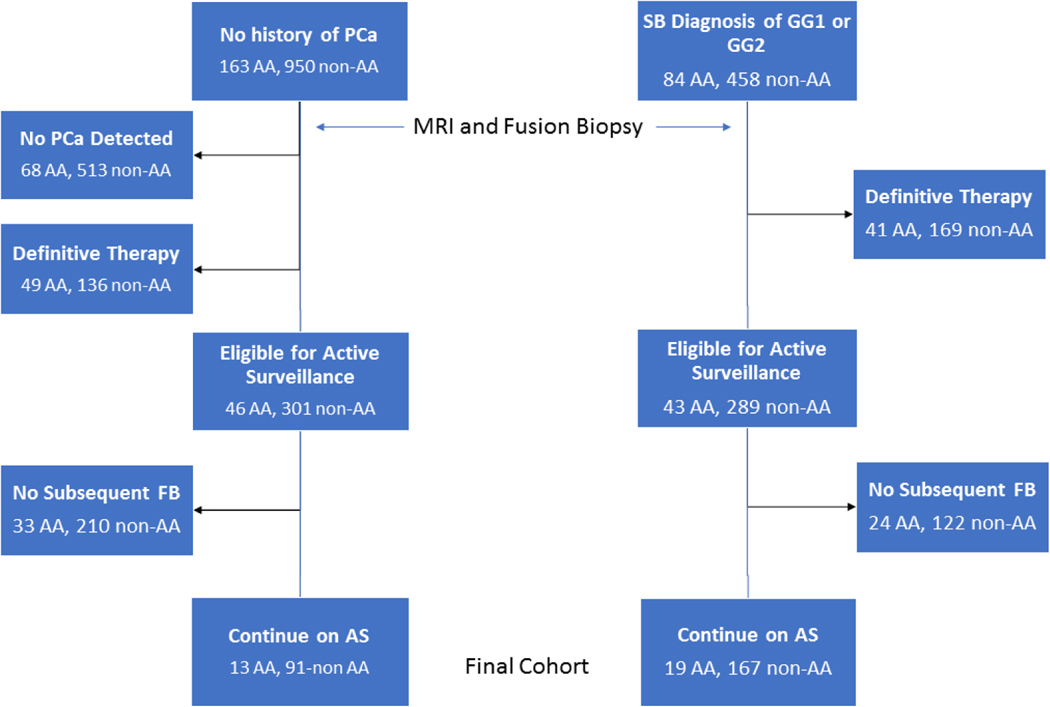
A total of 32 AA men, with either previously diagnosed (19) or newly diagnosed PCa (13), met our AS inclusion criteria and chose AS as their primary management strategy. In our non-AA cohort, a total of 258 men (245 Caucasian, 7 Asian, 2 Hispanic, 4 unidentified) met our AS inclusion criteria: 167 had a prior diagnosis and 91 had newly diagnosed PCa (Figure 1). Median follow-up times for the AA cohort and non-AA cohort was 44.4 (IQR 33.3–58.7) months and 38.6 (IQR 23.2–54.5) months, respectively. Including the initial FB, the AA group and non-AA group who pursued AS underwent an average of 2.6 ± 0.8 and 2.6 ± 1.0 biopsy sessions per patient, respectively, p=1.0.
Figure 1.
Flow chart of men included in our study who were followed on active surveillance
AA = African-American, FB = Fusion Biopsy, GG= Gleason Grade Group, PCa = Prostate Cancer, SB = Systematic Biopsy
Baseline characteristics of the AA and non-AA men who continued AS following FB are shown in Table 2. AA men were younger than non-AA men, 58.9 ± 8.4 vs. 63.0 ± 6.9, p<0.01; no other significant differences were seen. Of the AA men, 25/32 (78.1%) began AS with GG 1 PCa while 7/32 (21.9%) began AS with GG 2 disease. No significant differences were seen regarding the location of dominant tumors on mpMRI. The rate of anterior tumors in our AS cohorts was 40.6% vs. 36.8%, p=0.68, in AA and non-AA men, respectively.
Table 2.
