Abstract
Designing new cathodes with high capacity and moderate potential is the key to breaking the energy density ceiling imposed by current intercalation chemistry on rechargeable batteries. The carbonaceous materials provide high capacities but their low potentials limit their application to anodes. Here, we show that Fermi level tuning by p-type doping can be an effective way of dramatically raising electrode potential. We demonstrate that Li(Na)BCF2/Li(Na)B2C2F2 exhibit such change in Fermi level, enabling them to accommodate Li+(Na+) with capacities of 290–400 (250–320) mAh g−1 at potentials of 3.4–3.7 (2.7–2.9) V, delivering ultrahigh energy densities of 1000–1500 Wh kg−1. This work presents a new strategy in tuning electrode potential through electronic band structure engineering.
Keywords: p-type doping strategy, electrochemical potential tuning, Li(Na)BCF2/B2C2F2 cathodes, ultrahigh energy densities
Teaser text: Band structure engineering is used to design a new family of transition-metal-free Li(Na)BCF2/B2C2F2 cathodes for rechargeable batteries that achieve a record-breaking high energy density.
INTRODUCTION
The growing demand for batteries with exceedingly high energy density (ED) that can be used in electrical vehicles has inspired an active search—with regard to experiments and multi-scale calculations—for novel electrode materials [1–4]. The theoretical gravimetric energy density of a battery is defined as  , where n, F, E0 and ΣMi refer to the electron transfer, Faraday constant, electromotive force and formula weights of active materials participating in the cell reaction, respectively [5]. To achieve higher energy density, a battery must be built of well-matched cathodes and anodes with: (i) a maximized amount of ions available for (de)intercalation, (ii) large Gibbs free energy change when charge carriers are transferred between electrodes, and (iii) a light weight [6]. Since anodes often offer higher Li-ion storage capacities than cathodes [7], there is an immense effort from both academia and industry to boost the capacities of current widely used transition-metal oxide (TMO) cathodes to above ∼300 mAh g−1 (Fig. 1). For a long time, it was considered that transition-metals (TMs) are the sole source of electrochemical activity in cathodes with the specific capacity being limited by the electrons that TMs can exchange [8–10]. However, recent studies demonstrated that oxygen in oxide cathodes may also participate in the redox reaction. Many Li-rich materials, such as Li1+x(Ni1-y-zMnyCoz)1-xO2 [11,12], Li1.2Ni0.2Mn0.6O2 [5,13], Li2Ru0.5Sn0.5O2 [14] and Li1.3Mn0.4Nb0.3O2 [15], offer capacities up to 250–300 mAh g−1 through charge transfer via oxygen sites. However, the formation of highly reactive peroxide or superoxide species leads to potential safety issues, thus, the long-term practical application of these Li-rich oxides and viability of oxygen redox utilization is still under debate [16].
, where n, F, E0 and ΣMi refer to the electron transfer, Faraday constant, electromotive force and formula weights of active materials participating in the cell reaction, respectively [5]. To achieve higher energy density, a battery must be built of well-matched cathodes and anodes with: (i) a maximized amount of ions available for (de)intercalation, (ii) large Gibbs free energy change when charge carriers are transferred between electrodes, and (iii) a light weight [6]. Since anodes often offer higher Li-ion storage capacities than cathodes [7], there is an immense effort from both academia and industry to boost the capacities of current widely used transition-metal oxide (TMO) cathodes to above ∼300 mAh g−1 (Fig. 1). For a long time, it was considered that transition-metals (TMs) are the sole source of electrochemical activity in cathodes with the specific capacity being limited by the electrons that TMs can exchange [8–10]. However, recent studies demonstrated that oxygen in oxide cathodes may also participate in the redox reaction. Many Li-rich materials, such as Li1+x(Ni1-y-zMnyCoz)1-xO2 [11,12], Li1.2Ni0.2Mn0.6O2 [5,13], Li2Ru0.5Sn0.5O2 [14] and Li1.3Mn0.4Nb0.3O2 [15], offer capacities up to 250–300 mAh g−1 through charge transfer via oxygen sites. However, the formation of highly reactive peroxide or superoxide species leads to potential safety issues, thus, the long-term practical application of these Li-rich oxides and viability of oxygen redox utilization is still under debate [16].
Figure 1.
Schematic open-circuit voltage (Voc) of battery. The energy separation of the lowest-unoccupied-molecular-orbital (LUMO) and the highest-occupied-molecular-orbital (HOMO) is the electrolyte window. Electrochemical potential vs. capacity is presented for both graphite-anode and cathodes. The cathodes are commonly transition-metal (TM) compounds which have layered, spinel or olivine crystal structures.
