Abstract
Introduction
Glucose hypometabolism and insulin resistance increase risk for and accelerate progression in Parkinson's disease and neurocognitive disorders. We conducted a proof of concept trial to determine whether ketogenesis, a metabolic adaptation induced by dietary carbohydrate restriction, can improve cognitive performance in Parkinson's disease patients with mild cognitive impairment.
Methods
We enrolled patients with mild cognitive impairment associated with Parkinson's disease in an eight-week nutritional intervention with random assignment to either high-carbohydrate consumption typical of the Western dietary pattern (n = 7) or to a low-carbohydrate, ketogenic regimen (n = 7). We assessed changes in cognitive performance as well as motor function, anthropometrics, and metabolic parameters.
Results
Relative to the high-carbohydrate group, the low-carbohydrate group demonstrated improvements in lexical access (p = 0.02, Cohen's f effect size = 0.76) and memory (p = 0.01, f = 0.87) and as well as a trend for reduced interference in memory (p = 0.06, f = 0.60). The low-carbohydrate group also exhibited reduced body weight (p < 0.0001, f = 1.89) and increased circulation of beta-hydroxybutyrate (p = 0.01, f = 0.90). Change in body weight was strongly associated with memory performance (p = 0.001). Motor function was not affected by the intervention.
Conclusion
Nutritional ketosis enhanced cognitive performance in Parkinson's disease-associated mild cognitive impairment in this pilot study. This metabolic intervention and its mechanisms deserve further investigation in the context of neurodegeneration.
Keywords: Parkinson's disease, Mild cognitive impairment, Memory, Nutritional ketosis, Carbohydrate restriction
Highlights
-
•
The ketogenic group showed improved executive ability and memory.
-
•
No effect of the intervention on motor function
-
•
Change in body weight was strongly associated with cognitive benefit.
1. Introduction
Fifty to 80% of patients with Parkinson's disease (PD) exhibit abnormal glucose tolerance [1]. Peripheral insulin resistance is often an early abnormality in PD pathogenesis [2], and is related to impairment of central (brain) insulin signaling associated with neuronal maintenance and bioenergetics [3], suggesting that metabolic disturbance is a contributing etiological factor. Insulin resistance is associated with a more severe PD phenotype and rapid accrual of disease milestones, including mild cognitive impairment (MCI) and dementia [4].
Ketogenesis is a metabolic adaptation in which the liver produces ketone bodies contingent on low circulating levels of insulin, typically resulting from carbohydrate restriction. Nutritional ketosis is distinct from ketoacidosis, a consequence of severe insulin insufficiency often in the context of diabetes mellitus. In addition to carbohydrate restriction, feeding with medium chain triglycerides and ketone bodies can produce elevations of circulating ketones and benefits for brain function [5].
In a prior controlled trial, we demonstrated that short-term carbohydrate restriction designed to induce ketogenesis was effective in reducing glucose and insulin, elevating beta-hydroxybutyrate, and improving memory performance in older adults with MCI, a risk condition for dementia [6]. In a subsequent uncontrolled study, also with MCI participants, proton magnetic resonance spectroscopy imaging demonstrated significantly increased myo-inositol and marginal increases of N-acetyl-aspartate and creatine + phosphocreatine along with improved cognitive performance [7]. These findings suggested that the ketogenic intervention was associated with increased autophagy, improved neuronal integrity, and enhanced bioenergetic function. In the current pilot trial, we investigated the effects of a low carbohydrate, ketogenic intervention on cognitive performance in PD-MCI participants. In addition, we assessed effects on motor function, anthropometrics, and metabolic parameters.
2. Methods
2.1. Participants and study design
We recruited patients from the Gardner Center for Parkinson's disease and Movement Disorders at the University of Cincinnati Academic Health Center. We sought participants diagnosed with PD as per the UK Brain Bank criteria [8] and with cognitive signs and symptoms corresponding to MCI according to the Movement Disorder Society Task Force guidelines for Level I PD-MCI [9]. Inclusion criteria included either a total score between 20 and 25 on the Montreal Cognitive Assessment (MoCA) [10], reflecting mild overall cognitive decline, or a score <5 on the MoCA delayed recall task, the latter an indicator of hippocampal volume loss [11], which has been observed with verbal memory deficit in early PD [12]. In addition, patients were required to have been treated with a stable regimen of antiparkinsonian medications for at least six weeks and were expected to require no dose adjustment during the study period. Each participant also was required to have a study partner (typically spouse) involved in the education concerning the assigned dietary regimen and to assist with adherence to the protocol. Exclusion criteria included a diagnosis of dementia, a second neurological disorder, past or current substance abuse, unstable psychiatric disturbance, diabetes, or regular use of glucose or insulin modulating agents or medications that might affect the cognitive outcome measures such as benzodiazepines and stimulants.