Baseline clinical, imaging, and biopsy results
| AA Men (32) | Non-AA Men (258) | P-value | |
|---|---|---|---|
| Age (years) | 58.9 ± 8.4 | 63.0 ± 6.9 | <0.01* |
| PSA (ng/ml) | 6.3 ± 4.2 | 6.0 ± 3.8 | 0.30 |
| MRI Volume (cm3) | 56.6 ± 28.9 | 53.3 ± 24.4 | 0.48 |
| Largest Lesion Size (cm.) | 1.1 ± 0.5 | 1.2 ± 0.5 | 0.29 |
| High MRI Suspicion Score, n | 12 (37.5%) | 90 (34.9%) | 0.77 |
| No lesions seen on MRI, n | 1 (3.1%) | 8 (3.1%) | 0.99 |
| Dominant Anterior Tumor on MRI, n | 13 (40.6%) | 95 (36.8%) | 0.68 |
| GG 2, n | 7 (21.9%) | 76 (29.5%) | 0.17 |
| Mean Percentage of Total Biopsy Cores Positive | 11.6 ± 10.5% | 13.2 ± 11.9% | 0.49 |
| Mean Maximum Percentage of Core Positive | 13.5 ± 16.2% | 14.9 ± 16.2 | 0.65 |
P-values < 0.05 considered statistically significant and denoted with *.
AA = African-American, GG = Gleason Grade Group
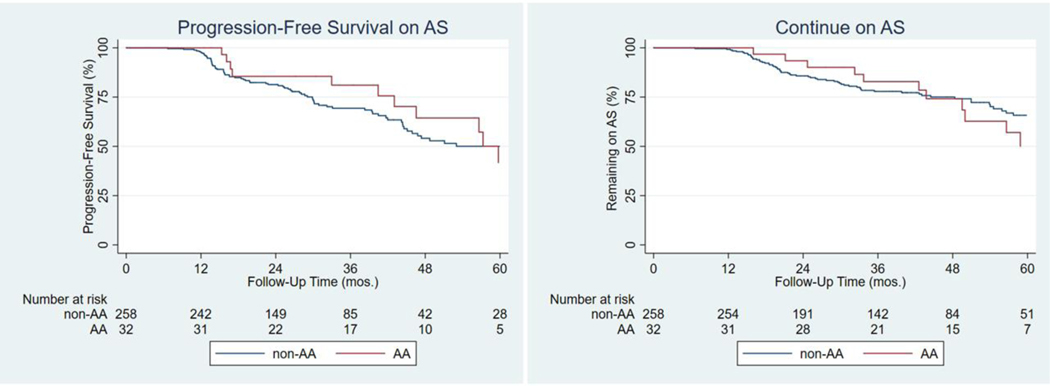
In the AA cohort, 13/32 men (40.6%) including 2/7 (28.6%) starting with GG 2, progressed to a higher GG on subsequent FB. In the non-AA group, 87/258 (33.7%), including 30/76 (39.5%) starting with GG 2, progressed to a higher GG on subsequent FB. Median GG progression-free survival (PFS) in the AA cohort was 59.7 months vs. 60.5 months among non-AA men on AS, p=0.26 (Figure 2).
Figure 2.
Median Progression-Free Survival (PFS) of African-American (AA) compared to all other men on AS (Active Surveillance). Median PFS was 59.7 months compared to 60.5 months for all other men, p=0.26. Median time continuing AS for AA men was 61.9 months and was not reached for non-AA men, p=0.80.
The median time for AA men to continue AS was 61.9 months; the median time to continue AS for non-AA men was not reached, p=0.80 (Figure 2). A total of 12/32 (37.5%) AA men discontinued AS to pursue definitive therapy including both men who progressed after beginning AS with GG 2. However, 3/13 (23.1%) AA men continued with AS despite GG progression on FB. 2/12 AA men who discontinued AS (16.7%) did so without GG progression. A total of 66/258 (25.6%) non-AA men, including 22/30 (73.3%) starting with GG 2, discontinued AS to pursue definitive therapy. Of these men, 31/87 (35.6%) continued AS despite GG progression on FB. Of those who discontinued AS, 9/66 (13.6%) of non-AA men did so without GG progression.