In light of the limited capacity of TMO cathodes, the advantages of light-weight carbonaceous materials as alternative electrodes are quite attractive. The variable orbital hybridization (sp, sp2, sp3) in those materials facilitates both structural stability and flexibility for Li+ storage [17]. With their operating potentials close to that of Li+/Li (0.2–1.5 V) and high theoretical capacity (e.g. 372 mAh g−1 for LiC6), they have served as ideal anode candidates for the past 25 years [18,19]. Other modified carbonaceous systems have also been proposed as high-capacity hosts for Li-ions, which can be classified into three types based on the carbon-hybridization nature (Table S1): 2D sp2, 3D sp2 and 3D sp2 + sp3 hybridization. Nevertheless, the Fermi levels of these modified carbon allotropes are all determined by the high-energy sp2-pz orbitals (Fig. S1), resulting in the invariably low potentials. An interesting question remains though: can the potential of carbonaceous materials be tuned to a level comparable to those of currently used TMO cathodes?
Fluorides possess higher electrochemical potential than oxide analogs due to the strongly inductive effect of fluorine [20], which opens opportunities for modifying the potential of carbonaceous electrodes. Indeed, fluorinated-graphite (CFx) has been used as a cathode in Li primary batteries (Table S1) [21], whose potential reaches about 2.5 V [22]. Unfortunately, the lithiated fluorinated-graphite is rarely rechargeable, and its thermodynamic instability leads to spontaneous decomposition into graphite and LiF [23–25]. It is known that the chemical potential of lithium can be separated into the (electro)chemical potentials of electrons  and ions
and ions 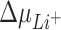 . Here,
. Here, 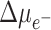 is determined by the Fermi level of electrode and plays a major role in the battery potential (
is determined by the Fermi level of electrode and plays a major role in the battery potential (  ) [26]. Rational tuning of ion intercalation potentials of carbonaceous electrodes hence relies on fine control of their electronic structure [4,27,28]. So far, there is no report on improving the energy density of fluorinated-graphite cathodes for rechargeable batteries.
) [26]. Rational tuning of ion intercalation potentials of carbonaceous electrodes hence relies on fine control of their electronic structure [4,27,28]. So far, there is no report on improving the energy density of fluorinated-graphite cathodes for rechargeable batteries.
In this work, we use the graphite anode as a starting point to demonstrate the feasibility of the p-type doping strategy for shifting the Fermi level and substantially enhancing the potential of cathodes in an electrochemical cell. It is shown that the Fermi level drops upon p-type doping in the newly designed Li(Na)BCF2 and Li(Na)B2C2F2 cathodes. They have average Li+(Na+) deintercalation potentials up to 3.49 (2.78) V and 3.63 (2.85) V, and deliver theoretical capacities of 395.4 (319.6) mAh g−1 and 295.8 (251.2) mAh g−1, respectively. As a result, the gravimetric energy densities of Li(Na)BCF2 and Li(Na)B2C2F2 reach the record level of 1379.9 (888.5) and 1073.8 (715.9) Wh kg−1 for Li+(Na+) storage, respectively. This finding reveals an exciting path for the design of next-generation cathodes with ultrahigh energy density based on band structure engineering.
RESULTS
The graphite anode exists in a relatively stable energy state with the Fermi level of −4.31 eV before Li+ intercalation (Fig. S2), which ultimately leads to its low potential (Fig. S1). Since the early 1970s there have been extensive efforts aimed at using fluorinated-graphite as cathodes for Li-ion batteries because of the strong electronegativity of fluorine [21]. The C-F sp3 σ bond has a deep energy level, resulting in the lower Fermi level of CF (−6.97 eV) (Fig. S2). Considering that CF has a wide band gap (∼3.0 eV), the electrons introduced by lithiation can fill into low energy levels if holes are created, which may result in a high electrochemical potential (Fig. 1). Achieving this goal is complicated by the fact that holes need to be created under the following conditions: (i) p-doping (or hole-doping) should not fundamentally modify the favorable orbital hybridizations and, more importantly, (ii) the structure should be stable during the electrochemical process.
Being adjacent in the Periodic Table to C, B is an ideal dopant for creating holes in CF. By using an unbiased swarm-intelligence structure searching method, as implemented in the CALYPSO code [29], we identified stable structures in the Li(Na)-B-C-F system. The effectiveness of the CALYPSO code in finding layered materials has been validated by successfully reproducing known materials [30]. Many new materials predicted by CALYPSO have been also experimentally confirmed [30,31]. Following the procedure described in Section S3, we reproduced the known CF structures and identified new stable Li(Na)BCF2 with P-3m1 symmetry (Fig. 2 and Table 1).