This was an eight-week randomized, controlled, parallel-group trial. Participants were assigned to either a high- or low-carbohydrate regimen according to a pre-determined schedule generated by a random number sequence. Assessments, including cognitive evaluation, motor evaluation, and anthropometric and metabolic assays, were performed at the enrollment visit and at the final study visit after 8 weeks. The study was approved by the University of Cincinnati Medical Institutional Review Board and registered at ClinicalTrials.gov (NCT00777010). Each participant provided informed consent.
2.2. Intervention
Each dietary regimen was presented as a healthy approach with emphasis on vegetable sources of carbohydrates, although the high carbohydrate participants were not restricted from any form of carbohydrate. The high-carbohydrate protocol was designed to maintain overall carbohydrate intake at the level typical of the Western diet [13] and well above the level required to induce ketone body production. For the low-carbohydrate group, we prescribed a target of 20 g carbohydrate per day exclusively from vegetables with total restriction from fruit, grain-based foods, and sweets. Participants and study partners were provided dietary education orally and with written information as to the macronutrient composition of common foods and were counseled concerning sources of carbohydrate, fat, and protein under the supervision of a registered dietitian. In order to provide individualized regimens that would be acceptable to participants, we discussed specific foods that might be included, substituted, or eliminated according to each individual's food preferences. Carbohydrate was the only macronutrient manipulated in the intervention. Consumption of fat and protein was measured but not controlled in view of evidence that moderate protein intake does not suppress ketogenesis or affect seizure control [14] and given our prior experience applying this protocol [6,7].
2.3. Dietary intake monitoring
In order to measure dietary intake and enhance adherence to the assigned regimen, all participants were instructed to keep three-day diet diaries during the week before enrollment and during each week of the intervention. The three-day diaries sampled consumption on two weekdays and one weekend day. Participants were provided with oral and written education as to how to complete the diet diary forms and instructed to record all intake including fluids with portion sizes. Each diary was reviewed with the participant and study partner when submitted to ensure completeness and clarify uncertainties regarding food preparation or portion size. Diet diaries submitted at baseline (pre-intervention) and after week 8 were analyzed at the Bionutrition Core of the University of Cincinnati Clinical Translational Research Center using the Nutrition Data System for Research (NDSR), version 2014 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN USA. For each time point, nutrient values were averaged across the three days. This method is appropriate when assessing energy and macronutrient intakes compared to specific dietary recommendations [15]. In addition, we performed point-of-care measurement of ketone bodies at brief study visits during weeks 2, 4, and 6 to assess adherence to the respective regimens.
2.4. Neurocognitive assessments
The neuropsychological protocol included tasks designed to assess domains typically affected in PD including aspects of executive ability and memory function. We administered the Controlled Oral Word Association task, which includes lexical access trials with phonological and categorical constraints [16] and assesses executive control operations and phonological and semantic information processing [17]. The California Verbal Learning Test [18] was used to obtain measures of recall and interference in memory. We also administered the Verbal Paired Associate Learning Test (VPAL) [19], which calls for learning new associations between arbitrary, semantically unrelated terms (for example, help-years), a more demanding verbal memory task.
2.5. Motor assessments
Motor function was evaluated with the motor subscale of the Movement Disorders Society-Unified Parkinson Disease Rating Scale (MDS-UPDRS-III) [20], which has been shown to have high inter-rater reliability and responsiveness to treatment. We also administered the Finger Tapping task [21], a timed measure of finger tapping movements of the dominant and non-dominant hands that calls for a rudimentary level of motor dexterity.
2.6. Anthropometric and metabolic measures
We measured body weight and waist circumference at the narrowest waist. Blood was obtained after overnight fast for serum assays of glucose, insulin, and β-hydroxybutyrate (β-OHB) that were performed at the University of Cincinnati Metabolic Diseases Institute. Glucose was determined using enzyme sensing with glucose oxidase to form gluconate and hydrogen peroxide. Insulin assays were performed using two antibodies and electrochemiluminescence detection. Β-OHB was measured through enzymatic quantification using β-hydroxybutyrate dehydrogenase.
2.7. Statistical analyses
The neuropsychological measures were chosen to be representative of cognitive domains vulnerable in PD including lexical access, memory interference, and memory recall. Using baseline scores, we examined inter-correlations within and between domains to corroborate inclusion of each measure in the respective composite outcomes. Test scores were standardized and means calculated to create each composite [22]. The lexical access composite incorporated the phonological and semantic production scores from the Controlled Oral Word Association task. The interference composite was derived from the CVLT intrusion and CVLT false positive scores. The memory composite included the CVLT short- and long-delay recall measures and the VPAL.