Of the 12 AA men who discontinued AS: 6 underwent radical prostatectomy (RP) with pathology available (Table 3); 4 had treatment at outside institutions, and two had radiation therapy (RT). The median time to RP was 46.6 months. All six men who underwent RP had organ-confined (pT2) disease; none had positive surgical margins and none had positive lymph nodes. With a median follow up time of 20.8 months after surgery, two patients had biochemical recurrence (BCR) after RP, including one patient who did not nadir after surgery.
Table 3.
Final Pathology of AA men initially on AS and Underwent Radical Prostatectomy
| Original FB GG | Last FB GG | Time until RP (months) | Pathology at RP | Time to BCR (months) |
|---|---|---|---|---|
| 1 | 4 | 74.6 | T2C, GG 4, margins neg. 0/23 nodes | 25.3 |
| 1 | 4 | 43.8 | T2C, GG 4, margins neg. 0/17 nodes | 1.3 |
| 1 | 1 | 32.3 | T2C, GG 3, margins neg. 0/8 nodes | N/A |
| 1 | 2 | 50.0 | T2C, GG 2, margins neg, 0/10 nodes | N/A |
| 1 | 4 | 49.5 | T2C, GG 2, margins pos., 0/32 nodes | N/A |
| 2 | 2 | 21.1 | T2C, GG 2, margins neg., 0/10 nodes | N/A |
AA = African-American, GG = Gleason Grade Group, FB = Fusion Biopsy, RP = Radical Prostatectomy
In the non-AA men cohort, a total of 34 underwent RP at our institution with a median time to RP of 22.4 months. Three patients had non-organ confined disease (≥ pT3), two had GG upgrading based on their last FB, one had positive surgical margins, and none had positive lymph nodes. Four of these patients had BCR with a median follow-up of 51.6 months.
Discussion:
AS in AA men remains a controversial topic.(17, 18) Although multiple studies have shown AS to provide excellent oncologic outcomes, AA men have comprised only about 5–10% of these cohorts, thereby limiting their applicability to this population.(7, 10, 19) However, prior studies have reported that AA men have a 3–4 fold greater risk of disease progression than their Caucasian counterparts.(7–9) Our results suggest that at least in short-term follow-up, AS in AA men staged with mpMRI and FB, a clear difference from prior studies, achieves similar results in terms of time to GG progression and the ability to defer definitive therapy compared to our non-AA, predominantly Caucasian cohort. When examining our FB results of AA and non-AA men referred with GG 1 disease based on SB, almost twice as many AA men and non-AA men were upgraded to GG ≥ 3 (22.1% vs 12.7%) and therefore ineligible for AS. These data support the role of MRI-TRUS FB in the AS selection process to filter out higher grade tumors that may be missed by standard extended template SB. This result again highlights that the integration of mpMRI and FB into clinical practice has led to improvements in the detection of clinically significant (≥ GG 2) PCa (csPCa).(13, 20) Furthermore, AA men have previously been shown to have higher rates of anterior tumors. Our results show that after selection with FB, there were no differences between the rates of dominant anterior lesions in our AA and non-AA men AS cohorts. These results suggest that mpMRI and FB may select out both harder to detect anterior tumors and more aggressive tumors leaving a more selective AS cohort.
Another hesitation about placing AA men on AS is the concern that these men may harbor a more aggressive phenotype. Sundi et al. examined over 1800 men with NCCN very low-risk (VLR) PCa who underwent immediate radical prostatectomy (RP) and found that AA men had significantly higher rates of adverse pathology (AP) (19.8% vs. 7.2%), Gleason Score upgrading (32.7% vs. 12.6%), and positive surgical margins (19.0% vs. 6.3%). (3) Maurice et al. queried the National Cancer Database for low-risk (LR) PCa and found AA men to have higher risks of pathologic upgrading and upstaging. (21) Another study of LR PCa found AA men to have higher rates of positive surgical margins compared to Caucasian men (31% vs 21%).(22) These clinical differences in the rates of adverse pathology may not be as generalizable today as these studies were performed prior to the incorporation of mpMRI and FB.