Figure 2.
(a) Crystal structure of Li(Na)BCF2 cathodes. The yellow, red, blue and green spheres are C, B, F and Li(Na) atoms, respectively. The layer-thickness (d1) and layer-distance (d2) are also marked. (b) Band structures of BCF2 (left) and LiBCF2 (right). The Fermi level (EF) is set to zero. (c) The differential-charge-density (Δρ) between BCF2 and LiBCF2. The total charge density isosurfaces (0.032 e Å−3) of both BCF2 and LiBCF2 are given in yellow. The positive and negative isosurfaces (0.003 e Å−3) of Δρ are given in blue and yellow, respectively.
Table 1.
Lattice constants a and c (Å), volume V (Å3), buckling (Å), F-C-C bond-angle in CF and F-B-C bond-angle in B-alloying system (°), d1 (Å) and d2 (Å) in the Li(Na)BCF2/Li(Na)B2C2F2 cathodes. The data for CF are also given for comparison.
| BCF2 | LiBCF2 | NaBCF2 | B2C2F2 | LiB2C2F2 | NaB2C2F2 | CF | |
|---|---|---|---|---|---|---|---|
| a = b | 2.713 | 2.802 | 2.856 | 2.696 | 2.720 | 2.752 | 2.599 |
| c | 11.630 | 11.479 | 12.451 | 15.895 | 15.759 | 16.798 | 11.403 |
| V | 74.113 | 78.072 | 87.935 | 100.042 | 100.944 | 110.188 | 66.701 |
| Buckling | 0.665 | 0.338 | 0.338 | 0.728 | 0.532 | 0.528 | 0.488 |
| Bond-angle | 113.01 | 101.81 | 101.58 | 115.07 | 108.72 | 108.37 | 108.03 |
| d1 | 3.364 | 3.539 | 3.527 | 5.502 | 5.627 | 5.615 | 3.249 |
| d2 | 2.451 | 2.201 | 2.699 | 2.445 | 2.252 | 2.784 | 2.452 |
The dynamical and thermodynamic stability of Li(Na)BCF2 was further verified by conducting phonon calculations and molecular dynamic simulations, as well as by calculating the grand potential phase diagrams under different synthesis conditions (Figs S10–S14 and Tables S5–S8). For dynamical stability, in Fig. S11, no imaginary frequency has been found to show good dynamic stability in the phonon dispersion curves for the lithiated and delithiated states. Furthermore, we also performed the molecular dynamics (MD) simulation of lithiated and delithiated states at 500 K (Fig. S12). The results show that the structures of lithiated and delithiated states maintain well. For the lithiated state LiBCF2, we further increased the MD simulation temperature to 800 K. The results show that the structure also maintains well. The combination of results indicates that, like many available cathodes, Li(Na)BCF2 structures are metastable and can be synthesized by considering the entropic effect. The Li/Na-ion migration behavior in Li(Na)BCF2 cathodes under fully charged and discharged states were also investigated by the first-principles calculations (Figs S15 and S16, and Table S9). As shown in Fig. S16, the energy barriers of Li ion migration in the lithiated state LiBCF2 and delithiated state BCF2 are 0.67 and 0.57 eV, respectively. For Na ion migration, the corresponding energy barriers are 0.70 and 0.48 eV, respectively. The energy barriers of the Li/Na ion diffusion in BCF2 systems are close to those in common cathode materials that are used in battery applications. Thus, the Li/Na ion diffusion in Li(Na)BCF2 structure is acceptable for battery applications.
Next, we investigated the electronic structure of the hole-containing BCF2 system compared to that of the CF system. The F-C-B bond angle in BCF2 and the F-C-C bond angle in CF, as given in Table 1, indicate that the sp3-hybridized CF framework is maintained after B-alloying. Thus, the concept of ‘p-type doping’ is proposed to emphasize that both the structure and the orbital hybridization in the BCF2/B2C2F2 system are unchanged from that of the pristine CF system. More importantly, the C-sp and F-p orbitals in CF form a stable state filled with electrons following the 8-electron rule (Section S2). As a result, the Fermi level of BCF2 shifts to −8.36 eV (Fig. S3), much lower than that of CF (−6.97 eV). Moreover, the Fermi level passes through the valence bands of BCF2, which proves the success of our strategy of creating holes in valence bands (Fig. 2b). It is also confirmed that lowering the Fermi level in the B-doped BCF2 system is caused by the electron loss on the sp3 σ orbitals, and the overall orbital hybridization pattern of the original CF system is not much affected (Section S2). Therefore, the B-alloyed CF structure is expected to have a substantially increased electrochemical potential. Also, the band structures of both Li(Na)BCF2 and Li(Na)B2C2F2 exhibit metallic characteristics (Figs S2 and S3), implying their good electrical conductivity as cathodes.