Group differences in cognitive, motor, anthropometric, and metabolic outcomes were evaluated with analyses of covariance (ANCOVA). These analyses involved between-group comparisons of the final visit scores with covariate control for enrollment visit values in order to isolate the effect of the intervention [23]. We calculated Cohen's f statistic to determine effect size estimates for significant effects. Cohen's f is applied in analysis of variance and is an extension of the effect size statistic (d) applied in the context of the t-test between means of two groups [24]. Cohen's f values are characterized as small (0.10), medium (0.25) and large (0.40) [24]. Given the sample size and large effects obtained, estimated power was >0.87 at alpha probability = 0.05. Regression analyses were used to investigate relationships between significant anthropometric or metabolic factors and the cognitive outcomes. Difference scores for the anthropometric and metabolic factors were calculated by subtracting baseline from final visit values. These difference scores, along with baseline cognitive scores, served as predictors in the regression and the final visit cognitive score was the dependent variable. These analyses determined the degree to which change in an anthropometric or metabolic factor was associated with the cognitive response to the intervention. We also examined within-group changes in anthropometric, metabolic, and dietary factors during the intervention protocol with dependent t-tests.
3. Results
3.1. Study participants
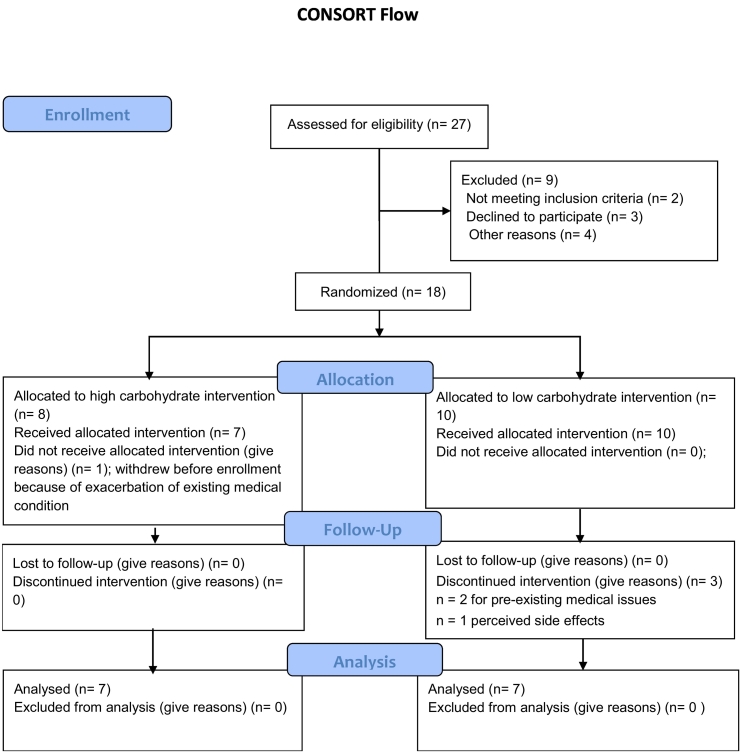
Eighteen patients met eligibility criteria and were enrolled. Four participants did not complete the trial, three because of complications associated with long-standing medical conditions and one because of perceived fatigue. Fourteen patients completed the trial, including 7 randomized to the high carbohydrate regimen and 7 to the low carbohydrate regimen (Fig. 1).
Fig. 1.
CONSORT flow diagram.
3.2. Pre-intervention nutritional parameters
There was no difference between groups with respect to demographic, anthropometric, metabolic, and nutrient intake data at the time of enrollment (Table 1). Total daily energy and macronutrient intake were similar for each group with no difference for any nutritional parameter. Prior to enrollment, carbohydrate and fat intake comprised, respectively, 44% and 40% of total daily kcal for all participants, reflecting the relatively high carbohydrate and fat intake levels characteristic of the Western dietary pattern [13]. Protein comprised 15% of total caloric intake, also consistent with typical US dietary intake data [25].
Table 1.
Baseline sample characteristic by group.
