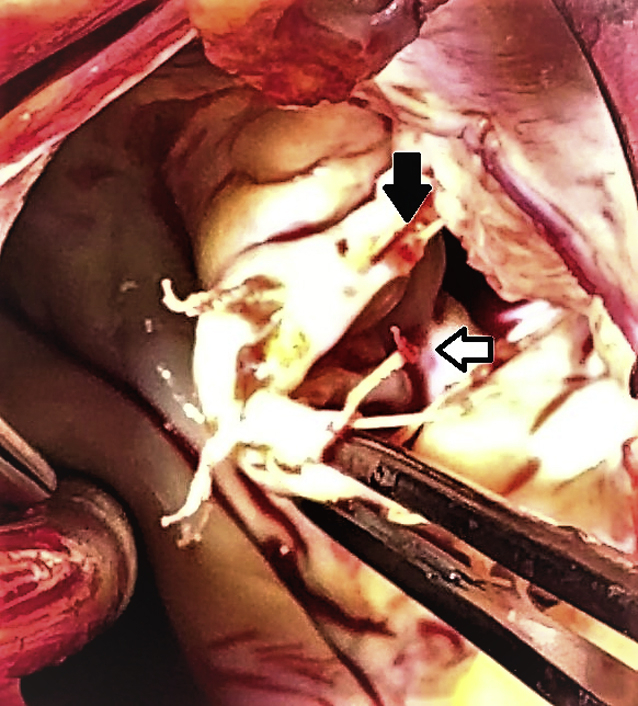
Calcified ruptured expanded polytetrafluoroethylene neochord (white arrow) and strained neochord with imminent rupture (black arrow).
Central Message.
ePTFE neochords can rarely undergo calcific degeneration and fracture causing a late failure of mitral valve repair.
Case Report
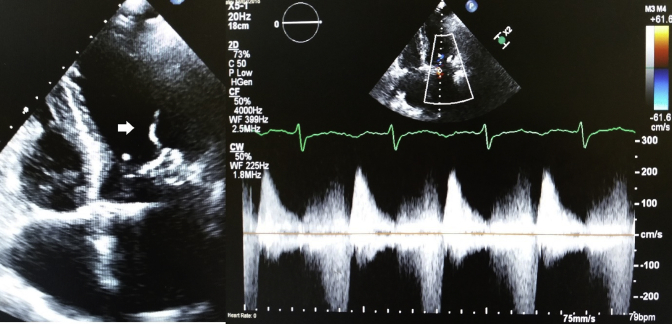
An 84-year-old woman represented with increasing shortness of breath and a new pan systolic murmur at the apex 12 years following a mitral valve repair (for severe mitral regurgitation due to P2 prolapse), tricuspid valve repair, and ablation of atrial fibrillation. At the initial operation, the flail posterior leaflet was supported with two CV 4 polytetrafluoroethylene (ePTFE) neochords (Gore-Tex; W. L. Gore & Associates Inc, Flagstaff, Ariz) and a 32-mm ring annuloplasty. The previous intraoperative, predischarge and follow-up echocardiograms confirmed no residual prolapsed/billowing or mitral regurgitation. Echocardiogram now confirmed severe mitral regurgitation from a flail P2 and dense echogenicity of chords (Figure 1). History and blood results did not show renal impairment or abnormality of calcium metabolism. Intraoperatively, the neochords were found to be stiff and calcified (Figure 2). One of the neochords was fractured and the other had signs of stiffening, calcific degeneration, and imminent fracture. The subchordal apparatus was thickened and restrictive hence the valve was deemed unrepairable. The pattern of calcification seen in the neochords was not replicated in the remaining subchordal apparatus and surrounding tissues. The annuloplasty ring was explanted and a 31-mm bioprosthesis was implanted with preservation of the posterior chordal apparatus.
Figure 1.
Calcified, ruptured expanded polytetrafluoroethylene neochord (white arrow) in the setting of severe mitral regurgitation.
Figure 2.
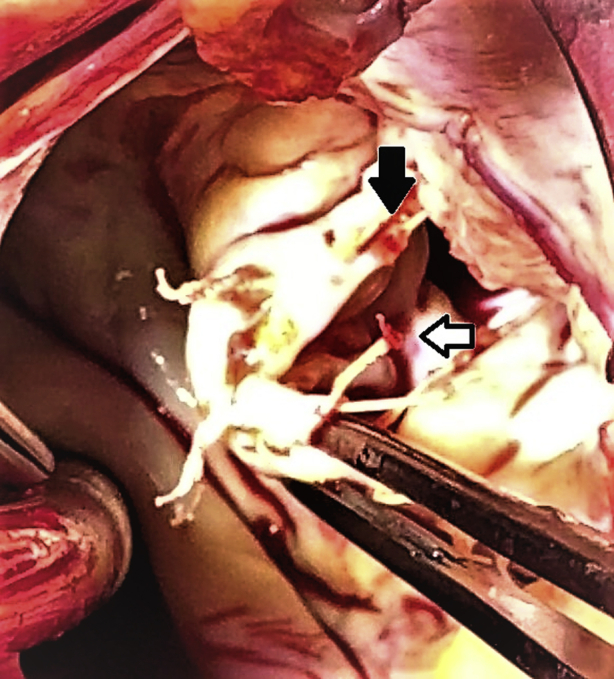
Calcified ruptured expanded polytetrafluoroethylene neochord (white arrow) and strained neochord with imminent rupture (black arrow).
Postoperative recovery was uneventful with satisfactory postoperative echocardiograms. Unfortunately, histopathology or electron microscopy was not obtained.