It is interesting to test if increasing the B/F ratio can further tune the electron chemical potential. On this basis, the B2C2F2 system, which was derived from another half-fluorinated graphite structure (C2F), was constructed. As shown in Table S2, the B-C hybridized orbitals are around the Fermi level in both BCF2 and B2C2F2 systems. Nevertheless, a longer B-C bond length (1.718 Å) was observed in B2C2F2 in comparison with that in BCF2 (1.702 Å). This is because half of the π bonds are passivated by F so the F-B interaction is relatively weak. Consequently, the Fermi level is slightly reduced from −8.36 eV in BCF2 to −8.48 eV in B2C2F2 (Fig. 3). The calculated grand potential phase diagram (Fig. S10) and phonon dispersion (Fig. S11) results also confirm that Li(Na)B2C2F2 can be experimentally prepared under certain pressure and temperature conditions.
Figure 3.

(a) Schematic of bonding modes between F, B and C orbitals in B2C2F2 system. All orbital energy levels are aligned with respect to the vacuum level. (b) Illustration of electronic structure tuning of carbonaceous electrode to address the high potential requirement for cathodes.
We further investigate the effect of the Fermi level tuning on the ions-(de)intercalation potentials of both BCF2 and B2C2F2 systems. A new strategy based on group-subgroup analysis was proposed for a rigorous search over the large configurational phase space with unit-cells of up to 54 Li/Na sites for different intercalation stages of Lix(Nax)BCF2 and Lix(Nax)B2C2F2 (0 < x < 1, see details in Section S6). Through the process of subgroup projection and Wyckoff position splitting, 16 220 and 79 050 Lix/NaxBCF2 and Lix/NaxB2C2F2 configurations in different concentrations are obtained, respectively. Notably, the linear fitting residual of Ewald and density functional theory (DFT) energies shown in Fig. S19 implies that it makes no sense to compare the trend between DFT and Ewald energies. However, considering that the electrostatic energies should not be changed for structures that are symmetrically related, it is feasible to judge the structural similarity by using the calculated Ewald energies to save the computational resources. As a result, after screening and eliminating the symmetry equivalent structures by using the Ewald electrostatic energy method (Section S6.3), total energies of 2347 and 1337 configurations were calculated for the Lix/NaxBCF2 and Lix/NaxB2C2F2 ground state hull, respectively.
Figure 4 and Tables S11–S14 show the stable configurations and corresponding formation energies (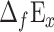 defined in Eq. S5) of Lix(Nax)BCF2 and Lix(Nax)B2C2F2. Some characteristics can be identified. First, the formation energies for all concentrations are negative, indicating the energetic stability of Lix(Nax)BCF2/B2C2F2. Second, different stages (defined as stage x, as discussed in Section S6.4) were found during both Li+ and Na+ intercalation into BCF2/B2C2F2 systems. This phenomenon has been reported in other 2D materials [32], and also has been experimentally confirmed in graphitic carbon [33]. Importantly, the average electrochemical potentials for Li+ (or Na+) intercalation in Lix(Nax)BCF2 and Lix(Nax)B2C2F2 were predicted to be 3.49 (2.78) V and 3.63 (2.85) V, respectively (Fig. 4 and Fig. S22). This confirms that the tuning of Fermi level indeed dramatically enhances the intercalation potentials of Lix(Nax)BCF2 and Lix(Nax)B2C2F2.
defined in Eq. S5) of Lix(Nax)BCF2 and Lix(Nax)B2C2F2. Some characteristics can be identified. First, the formation energies for all concentrations are negative, indicating the energetic stability of Lix(Nax)BCF2/B2C2F2. Second, different stages (defined as stage x, as discussed in Section S6.4) were found during both Li+ and Na+ intercalation into BCF2/B2C2F2 systems. This phenomenon has been reported in other 2D materials [32], and also has been experimentally confirmed in graphitic carbon [33]. Importantly, the average electrochemical potentials for Li+ (or Na+) intercalation in Lix(Nax)BCF2 and Lix(Nax)B2C2F2 were predicted to be 3.49 (2.78) V and 3.63 (2.85) V, respectively (Fig. 4 and Fig. S22). This confirms that the tuning of Fermi level indeed dramatically enhances the intercalation potentials of Lix(Nax)BCF2 and Lix(Nax)B2C2F2.
Figure 4.