| High carbohydrate (n = 7) | Low carbohydrate (n = 7) | t(12); p | |
|---|---|---|---|
| Age, y | 65.4 (6.5) | 66.0 (5.5) | 0.17; 0.86 |
| Education, y | 15.7 (3.4) | 17.7 (2.2) | 1.30; 0.22 |
| PD duration, y | 2.9 (2.1) | 2.6 (2.8) | 0.19; 0.84 |
| MoCA | 24.2 (2.4) | 25.4 (1.1) | 1.12; 0.28 |
| MoCA recall score | 3.0 (1.4) | 2.5 (0.9) | 0.64; 0.53 |
| Body weight, kg | 96.1 (13.3) | 89.9 (12.1) | 0.89; 0.38 |
| Waist circumference, cm | 105.9 (8.5) | 102.6 (16.8) | 0.46; 0.65 |
| BMI | 31.2 | 27.9 | 0.97; 0.34 |
| Fasting glucose, mg/dL | 91.3 (12.1) | 93.4 (9.9) | 0.35; 0.72 |
| Fasting insulin, μU/L | 8.7 (3.8) | 8.2 (4.4) | 0.26; 0.79 |
| βHB, mM | 0.11 (0.06) | 0.08 (0.04) | 1.27; 0.22 |
| Total energy, kcal/day | 2623 (1586) | 2205 (624) | 0.60; 0.55 |
| Carbohydrate, g/day | 285 (179) | 248 (83) | 0.46; 0.64 |
| Protein, g/day | 101 (46) | 82 (28) | 0.85; 0.40 |
| Fat, g/day | 118 (87) | 95 (29) | 0.61; 0.54 |
| Fiber, g/day | 18.5 (7.4) | 20.2 (7.1) | 0.43; 0.67 |
| Carbidopa | 1 | 0 | |
| Carbidopa/levodopa | 6 | 6 | |
| Tolcapone | 1 | 1 | |
| Ropinirole | 1 | 1 | |
| Selegiline | 0 | 2 | |
| Pramipexole | 0 | 1 | |
| Amantadine | 1 | 0 | |
| Rasagiline | 2 | 0 | |
| Rotigotine | 1 | 0 | |
| Entacapone | 0 | 1 |
Note: Values are M (SD) except where noted otherwise. PD duration = period from Parkinson's disease diagnosis to date of study enrollment. MoCA = Montreal Cognitive Assessment. BMI = body mass index. βHB = beta-hydroxybutyrate. Nutritional information derived from diet diary records obtained during the week prior to the enrollment visit. Values for medications indicate the number of participants within group treated with each drug.
3.3. Between group analyses of cognitive performances
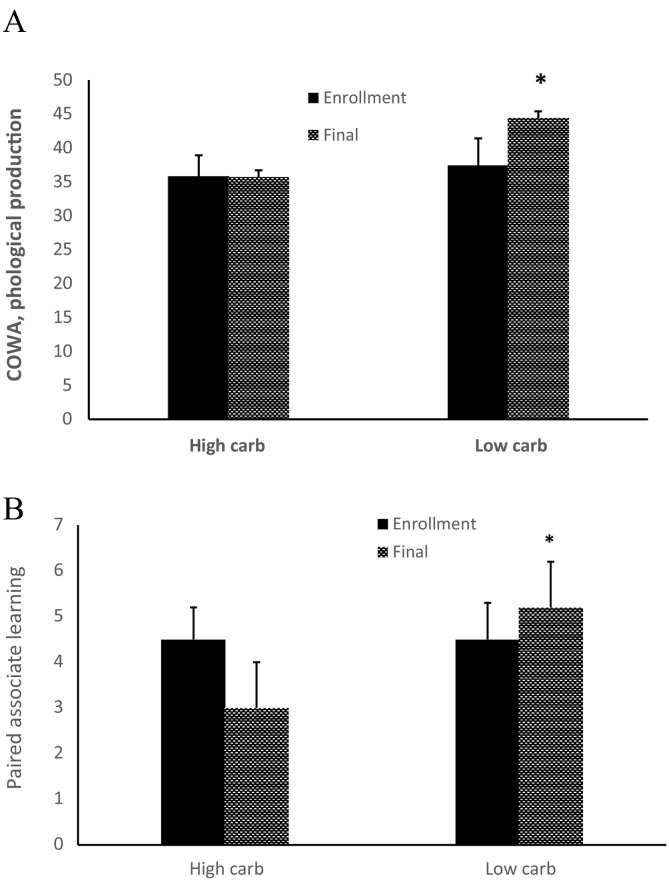
As compared with the high carbohydrate group, the low-carbohydrate group exhibited improved performances on the lexical access composite (F(1,11) = 6.55, p = 0.02, Cohen's f effect size = 0.76; Fig. 2A), and on the memory composite (F(1,11) = 8.42, p = 0.01, f = 0.87; Fig. 2B). The low carbohydrate group also exhibited a trend for reduced interference in memory (F(1,11) = 4.03, p = 0.06, f = 0.60).
Fig. 2.
Enhanced cognitive performances for the low carbohydrate group shown for representative tasks. Panel A: lexical access was enhanced for the low carbohydrate group as reflected in comparatively improved phonological production on the Controlled Oral Word Association (COWA) task (F(1,11) = 6.55, p < 0.02, Cohen's f = 0.79). Panel B shows improved Verbal Paired Associate Learning Test performance for the low carbohydrate group, (F(1,11) = 5.06, p < 0.04, Cohen's f = 0.76).
3.4. Motor function
There was no effect for motor function assessed by the MDS-UPDRS-III (34.5 (10.7) vs 26.5 (5.3), p = 0.42) or for finger tapping for the dominant (45.9 (12.6) vs 43.3 (5.4), p = 0.92) hand and non-dominant (36.6 (12.6) vs 41.3 (4.6), p = 0.58) hand.