Another factor that may contribute to the observation that AA men tend to have more aggressive prostate cancer is delayed diagnosis due to poor access to healthcare and other socioeconomic factors. In the PLCO cancer screening trial, Barocas et al. found that non-Hispanic black men were 45% less likely to undergo a repeat PSA screening test or prostate biopsy. (23) AA men have also been shown to have lower rates of health insurance and even AA men with health insurance are less likely to receive treatment than their Caucasian counterparts.(24, 25) Our study suggests that if sufficient medical resources are available to perform a FB, AA men can be safely selected for AS without fear of disparate rates of upgrading. Additionally, Leapman et al. examined 895 men with LR PCa and equal access to medical care, of whom 40% were AA, and found no significantly higher risks of pathologic or stage upgrading, positive surgical margins, or biochemical recurrence (BCR) were observed.(4) Schulman et al. examined an equal-access health system with over 2500 men diagnosed with PCa, of whom 41% were AA and found race to not be predictive of PCa outcomes. (26)
While a delay in diagnosis or difference in anatomic locations may play a role in the disparities observed between AA and Caucasian men in prior reported outcomes there is evidence that suggests a more aggressive phenotype in AA men. Mahal et al. used a commercially available 22 gene panel risk-classifier, Decipher score, to determine the difference in the risk of development of metastases in both African-American and Caucasian men who underwent RP.(27) While no differences were seen in higher grade Gleason Scores (7–10), AA men with Gleason 6 disease were almost twice as likely (25% vs. 13%) to have intermediate or high risk scores as defined as determined by a Decipher score ≥ 0.45.(27) These findings may help explain a review of the SEER database which found a difference in the 12-year prostate cancer specific mortality (2.2% vs. 1.4%) between AA and white men with Gleason 6 disease while no differences were seen in Gleason 7–10 disease.(5) Other studies looking at the genomic sequencing of PCa in AA men have found specific genetic alterations which correlate with advanced or metastatic disease to occur at higher rates in AA men as compared to Caucasian men.(28, 29) While our results show that AS may be a reasonable option for appropriately selected AA men, these biologically more aggressive tumors must be kept in mind when selecting candidates for AS, determining length between follow-up, and deciding when patients should discontinue surveillance.
Our study is limited by its retrospective nature and our reliance on clinical judgment regarding the timing of follow-up FBs, rather than a protocol-driven follow-up biopsy schedule. Furthermore, our study has limited power to draw definitive conclusions regarding AS in AA men due to the short duration of follow-up and the relatively small number of AAs in our cohort. A major strength of this study is our institutional experience with prostate imaging and MRI-TRUS guided prostate biopsies, both of which entail substantial learning curves.
Conclusion:
AS is a useful strategy to delay or prevent the morbidity of definitive therapy in many men with localized prostate cancer. However, concerns remain regarding the advisability of this approach in AA men due to the fear that they may be more likely to harbor an aggressive disease phenotype. Our study suggests that some AA men may have previously been placed on AS based on SBs that missed higher grade PCa that could have been detected with FB. This may have contributed to the poorer oncological outcomes reported for AA men on AS compared to Caucasians. MRI-TRUS FB has the potential to mitigate these concerns by identifying men (both AA and Caucasian) with unfavorable intermediate and high-risk disease for whom AS would not represent optimal management.
Acknowledgements:
This research was supported by the Intramural Research Program of the National Cancer Institute, NIH.
Sherif Mehralivand’s postdoctoral fellowship was funded by a research grant from the “Dr. Mildred Scheel” foundation, Bonn, Germany.