Formation energies (ΔfEx) and electrochemical potential of (a) LixBCF2 and (b) NaxBCF2 as a function of Li+/Na+ concentration, respectively. Red and green solid lines indicate the constructed convex-hull of Stage I and Stage II phases, respectively.
The stabilities of their deintercalated states Lix(Nax)BCF2/Lix(Nax)B2C2F2 (0 ≤ x ≤ 1) have been evaluated by calculating the formation energy of F vacancy (VF) (Section S6.5). In the temperature range of 200 to 500 K (a much wider range than the battery operating condition), where possible phase-transitions are solid-state reactions involving the absorption or loss of fluorine (μ(F) = 0 eV for Eq. S7), Lix(Nax)BCF2 and Lix(Nax)B2C2F2 cathodes remain stable [ΔH(VF) > 0] at all Li/Na concentrations (Fig. S25). Moreover, the compatibility of Lix(Nax)BCF2/Lix(Nax)B2C2F2 cathodes with the commonly used electrolyte is investigated. Here we take the classical 1 M LiPF6 in EC: DEC (1:1) electrolyte as an example. Its electrochemical window is reported to be larger than 4.5 V [4]. This is much higher than the Li+(Na+)-intercalation potentials of Li(Na)BCF2 and Li(Na)B2C2F2 cathodes (2.7–3.7 V) designed in our work, suggesting good compatibility between these cathodes and electrolyte.
Considering that the intercalation process is mainly determined by charge transfer from Li/Na to BCF2 and B2C2F2 (Fig. S5), the energy gained in this transfer can serve as a descriptor to quantitatively illustrate the effect of p-type doping on the Li+/Na+ intercalation potentials. Also, the electrode potentials μA and μC constrain the open circuit voltage Voc of a battery cell to 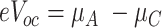 , where the energy of a given μA or μC corresponds to the Fermi energy in an itinerant-electron band, as is the case for Li metal, or the energy of a redox couple of the covalently bonded B-C cation in Lix(Nax)BCF2/Lix(Nax)B2C2F2. Thus, here we used the state-filling model [34] to calculate the energy acquired when Li/Na electron is transferred into an empty state above the Fermi level of pristine BCF2/B2C2F2. This can be described as
, where the energy of a given μA or μC corresponds to the Fermi energy in an itinerant-electron band, as is the case for Li metal, or the energy of a redox couple of the covalently bonded B-C cation in Lix(Nax)BCF2/Lix(Nax)B2C2F2. Thus, here we used the state-filling model [34] to calculate the energy acquired when Li/Na electron is transferred into an empty state above the Fermi level of pristine BCF2/B2C2F2. This can be described as 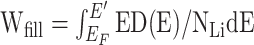 , where E is the energy referenced to the vacuum level,
, where E is the energy referenced to the vacuum level, 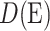 is the density of states of BCF2/B2C2F2 and
is the density of states of BCF2/B2C2F2 and 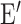 is derived from
is derived from 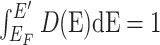 , which assumes that one Li/Na electron is transferred into BCF2/B2C2F2 per formula unit. Note that the amount of charge transferred may not be precisely 1, as was previously demonstrated for other layered electrodes [35,36]. The charge transfer in BCF2/B2C2F2 was quantified by Bader analysis and the results are shown in Table S3. The amount of charge transferred from Na and Li to the B-C covalent bonds in BCF2/B2C2F2 is ∼0.78 and ∼0.86 in all cases, very close to 1. The Wfill results shown in Fig. 5 indicate that the high Fermi level (−4.31 eV) caused by the C-C sp2 hybridization in the pristine graphite leads to high Wfill (−3.0 eV) and ultimately lower electrochemical potential for Li+/Na+ (de)intercalation (<0.2 V). In contrast, p-type doping effectively lowers the Fermi level of BCF2 and B2C2F2 to −8.36 eV and −8.48 eV, respectively, and thus enables the systems to have lower Wfill (−7.05 eV for BCF2 and −7.23 eV for B2C2F2) and higher potentials. The relationship between the Fermi level and electrochemical potential quantitatively confirms the tuning effect of Fermi level on the intercalation potentials of Li+/Na+ in BCF2/B2C2F2 systems.