3.5. Between-group anthropometric and metabolic parameters
Compared to the high-carbohydrate group, the low-carbohydrate group had greater body weight reduction (95 (12.1) kg vs 83 (12.5) kg; F(1,11) = 39.4, p < 0.0001, f = 1.89), and there was a trend for reduction of waist circumference (104 (8.6) vs 94 (15.3); F(1,11) = 3.54, p = 0.10, f = 0.55). We also observed an effect for β-OHB (0.08 (0.01) mM vs 0.31 (0.19) mM; F(1,11) = 7.74, p = 0.01, f = 0.90). However, there was no between-group difference for fasting glucose (92.6 (17.6) mg/dL vs 91.3 (8.4) mg/dL, p = 0.50) or fasting insulin (6.5 (3.0) μU/L vs 5.8 (3.2) μU/L, p = 0.74).
3.6. Regression analyses
Change in body weight was strongly associated with memory performance (standardized beta (βstd) = − 0.59, p < 0.001) and marginally predicted lexical access (βstd = − 0.34, p = 0.06). Further, change in body weight was related to β-OHB (βstd = − 0.61, p = 0.02).
3.7. Within-group changes in dietary intake
For the high-carbohydrate group, total daily energy intake did not change significantly during the intervention despite the apparently large difference (2623 (1568) kcal to 2016 (671) kcal), and there was no change for any macronutrient. Caloric intake from carbohydrate (43.5% to 39%) and from fat (40% to 43%) were maintained at pre-intervention proportions for the high carbohydrate participants and comprised the largest components of energy intake. The relative proportion of daily energy from protein (15% to 17%) and intake of fiber (18.5 (7.4) g to 17.6 (6.9) g) also did not change during the intervention.
However, for the low carbohydrate group, daily energy intake declined significantly (2205 (624) kcal to 1667 (404) kcal, p = 0.004) as did carbohydrate intake (from 44.9% to 8% of total kcal, p = 0.001). Intake of protein (14% to 29%, p = 0.009) and fat (38% to 61%, p = 0.04) increased relative to pre-intervention levels, and there was a reduction of fiber consumption (20.2 (7.1) g to 6.8 (4.1) g, p = 0.007).
3.8. Within-group anthropometric and metabolic parameters
At week 8, the high-carbohydrate group was unchanged with respect to body weight, waist circumference, fasting glucose, and β-OHB relative to baseline levels (Table 2). However, fasting insulin declined significantly (p = 0.01). In contrast, within the low-carbohydrate group, several anthropometric and metabolic factors were modified by the intervention including reductions in body weight (p < 0.001), waist circumference (p = 0.05), and fasting insulin (p = 0.04) along with an increase in β-OHB (p = 0.02).
Table 2.
Within group changes in anthropometric and metabolic parameters and energy and macronutrient intake during intervention.
| High carbohydrate |
Low carbohydrate |
|||||
|---|---|---|---|---|---|---|
| Enrollment | Final | t(7); p | Enrollment | Final | t(7); p | |
| Body weight, kg | 96.1 (13.3) | 95.1 (12.1) | 1.4; 0.20 | 89.9 (12.1) | 83.4 (12.5) | 11.2; <0.001⁎ |
| Waist, cm | 105.9 (8.5) | 104.2 (8.6) | 4.1; 0.36 | 102.6 (16.8) | 94.7 (15.3) | 2.34; 0.05⁎ |
| Glucose, mg/dL | 91.3 (12.1) | 92.6 (17.6) | 0.31; 0.76 | 93.4 (9.9) | 91. (8.4) | 1.26; 0.25 |
| Insulin, μU/L | 8.7 (3.8) | 6.5 (3.0) | 3.23; 0.01⁎ | 8.2 (4.4) | 5.8 (3.2) | 2.54; 0.04⁎ |
| βHB, mM | 0.11 (0.06) | 0.08 (0.01) | 1.50; 0.18 | 0.08 (0.04) | 0.31 (0.19) | 3.03; 0.02⁎ |
| Total energy, kcal | 2623 (1586) | 2016 (671) | 1.45; 0.19 | 2205 (624) | 1667 (404) | 4.90; 0.004⁎ |
| Carbohydrate, g; % total kcal | 285; 43% | 197; 39% | 1.39; 0.21 | 248; 44% | 36; 8% | 6.39; 0.001⁎ |
| Protein, g; % total kcal | 101; 15% | 89; 17% | 0.84; 0.42 | 82; 14% | 123; 29% | 4.08; 0.009⁎ |
| Fat, g; % total kcal | 118; 40% | 97; 43% | 1.30; 0.23 | 95; 38% | 113; 61% | 2.59; 0.04⁎ |
| Fiber, g | 18.5 (7.4) | 17.6 (6.9) | 0.57; 0.58 | 20.2 (7.1) | 6.8 (4.1) | 4.36; 0.007⁎ |
Note. Values are M (SD) except where indicated otherwise.
Indicates p < 0.05.