Footnotes
Conflicts of Interest: None
References
- 1.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314(1):80–2. [DOI] [PubMed] [Google Scholar]
- 2.Krishna S, Fan Y, Jarosek S, Adejoro O, Chamie K, Konety B. Racial Disparities in Active Surveillance for Prostate Cancer. J Urol. 2017;197(2):342–9. [DOI] [PubMed] [Google Scholar]
- 3.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013;31(24):2991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leapman MS, Freedland SJ, Aronson WJ, Kane CJ, Terris MK, Walker K, et al. Pathological and Biochemical Outcomes among African-American and Caucasian Men with Low Risk Prostate Cancer in the SEARCH Database: Implications for Active Surveillance Candidacy. J Urol. 2016;196(5):1408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate Cancer-Specific Mortality Across Gleason Scores in Black vs Nonblack Men. JAMA. 2018;320(23):2479–81. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–7. [DOI] [PubMed] [Google Scholar]
- 7.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187(5):1594–9. [DOI] [PubMed] [Google Scholar]
- 8.Abern MR, Bassett MR, Tsivian M, Banez LL, Polascik TJ, Ferrandino MN, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16(1):85–90. [DOI] [PubMed] [Google Scholar]
- 9.Odom BD, Mir MC, Hughes S, Senechal C, Santy A, Eyraud R, et al. Active surveillance for low-risk prostate cancer in African American men: a multi-institutional experience. Urology. 2014;83(2):364–8. [DOI] [PubMed] [Google Scholar]
- 10.Sundi D, Faisal FA, Trock BJ, Landis PK, Feng Z, Ross AE, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85(1):155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black American men. J Urol. 2014;191(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014;114(6b):E43–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quintana L, Ward A, Gerrin SJ, Genega EM, Rosen S, Sanda MG, et al. Gleason Misclassification Rate Is Independent of Number of Biopsy Cores in Systematic Biopsy. Urology. 2016;91:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto PA, Chung PH, Rastinehad AR, Baccala AA Jr., Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186(4):1281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gokce MI, Sundi D, Schaeffer E, Pettaway C. Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis. 2017;20(2):127–36. [DOI] [PubMed] [Google Scholar]
- 18.Sundi D, Schaeffer EM. Active surveillance for African-American men with prostate cancer: proceed with caution. Con. Oncology (Williston Park). 2014;28(1):83, 5. [PubMed] [Google Scholar]
- 19.Tosoian JJ, Mamawala M, Epstein JI, Landis P, Wolf S, Trock BJ, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015;33(30):3379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurice MJ, Sundi D, Schaeffer EM, Abouassaly R. Risk of Pathological Upgrading and Up Staging among Men with Low Risk Prostate Cancer Varies by Race: Results from the National Cancer Database. J Urol. 2017;197(3 Pt 1):627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jalloh M, Myers F, Cowan JE, Carroll PR, Cooperberg MR. Racial variation in prostate cancer upgrading and upstaging among men with low-risk clinical characteristics. Eur Urol. 2015;67(3):451–7. [DOI] [PubMed] [Google Scholar]
- 23.Barocas DA, Grubb R 3rd, Black A, Penson DF, Fowke JH, Andriole G, et al. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer. 2013;119(12):2223–9. [DOI] [PubMed] [Google Scholar]
- 24.Smith ZL, Eggener SE, Murphy AB. African-American Prostate Cancer Disparities. Curr Urol Rep. 2017;18(10):81. [DOI] [PubMed] [Google Scholar]
- 25.Major JM, Norman Oliver M, Doubeni CA, Hollenbeck AR, Graubard BI, Sinha R. Socioeconomic status, healthcare density, and risk of prostate cancer among African American and Caucasian men in a large prospective study. Cancer Causes Control. 2012;23(7):1185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulman AA, Howard LE, Tay KJ, Tsivian E, Sze C, Amling CL, et al. Validation of the 2015 prostate cancer grade groups for predicting long-term oncologic outcomes in a shared equal-access health system. Cancer. 2017;123(21):4122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahal BA, Alshalalfa M, Spratt DE, Davicioni E, Zhao SG, Feng FY, et al. Prostate Cancer Genomic-risk Differences Between African-American and White Men Across Gleason Scores. Eur Urol. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Khani F, Mosquera JM, Park K, Blattner M, O’Reilly C, MacDonald TY, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20(18):4925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang FW, Mosquera JM, Garofalo A, Oh C, Baco M, Amin-Mansour A, et al. Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function ERF Mutations. Cancer Discov. 2017;7(9):973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]