, which assumes that one Li/Na electron is transferred into BCF2/B2C2F2 per formula unit. Note that the amount of charge transferred may not be precisely 1, as was previously demonstrated for other layered electrodes [35,36]. The charge transfer in BCF2/B2C2F2 was quantified by Bader analysis and the results are shown in Table S3. The amount of charge transferred from Na and Li to the B-C covalent bonds in BCF2/B2C2F2 is ∼0.78 and ∼0.86 in all cases, very close to 1. The Wfill results shown in Fig. 5 indicate that the high Fermi level (−4.31 eV) caused by the C-C sp2 hybridization in the pristine graphite leads to high Wfill (−3.0 eV) and ultimately lower electrochemical potential for Li+/Na+ (de)intercalation (<0.2 V). In contrast, p-type doping effectively lowers the Fermi level of BCF2 and B2C2F2 to −8.36 eV and −8.48 eV, respectively, and thus enables the systems to have lower Wfill (−7.05 eV for BCF2 and −7.23 eV for B2C2F2) and higher potentials. The relationship between the Fermi level and electrochemical potential quantitatively confirms the tuning effect of Fermi level on the intercalation potentials of Li+/Na+ in BCF2/B2C2F2 systems.
Figure 5.

Electrochemical potentials as a function of Wfill for Li(Na)BCF2/Li(Na)B2C2F2 cathodes. Blue and yellow symbols represent Li+ and Na+ intercalation potentials, respectively. The dashed lines illustrate the dramatic increase of potentials of Li(Na)BCF2/Li(Na)B2C2F2 cathodes compared with that of graphite anode.
CONCLUSION
In summary, we demonstrate how the rational p-type doping of full shell bonding-orbital in a CF system allows us to drastically shift Fermi level of structure to a lower energy, thus dramatically raising the electrochemical potential of the mother material. Adopting this concept, new cathode candidates Li(Na)BCF2 and Li(Na)B2C2F2 with average Li+(Na+) deintercalation potentials of 3.49(2.78) V and 3.63(2.85) V, respectively, were successfully designed. Most importantly, the theoretical energy densities of these cathodes reach 1379.9(888.5) and 1073.8(715.9) Wh kg−1 for Li+(Na+)-storage, which represent the highest values compared with any commercial cathodes currently used in Li-ion (or Na-ion) batteries.
We further suggest that this full shell p-type doping (or hole-doping) strategy can be applied to other charge transfer-dominated ion-intercalation systems. Indeed, a wide variety of the existing rigid-band transition metal dichalcogenides (TMD) can be considered for band structure tuning, including MX2 (M = Mo, W, Nb, Ta; X = S, Se) (Table S4). For example, Mo has six valence electrons (5s24d4), which bond with 12 electrons of two stoichiometric S atoms (2 × 3s23p4) to form an 18-electron full-shell configuration (Fig. S6). Notably, Nb has one less electron than Mo, thus Nb substituting on Mo site can be regarded as p-type dopant for MoS2, shifting the Fermi level down as expected (Fig. S7). Upon Li+ intercalation process, the electron of Li fills lower energy levels, resulting in a higher potential in NbS2 (2.90 V) than in MoS2 (0.90 V) (Table S4). Thus, the full-shell doping strategy works well for both p and d elements. The findings in this work are expected to motivate more researchers to directly evaluate the link between the electrochemical potential and the band structure tuning of electrodes.
Supplementary Material
Acknowledgements
We would like to thank Profs. Ruqian Wu, Su-Huai Wei, Wenqing Zhang and Jian Wang for fruitful discussions. We appreciate the High Performance Computing Center of Shanghai University and Shanghai Engineering Research Center of Intelligent Computing System (19DZ2252600) for providing computing resources and technical support.
Contributor Information
Zhiqiang Wang, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China; State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China.
Da Wang, State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China.
Zheyi Zou, State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China.
Tao Song, State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China.
Dixing Ni, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China.
Zhenzhu Li, Soochow Institute for Energy and Materials Innovations (SIEMIS), College of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215006, China.
Xuecheng Shao, State Key Lab of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China.
Wanjian Yin, Soochow Institute for Energy and Materials Innovations (SIEMIS), College of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215006, China.
Yanchao Wang, State Key Lab of Superhard Materials, College of Physics, Jilin University, Changchun 130012, China.
Wenwei Luo, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China.
Musheng Wu, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China.
Maxim Avdeev, Australian Nuclear Science and Technology Organisation, Kirrawee DC, NSW 2232, Australia; School of Chemistry, University of Sydney, Sydney 2006, Australia.
Bo Xu, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China.
Siqi Shi, State Key Laboratory of Advanced Special Steel, School of Materials Science and Engineering, Shanghai University, Shanghai 200444, China; Materials Genome Institute, Shanghai University, Shanghai 200444, China.
Chuying Ouyang, Department of Physics, Laboratory for Computational Materials Physics, Jiangxi Normal University, Nanchang 330022, China.
Liquan Chen, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China.
SUPPLEMENTARY DATA
Supplementary data are available at NSR online.