4. Discussion
This proof of concept trial demonstrated that carbohydrate restriction with ketogenesis improved performances in aspects of executive ability and memory in PD-MCI participants. Executive abilities represent a key domain vulnerable to early PD-MCI [26]. In this study, we observed better lexical access and fewer intrusion errors for the low carbohydrate group, reflecting improved executive control processes including inhibition, set shifting, and access to semantic knowledge [27].
In addition, the effect for memory supports the notion of improved memory encoding among the low carbohydrate participants, implying enhanced function of the hippocampus and associated medial temporal lobe structures [28]. This finding recapitulates our prior observation of improved paired associate learning with nutritional ketosis in non-PD MCI participants [6]. Ketone metabolism may have particular benefit for medial temporal lobe structures including hippocampus [29], at least in part because these structures are more vulnerable to aging and metabolic disturbance, underscoring the salience of a metabolic intervention.
The strong inverse association between the intervention-related change in body weight and memory enhancement was not anticipated, although we had expected a positive association of memory with β-OHB [6]. There is evidence that BMI is inversely related to central ATP production [30] and that obesity is related to brain atrophy and neurocognitive decline [31], emphasizing the importance of body weight regulation for brain bioenergetics and structural integrity. Accordingly, weight loss was not merely a side effect of carbohydrate restriction. Body weight reduction, particularly in overweight individuals would be expected to be related to reductions of insulin and inflammatory mediators [31], which also would contribute to improved central bioenergetic function. An important counterpoint to the apparent benefit associated with weight loss is concern regarding loss of lean mass. While we did not measure lean mass in this study, one might expect at least modest muscle loss in the context of decline in body weight in the low carbohydrate group, even though ketosis generally is muscle sparing. This would be an important issue in longer trials and with respect to individuals with PD who might be more vulnerable to muscle atrophy. The future application of the ketogenic approach in this population, especially for longer duration, should monitor body composition and caloric intake.
It is of interest that the high carbohydrate group exhibited significant reduction of insulin during the course of the intervention, even while maintaining overall high carbohydrate intake. This implies that the high carbohydrate participants reduced high glycemic carbohydrates in favor of vegetables as recommended in the nutritional education. However, this effect in the context of a relatively brief intervention was not sufficient to enhance cognitive performance.
There is no approved pharmacologic intervention to treat cognitive decline in PD. As such, these preliminary findings may have implications for application of a worthwhile non-pharmacologic management strategy for patients with PD-MCI. Randomized trials employing nutritional interventions have demonstrated mitigation of dementia risk [32]. Other nutritional approaches have included supplementation with bioactive compounds such as flavonoids for PD [33] and alteration of overall dietary pattern such as carbohydrate restriction and the Mediterranean diet for Alzheimer's disease [34]. While nutritional interventions have implications for improved metabolic function, one might consider ketosis to be among the most potent because it is associated with metabolic benefits and enhanced cerebral bioenergetics, the latter demonstrated to offset glucose hypometabolism associated with neurodegeneration [35].
This study has limitations inherent to its pilot nature and relatively brief duration. First, the sample size was small, which may have precluded observation of a modest change in motor function, although we obtained large effect sizes for the cognitive findings. This factor, along with evidence of similar benefit in previous studies [5,6], contributes to emerging evidence supporting further investigation of this intervention. The gender proportions of the final cohort were overwhelmingly male. Prospective participants who were screened and did not qualify for enrollment or declined to participate also were predominantly male. However, the clinic population from which the sample was drawn generally reflects national gender proportions for PD (2:1 male to female). We are not aware of a systematic reason for the gender inequality other than a tendency for greater cognitive impairment in men with PD observed in other research [36]. The unbalanced gender proportions may have been a random effect that would have been mitigated in a larger sample.
The brief duration of the intervention may have enhanced adherence but limited the benefit of a longer-term protocol. Notably, we did not observe benefit for motor function in this trial, which may have occurred with a longer intervention period. Future investigations with longer intervention periods will be important as will studies that assess the duration of benefit following discontinuation of the therapy. Finally, we were not able to include biomarkers or metabolic measures through neuroimaging or cerebrospinal fluid that could have provided direct evidence of central nervous system mechanisms.
In sum, we have shown that short-term nutritional ketosis is capable of enhancing cognitive performance in PD-MCI patients. The findings extend prior observations of neurocognitive benefits in at-risk individuals and in those with early neurodegeneration. However, further investigation is needed with larger samples in order to demonstrate the reproducibility of the findings. In addition, it will be necessary to investigate whether longer duration intervention might provide similar or greater benefit and whether benefit is sustained after termination of the treatment. Future studies of nutritional ketosis also will be of interest to evaluate the effects of genetic and epigenetic factors and endophenotypes of PD and to investigate central mechanisms.
Documentation of author roles
-
1)
Research project: A. Conception, B. Organization, C. Execution;
-
2)
Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
-
3)
Manuscript: A. Writing of the first draft, B. Review and Critique.