Discussion
ePTFE neochords are widely used in mitral valve repair due to its strength, inertness, hydrophobic properties and nonimmunogenicity. The structure of ePTFE is made of highly microporous sheets of ePTFE that allow water vapor to pass through but retain hydrophobic properties due to the strong electronegativity of fluorine atoms. ePTFE is chemically inert. Unlike the biological substitutes with phospholipid cell membranes that undergo amorphous dystrophic calcification and degeneration over time, ePTFE is not known to imbibe calcium or undergo calcific degeneration.
ePTFE patches have been used extensively in congenital cardiac surgery for ventricular and atrial septal defect closure and right ventricular outflow and pulmonary valve reconstructions. Kumar and colleagues1 reported excellent results with right ventricular outflow tract reconstruction with a PTFE monocusp valve over a 20-year period. The sheer and compressive stresses on patches in these reconstructions differ from the tensile and frictional stresses experienced by neochords in mitral valve repairs. Excellent long-term results with ePTFE neochords in mitral valve repair have been reported.2,3 There have been, however, isolated reports of early and late failures.4,5
ePTFE carries an electrostatic negative charge on the surface and initially repels blood elements and bacteria. The microporous structure is gradually filled in by platelets and clots that organize to form a dense fibrous tissue ingrowth covered later by endothelial cells. Structural analysis up to a decade later shows that neochords remain supple and retain their flexibility and strength after this process completes.6 ePTFE repels bacteria due to its surface electronegativity. However, due to its highly porous microstructure, it is extremely difficult to eradicate infection once it sets in. It is extremely resistant to chemicals and does not lyse or degrade on exposure to microbiological enzymes that normally destroy the surrounding biological tissues leading to loosening and extrusion of implants. Dystrophic calcification of infection-related fibrin deposits can potentially weaken the structure. There was no evidence of infection in this case.
The extensive calcific degeneration as a mechanism of rupture has been rarely reported.7 It is possible that fibrosis in the interstices of the microporous structure attracts calcium from blood despite the endothelial cover. The CV 4 ePTFE neochords can withstand 1000 g tensile load.8,9 Even after being crushed by forceps, this strength is at least 500 g.8,9 The typical tensile strain on the chords in mitral valve repairs at the site of primary chordal attachment in the P2 segment is 50 g.8,9 Slow calcification of the interstitial fibrosa in the neochord can potentially cause stiffening and loss of tensile strength in the weakened microstructure. These calcified neochords possibly can fracture under even normal tensile loads as seen in our case. This is distinctly different from early fracture related to ePTFE injury by forceps or mechanical stress induced by chordal length.
Concerns have been raised regarfing neochord damage by energy transfer during atrial fibrillation ablation. ePTFE is electrically inert and thermally stable. Ablation is performed before placement of neochords and is unlikely to cause damage.
The cause of this rare calcification in our case is unclear and nonetheless calls for yearly echocardiographic follow-up.
Footnotes
Disclosures: Authors have nothing to disclose with regard to commercial support.
References
- 1.Kumar M., Turrentine M.W., Rodefeld M.D., Bell T., Brown J.W. Right ventricular outflow tract reconstruction with a polytetrafluoroethylene monocusp valve: a 20-year experience. Semin Thorac Cardiovasc Surg. 2016;28:463–470. doi: 10.1053/j.semtcvs.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 2.David T.E., Armstrong S., Ivanov J. Chordal replacement with polytetrafluoroethylene sutures for mitral valve repair: a 25-year experience. J Thorac Cardiovasc Surg. 2013;145:1563–1569. doi: 10.1016/j.jtcvs.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 3.Salvador L., Mirone S., Bianchini R., Regesta T., Patelli F., Minniti G., et al. A 20-year experience with mitral valve repair with artificial chordae in 608 patients. Thorac Cardiovasc Surg. 2008;135:1280–1287. doi: 10.1016/j.jtcvs.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Yamashita M.H., Skarsgard P.L. Intermediate and early rupture of expanded polytetrafluorethylene neochordae after mitral valve repair. Ann Thorac Surg. 2011;92:341–343. doi: 10.1016/j.athoracsur.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 5.Castillo J.G., Anyanwu A.C., El-Eshmawi A., Gordon R.E., Adams D.H. Early rupture of an expanded polytetrafluoroethylene neochord after complex mitral valve repair: an electron microscopic analysis. J Thorac Cardiovasc Surg. 2013;145:e29–e31. doi: 10.1016/j.jtcvs.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Minatoya K., Kobayashi J., Sesako Y., Ishibashi-Ueda H., Yutani C., Kitamura S. Long-term pathological changes of expanded polytetrafluorethylene suture in the human heart. J Heart Valve Dis. 2001;10:139–142. [PubMed] [Google Scholar]
- 7.Farivar R.S., Shernan S.K., Cohn L.H. Late rupture of polytetrafluoroethylene neochordae after mitral valve repair. J Thorac Cardiovasc Surg. 2009;137:504–506. doi: 10.1016/j.jtcvs.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 8.Hertwick S.P., von Fraunhofer J.A., Materson B.J. Tensile characteristics of PTFE sutures. Biomaterials. 1986;9:457–459. doi: 10.1016/0142-9612(88)90013-0. [DOI] [PubMed] [Google Scholar]
- 9.Salisbury P.F., Cross C.E., Reuben P.A. Chorda tendinea tension. Am J Physiol. 1965;206:385–392. doi: 10.1152/ajplegacy.1963.205.2.385. [DOI] [PubMed] [Google Scholar]