FUNDING
This work was supported by the National Natural Science Foundation of China (51802187, 51962010, 11874254, 51622207 and U1630134) and the Shanghai Sailing Program (18YF1408700).
AUTHOR CONTRIBUTIONS
Zhiqiang Wang, Da Wang, Siqi Shi and Chuying Ouyang designed the research; Zhiqiang Wang, Da Wang, Siqi Shi, Chuying Ouyang and Liquan Chen performed the research; Zhiqiang Wang, Da Wang, Siqi Shi, Chuying Ouyang, Zheyi Zou, Tao Song, Dixing Ni, Zhenzhu Li, Xuecheng Shao, Wanjian Yin, Yanchao Wang, Wenwei Luo, Musheng Wu, Maxim Avdeev and Bo Xu analyzed the data; Zhiqiang Wang, Da Wang, Siqi Shi and Chuying Ouyang wrote the paper.
Conflict of interest statement. None declared.
REFERENCES
- 1. Armand M, Tarascon JM. Building better batteries. Nature 2008; 451: 652–7. 10.1038/451652a [DOI] [PubMed] [Google Scholar]
- 2. Chiang Y-M. Building a better battery. Science 2010; 330: 1485–6. 10.1126/science.1198591 [DOI] [PubMed] [Google Scholar]
- 3. Shi S, Gao J, Liu Y et al. Multi-scale computation methods: their applications in lithium-ion battery research and development. Chin Phys B 2016; 25: 018212. 10.1088/1674-1056/25/1/018212 [DOI] [Google Scholar]
- 4. Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater 2010; 22: 587–603. 10.1021/cm901452z [DOI] [Google Scholar]
- 5. Whittingham MS. Lithium batteries and cathode materials. Chem Rev 2004; 104: 4271–302. 10.1021/cr020731c [DOI] [PubMed] [Google Scholar]
- 6. Poizot P, Laruelle S, Grugeon S et al. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000; 407: 496–9. 10.1038/35035045 [DOI] [PubMed] [Google Scholar]
- 7. Manthiram A, Vadivel Murugan A, Sarkar A et al. Nanostructured electrode materials for electrochemical energy storage and conversion. Energ Environ Sci 2008; 1: 621–38. 10.1039/b811802g [DOI] [Google Scholar]
- 8. Mizushima K, Jones PC, Wiseman PJ et al. LixCoO2 (0 < x < −1): a new cathode material for batteries of high energy density. Mater Res Bull 1980; 15: 783–9. [Google Scholar]
- 9. Kang B, Ceder G. Battery materials for ultrafast charging and discharging. Nature 2009; 458: 190–3. 10.1038/nature07853 [DOI] [PubMed] [Google Scholar]
- 10. Seo D-H, Lee J, Urban A et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat Chem 2016; 8: 692–7. 10.1038/nchem.2524 [DOI] [PubMed] [Google Scholar]
- 11. Petersburg CF, Li Z, Chernova NA et al. Oxygen and transition metal involvement in the charge compensation mechanism of LiNi1/3Mn1/3Co1/3O2 cathodes. J Mater Chem 2012; 22: 19993–20000. 10.1039/c2jm33392a [DOI] [Google Scholar]
- 12. Oishi M, Fujimoto T, Takanashi Y et al. Charge compensation mechanisms in Li1.16Ni0.15Co0.19Mn0.50O2 positive electrode material for Li-ion batteries analyzed by a combination of hard and soft X-ray absorption near edge structure. J Power Sources 2013; 222: 45–51. 10.1016/j.jpowsour.2012.08.023 [DOI] [Google Scholar]
- 13. Sathiya M, Rousse G, Ramesha K et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat Mater 2013; 12: 827–35. 10.1038/nmat3699 [DOI] [PubMed] [Google Scholar]
- 14. Koga H, Croguennec L, Ménétrier M et al. Reversible oxygen participation to the redox processes revealed for Li1.20Mn0.54Co0.13Ni0.13O2. J Electrochem Soc 2013; 160: A786–92. 10.1149/2.038306jes [DOI] [Google Scholar]
- 15. Yabuuchi N, Takeuchi M, Nakayama M et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc Natl Acad Sci USA 2015; 112: 7650–5. 10.1073/pnas.1504901112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang P, Wei S-H. Origin of charge compensation and its effect on the stability of oxide cathodes for Li-ion batteries: the case of orthosilicates. Electrochim Acta 2018; 270: 409–16. 10.1016/j.electacta.2018.03.105 [DOI] [Google Scholar]
- 17. Ji J, Ji H, Zhang LL et al. Graphene-encapsulated Si on ultrathin-graphite foam as anode for high capacity lithium-ion batteries. Adv Mater 2013; 25: 4673–7. 10.1002/adma.201301530 [DOI] [PubMed] [Google Scholar]