RK: 1A, 1B, 2A, 2B, 2C, 3A, 3B
MDS: 1B, 1C
SSS: 1C, 3B
PGS: 3B
APD: 1C, 3B
RSI: 3B
AJE: 1C, 2C, 3B
Financial disclosure related to this research
Funding: This work was supported by the Center for Clinical and Translational Science, University of Kentucky & Center for Clinical and Translational Science, University of Cincinnati (NIH CTSA #UL1TR000117), the Gardner Family Fund of the University of Cincinnati Neuroscience Institute, and from the Lurie Family Gift to support dementia prevention research.
Financial disclosures of authors
RK received research support from the US Highbush Blueberry Council, the California Strawberry Commission, Roche Pharmaceuticals, and the Lurie Family Gift.
MDS received support from the US Highbush Blueberry Council, the California Strawberry Commission, and Roche Pharmaceuticals.
SSS None.
PGS has received grant support from the NIH, the Kentucky Spinal Cord and Head Injury Research Trust, and the Veterans Administration BLRD.
APD received grant support from NeuroNEXT and the Michael J. Fox Foundation for Parkinson's Research.
RSI received research support from the NIH, the Zuckerman Family Foundation, and the Women's Alzheimer's Movement.
AJE received grant support from the NIH, Great Lakes Neurotechnologies, and the Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, ACADIA, Acorda, Cynapsus/Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, ACADIA, the American Academy of Neurology, and the Movement Disorders Society.
References
- 1.Sandyk R. The relationship between diabetes mellitus and Parkinson's disease. Int. J. Neurosci. 1993;69:125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- 2.Dunn L., Allen G.F., Mamais A., Ling H., Li A., Duberley K.E., Hargreaves I.P., Pope S., Holton J.L., Lees A., Heales S.J., Bandopadhyay R. Dysregulation of glucose metabolism is an early event in sporadic Parkinson's disease. Neurobiol. Aging. 2014;35:1111–1115. doi: 10.1016/j.neurobiolaging.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schapira A.H. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;1:97–109. doi: 10.1016/S1474-4422(07)70327-7. [DOI] [PubMed] [Google Scholar]
- 4.Bosco D., Plastino M., Cristiano D., Colica C., Ermio C., DeBartolo M., Mungari P., Fone G., Consoli D., Consoli A., Fava A. Dementia is associated with insulin resistance in Parkinson's disease. J. Neurol. Sci. 2012;315:39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Croteau E., Castellano C.-A., Richard M.A., Fortier M., Nugent S., Lepage M., Duchesne S., Whittingstall K., Turcotte E.E., Bocti C., Fulop T., Cunnane S.C. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer's disease. J. Alzheimers Dis. 2018;64:551–561. doi: 10.3233/JAD-180202. [DOI] [PubMed] [Google Scholar]
- 6.Krikorian R., Shidler M.D., Dangelo K., Couch S.C., Benoit S.C., Clegg D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging. 2012;33:425.e19–425.e27. doi: 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krikorian R., Boespflug E.L., Dudley J.A., Norris M.M., Chu W.-J., Summer S.S., Eliassen J.C. Enhanced cerebral bioenergetics with dietary ketosis in mild cognitive impairment. Nutri. Aging. 2014;2:223–232. [Google Scholar]
- 8.Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson's disease. A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litvan I., Goldman J.G., Troster A.I., Schmand B.A., Weintraub D., Petersen R.C., Mollenhauer B., Adler C.H., Marder K., Williams-Gray C.H., Aarsland D., Kulisevsky J., Rodriguez-Oroz M.C., Burn D.J., Barker R.A., Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement Disorder Society task force guidelines. Mov. Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalrymple-Alford J.C., MacAskill M., Nakas C.T., Livingston L., Graham C., Crucian G.P., Melzer T.R., Kirwan J., Keenan R., Wells S., Porter R.J., Watts R., Anderson T.J. The MoCA: well-suited screen for cognitive impairment in Parkinson's disease. Neurol. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 11.Ritter A., Hawley N., Banks S.J., Miller J.B. The association between Montreal Cognitive Assessment memory scores and hippocampal volume in a neurodegenerative disease sample. J. Alzheimers Dis. 2017;58:695–699. doi: 10.3233/JAD-161241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyer M.K., Bronnick K.S., Hwang K.S., Bergsland N., Tysnes O.B., Larsen J.P., Thompson P.M., Somme J.H., Apostolova L.G. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2013;84:23–28. doi: 10.1136/jnnp-2012-303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordain L., Eaton B.S., Sebastian A., Mann N., Lindeberg S., Watkins B.W., O'Keefe J.H., Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 14.Kosoff E.A., Dorward J.L. The modified Atkins diet. Epilepsia. 2008;8:37–41. doi: 10.1111/j.1528-1167.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 15.Willet W. Nutritional Epidemiology, Monographs in epidemiology and biostatistics. Oxford University Press; New York: 2013. 24-hour recall and diet record methods; pp. 49–69. [Google Scholar]
