Abstract
High-definition mapping of atrial fibrillation is most commonly performed from the endocardial surface. This report describes an example of a case in which combined mapping of the endocardium and epicardium of the left atrium demonstrated electrical dissociation between the 2 surfaces and implies that endocardial mapping alone may not provide sufficient information. (Level of Difficulty: Advanced.)
Key Words: ablation, atrial fibrillation, electroanatomical mapping
Abbreviations and Acronyms: AF, atrial fibrillation; HD, high definition; LA, left atrium; PeAF, persistent atrial fibrillation
Graphical abstract
High-definition mapping of atrial fibrillation is most commonly performed from the endocardial surface. This report describes an example of a case…
Catheter ablation for the treatment of persistent atrial fibrillation (PeAF) is a complex procedure with a recurrence rate of 41% to 54% at 18 months depending on the precise procedural technique used (1). High-definition (HD) mapping during PeAF often reveals specific regions of organized activity and continuous fractionated signals which may reflect transient rotational activation and colliding wavefronts. Mapping is generally confined to the endocardial surface in most cases, and patterns of epicardial activation are generally unknown. Epicardial activation may differ significantly from endocardial recordings and may further partly explain limitations of the currently available strategies.
Presentation
A 59-year-old male with a 10-year history of symptomatic PeAF refractory to antiarrhythmic drug therapy and a prior subarachnoid hemorrhage was referred for hybrid ablation and epicardial left atrium (LA) appendage closure. With the patient under general anesthesia, LA endocardial access was obtained using a transseptal approach and a HD Grid catheter (Abbott, Inc., St. Paul, Minnesota) advanced into the LA. At the same time, epicardial mapping was performed using a right thoracoscopic approach in which a second HD Grid catheter (Abbott, Inc.) was advanced through both the transverse and the oblique pericardial sinuses.
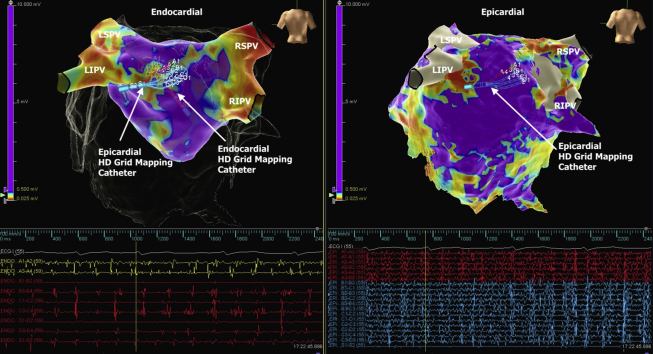
As shown in Figure 1, simultaneous recordings from the posterior wall of the LA show continual fractionated signals on the epicardial surface with slower and more organized signals from the equivalent location on the endocardium. This demonstrates electrical dissociation between the 2 surfaces and implies that endocardial mapping alone may not provide sufficient information to develop an ablation strategy. Epicardial ablation resulted in isolation of the posterior wall of the LA with restoration of sinus rhythm and electrical silence of both the endocardial and the epicardial surfaces.
Figure 1.
Endocardial Voltage Map
(Top left) Endocardial voltage map with signals recorded on the posterior wall of the LA (bottom left) showing organized and slower electrical activation when compared with simultaneous continuous highly fractionated signals (bottom right) recorded from a similar location on the epicardial surface (top right). Voltage settings at 0.5 mV. LA = left atrium; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; RSPV = right superior pulmonary vein; RIPV = right inferior pulmonary vein.
Dissociation of electrical activity between the endocardial and epicardial surfaces is a finding which the present authors have observed repeatedly in cases of combined endocardial and epicardial HD mapping, and epicardial connections in patients with endocardial posterior wall isolation were previously published (2).
Discussion
Prior preclinical studies have demonstrated electrical dissociation which appears to progress over time (3). Despite the fact that the LA wall is extremely thin, measuring 2.3 ± 0.9 mm there is a considerable difference in the electrical activation of both surfaces, as demonstrated in the present case (4). Epicardial activation may be an independent source of fibrillatory activity which cannot be adequately mapped from the endocardial surface, and that may help to explain some cases of AF recurrences following catheter ablation of PeAF.
Footnotes
Dr. Baley is an employee of Abbott Medical Canada, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Verma A., Jiang C.Y., Betts T.R. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 2.Glover B.M., Hong K.L., Baranchuk A., Bakker D., Chacko S., Bisleri G. Preserved left atrial epicardial conduction in regions of endocardial “isolation.”. J Am Coll Cardiol EP. 2018;4:557–558. doi: 10.1016/j.jacep.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 3.de Groot N.M., Houben R.P., Smeets J.L. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–1682. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 4.Platonov P.G., Ivanov V., Ho S.Y., Mitrofanova L. Left atrial posterior wall thickness in patients with and without atrial fibrillation: data from 298 consecutive autopsies. J Cardiovasc Electrophysiol. 2008;19:689–692. doi: 10.1111/j.1540-8167.2008.01102.x. [DOI] [PubMed] [Google Scholar]