- 18. El-Kady MF, Shao Y, Kaner RB. Graphene for batteries, supercapacitors and beyond. Nat Rev Mater 2016; 1: 16033. 10.1038/natrevmats.2016.33 [DOI] [Google Scholar]
- 19. Raccichini R, Varzi A, Passerini S et al. The role of graphene for electrochemical energy storage. Nat Mater 2014; 14: 271–9. 10.1038/nmat4170 [DOI] [PubMed] [Google Scholar]
- 20. Melot BC, Tarascon JM. Design and preparation of materials for advanced electrochemical storage. Acc Chem Res 2013; 46: 1226–38. 10.1021/ar300088q [DOI] [PubMed] [Google Scholar]
- 21. Watanabe N, Fukuda M. US Patent. 1972; 3: 502. [Google Scholar]
- 22. Liu W, Li H, Xie J-Y et al. Rechargeable room-temperature CFx-sodium battery. ACS Appl Mater Inter 2014; 6: 2209–12. 10.1021/am4051348 [DOI] [PubMed] [Google Scholar]
- 23. Placke T, Kloepsch R, Dühnen S et al. Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J Solid State Electrochem 2017; 21: 1939–64. 10.1007/s10008-017-3610-7 [DOI] [Google Scholar]
- 24. Cherkashinin G, Hausbrand R, Jaegermann W. Performance of Li-ion batteries: contribution of electronic factors to the battery voltage. J Electrochem Soc 2019; 166: A5308–12. 10.1149/2.0441903jes [DOI] [Google Scholar]
- 25. Armand M. Matériaux d'électrodes à couple redox interne. Thesis, Grenoble, 1978. [Google Scholar]
- 26. Urban A, Seo D-H, Ceder G. Computational understanding of Li-ion batteries. npj Comput Mater 2016; 2: 16002. 10.1038/npjcompumats.2016.2 [DOI] [Google Scholar]
- 27. Schuld S, Hausbrand R, Fingerle M et al. Experimental studies on work functions of Li+ ions and electrons in the battery electrode material LiCoO2: a thermodynamic cycle combining ionic and electronic structure. Adv Energy Mater 2018; 8: 1703411. 10.1002/aenm.201703411 [DOI] [Google Scholar]
- 28. Gerischer H, Decker F, Scrosati B. The electronic and the ionic contribution to the free energy of alkali metals in intercalation compounds. J Electrochem Soc 1994; 141: 2297–300. 10.1149/1.2055115 [DOI] [Google Scholar]
- 29. Wang Y, Lv J, Zhu L et al. CALYPSO: a method for crystal structure prediction. Comput Phys Commun 2012; 183: 2063–70. 10.1016/j.cpc.2012.05.008 [DOI] [Google Scholar]
- 30. Zhu L, Wang H, Wang Y et al. Substitutional alloy of Bi and Te at high pressure. Phys Rev Lett 2011; 106: 145501. 10.1103/PhysRevLett.106.145501 [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Naumov II, Hoffmann R et al. Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure. Proc Natl Acad Sci USA 2017; 114: 6990–5. 10.1073/pnas.1704505114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouvetakis J, Sasaki T, Shen C et al. Novel aspects of graphite intercalation by fluorine and fluorides and new B/C, C/N and B/C/N materials based on the graphite network. Synthetic Met 1989; 34: 1–7. 10.1016/0379-6779(89)90355-X [DOI] [Google Scholar]
- 33. Persson K, Hinuma Y, Meng YS et al. Thermodynamic and kinetic properties of the Li-graphite system from first-principles calculations. Phys Rev B 2010; 82: 125416. 10.1103/PhysRevB.82.125416 [DOI] [Google Scholar]
- 34. Liu Y, Wang YM, Yakobson BI et al. Assessing carbon-based anodes for lithium-ion batteries: a universal description of charge-transfer binding. Phys Rev Lett 2014; 113: 028304. 10.1103/PhysRevLett.113.028304 [DOI] [PubMed] [Google Scholar]
- 35. Zhang J-J, Dong S. Prediction of above 20 K superconductivity of blue phosphorus bilayer with metal intercalations. 2D Mater 2016; 3: 035006. 10.1088/2053-1583/3/3/035006 [DOI] [Google Scholar]
- 36. Fan S, Zou X, Du H et al. Theoretical investigation of the intercalation chemistry of lithium/sodium ions in transition metal dichalcogenides. J Phys Chem C 2017; 121: 13599–605. 10.1021/acs.jpcc.7b05303 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