- 16.Miceli G., Caltagirone C., Gainotti G., Masullo C., Silveri M.C., Villa G. Influence of age, sex, literacy and pathologic lesion on incidence, severity and type of aphasia. Acta Neurol. Scand. 1981;64:370–382. doi: 10.1111/j.1600-0404.1981.tb04416.x. [DOI] [PubMed] [Google Scholar]
- 17.Troyer A.K., Moscovitch M., Winocur G., Alexander M.P., Stuss D. Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- 18.Delis D.C., Kramer J.H., Kaplan E., Ober B.A. second edition. Psychological Corporation; San Antonio, TX: 2000. California Verbal Learning Test. [Google Scholar]
- 19.Krikorian R. Independence of verbal and spatial paired associate learning. Brain Cogn. 1996;32:219–223. [Google Scholar]
- 20.Goetz C.G., Tilley B.C., Shaftman S.R., Stebbins G.T., Fahn S., Martinez-Martin P., Poewe W., Sampaio C., Stern M.B., Dodel R., Dubois B., Holloway R., Jankovic J., Kulisevsky J., Lang A.E., Lees A., Leurgans S., LeWitt P.A., Nyenhuis D., Olanow C.W., Rascol O., Schrag A., Teresi J.A., van Hilten J.J., LaPelle N., Movement Disorder Society UPDRS Revision Task Force Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 21.Reitan R.M., Wolfson D. 2nd edition. Neuropsychology Press; Tucson AZ: 1993. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Applications. [Google Scholar]
- 22.Riordan H.J. Constructing composites to optimize cognitive outcomes. J. Clin. Studies. 2017;9:42–46. [Google Scholar]
- 23.Sheeber L.B., Sorensen E.D., Howe S.R. Data analytic techniques for treatment outcome studies with pretest/posttest measurements: an extensive primer. J. Psychiatr. Res. 1996;30:185–199. doi: 10.1016/0022-3956(96)00012-X. [DOI] [PubMed] [Google Scholar]
- 24.Cohen J. 2nd edition. Lawrence Erlbaum Associates; New York: 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 25.Fulgoni V.L. Current protein intake in America: analysis of the National Health and Nutrition Examination survey 2003-2004. Am. J. Clin. Nutr. 2008;87:15545–15575. doi: 10.1093/ajcn/87.5.1554S. [DOI] [PubMed] [Google Scholar]
- 26.Dimberger G., Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J. Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- 27.Fisk J.E., Sharp C.A. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J. Clin. Exp. Neuropsychol. 2003;26:874–890. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- 28.Clark I.A., Kim M., Maguire E.A. Verbal paired associates and the hippocampus: the role of scenes. J. Cogn. Neurosci. 2018;30:1821–1845. doi: 10.1162/jocn_a_01315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elamin M., Ruskin D.N., Masio S.A., Sacchetti P. Ketone-based metabolic therapy: is increased NAD+ a primary mechanism? Front. Mol. Neurosci. 2017;10 doi: 10.3389/fnmol.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmoller A., Hass T., Strugovashchikova O., Melchert U.H., Scholand-Engler H.G., Peters A., Schweiger U., Hohagen F., Oltmanns K.M. Evidence for a relationship between body mass and energy metabolism in the human brain. J. Cereb. Blood Flow Metab. 2010;30:1403–1410. doi: 10.1038/jcbfm.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Brien P.D., Hinder L.M., Callaghan B.C., Feldman E.L. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–477. doi: 10.1016/S1474-4422(17)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Bäckman L., Hänninen T., Jula A., Laatikainen T., Lindström J., Mangialasche F., Paajanen T., Pajala S., Peltonen M., Rauramaa R., Stigsdotter-Neely A., Strandberg T., Tuomilehto J., Soininen H., Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 33.Gao X., Cassidy A., Schwarzschild M.A., Rimm E.B., Ascherio A. Habitual intake of dietary flavonoids and risk of Parkinson's disease. Neurology. 2012;78 doi: 10.1212/WNL.0b013e31824f7fc4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psaltopoulou T., Sergentanis T.N. Mediterranean diet may reduce Alzheimer's risk. Evid. Based Med. 2015;20:202. doi: 10.1136/ebmed-2015-110237. [DOI] [PubMed] [Google Scholar]
- 35.Cunnane S.C., Courchesne-Loyer A., Vandenberghe C. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front. Mol. Neurosci. 2016;9:53. doi: 10.3389/fnmol.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller J., Dogan I., Schulz J.B., Reetz K. Evidence for gender differences in cognition, emotion, and quality of life in Parkinson's disease? Aging Dis. 2014;5:63–75. doi: 10.14366/AD.2014.050063. [DOI] [PMC free article] [PubMed] [Google Scholar]