Abstract
A 75-year-old patient with previous mitral and tricuspid reconstruction experienced severe tricuspid regurgitation. On the basis of a prohibitive surgical risk, an interventional heterotopic tricuspid valve implantation was planned. Implantation was performed using fusion-imaging, which facilitated intraprocedural navigation. At 6-month follow-up, the patient presented in improved condition with reduced symptoms. (Level of Difficulty: Advanced.)
Key Words: structural heart interventions, transcatheter caval valve implantation, tricuspid regurgitation
Abbreviations and Acronyms: IVC, inferior vena cava; PM, pacemaker; RA, right atrium; RV, right ventricle; RVF, right ventricular function; SVC, superior vena cava; TR, tricuspid regurgitation; TV, tricuspid valve
Graphical abstract

A 75-year-old patient with previous mitral and tricuspid reconstruction experienced severe tricuspid regurgitation. On the basis of a prohibitive…
History of Presentation
A 75-year-old female patient presented to the outpatient clinic with worsening dyspnea (New York Heart Association [NYHA] functional class III), fatigue, and peripheral edema.
Learning Objectives
-
•
Caval valve implantation represents a useful addition to the invasive armamentarium for treatment of severe symptomatic tricuspid regurgitation in patients with prohibitive surgical risk and contraindications for other transcatheter options.
-
•
Several patient-dependent parameters need to be considered during the evaluation process for caval valve implantation, with specific attention to the central venous anatomy, size, and right ventricular function.
-
•
Software-based pre-operative planning allows facilitation of intraprocedural imaging projecting pre-procedural–defined markers and structures within live fluoroscopy.
Past Medical History
The patient had undergone surgical aortic valvuloplasty in 1982 with iatrogenic third-degree atrioventricular block followed by dual-chamber (DDDR) pacemaker (PM) implantation with endocardial leads. Due to multiple lead revisions, she ultimately received an epicardial PM system.
In 2007, she underwent minimally invasive tricuspid valve (TV) repair with a sutured annuloplasty and bicuspidalization for severe tricuspid regurgitation (TR). Two years later, she underwent mechanical aortic valve replacement, myxoma resection with associated mitral valve repair, and TV reconstruction with anterior leaflet mobilization (Figure 1) for aortic and mitral endocarditis associated with recurrent TR.
Figure 1.
Pre-Interventional Chest X-Ray
Pre-interventional chest x-ray showing endocardial and epicardial pacemaker leads, a mechanical aortic valve prosthesis, and a mitral annuloplasty ring.
Further comorbidities were hypertension, dyslipidemia, chronic obstructive pulmonary disease, autoimmune thrombocytopenia, and renal failure.
Differential Diagnosis
The differential diagnosis included congestive heart failure, recurrent mitral regurgitation and/or TR, aortic valve prosthesis dysfunction, and progressive kidney failure.
Investigations
Pre-operative echocardiography revealed severe TR with annular dilation, leaflet restriction, and an effective regurgitant orifice of 0.62 cm2 and regurgitant volume of 73.7 ml. Right ventricular (RV) function (RVF) was reduced with a tricuspid annular plane excursion of 11 mm. Catheterization revealed a mean pulmonary artery pressure of 39 mm Hg with a pulmonary capillary wedge pressure of 15 mm Hg and an elevated pulmonary vascular resistance of 449,65 dynes/s/cm−5. The calculated EuroSCORE II mortality was 7.6%.
Management
Due to previous surgical procedures and reduced RVF, the heart team refused surgery. The patient was evaluated for percutaneous edge-to-edge repair using the MitraClip XTR device (Abbott, Abbott Park, Illinois) but deemed ineligible due elevated pulmonary pressures and previous tricuspid annuloplasty. Interventional heterotopic TV replacement was eventually chosen as the therapeutic option in this advanced stage of TV disease. The device, the Tricento valve (New Valve Technology, Muri, Switzerland), consists of a customized covered stent (maximum length 13.5 cm) with a bicuspid valve opening in its inferior part (Figure 2). Designated landing zones are the superior vena cava (SVC) and inferior vena cava (IVC).
Figure 2.
Schematic View of the Tricento Valve
(A) Schematic view of the Tricento valve with the intra-atrial valve segment and the red arrows indicating the right atrial inflow through the valve segment; and (B) the customized prosthesis used in the presented patient.
Pre-operative planning was performed using a computed tomography–based segmentation software (Heart-Navigator; Philips, Amsterdam, the Netherlands). After segmentation (Figures 3A to 3C), 3-dimensional reconstructions of cardiac structures such as the right atrium (RA), the SVC and IVC, and the RV were created (Figure 3D). A marker was placed at the medial inferior atriocaval boarder for intraprocedural orientation during deployment (Figure 3A).
Figure 3.
CT-Based Segmentation of the RA, the Caval Veins, and the Mitral Ring
(A to C) Computed tomography–based segmentation of the right atrium (red), the caval veins (blue), and the mitral ring (yellow). A marker is placed at the medial atriocaval border as an intraprocedural landmark (asterisk). (D) 3-Dimensional reconstruction of anatomic structures and mitral ring (triangle).
The intervention (Video 1) was conducted under general anesthesia with intubation and availability of fluoroscopy. Transfemoral endovascular access was gained and a pigtail catheter was positioned within the RA. After obtainment of 2 contrast agent sequences, registration for intraprocedural projection of 3-dimensional reconstructions and markers was performed (Figure 4, Video 1). An ablation catheter was positioned as an additional landmark at the inferior landing zone according to the projected IVC reconstruction. Meanwhile, crimping and loading of the stent was performed. Following introduction of the sheath (26-F), the deployment apparatus was inserted and its tip positioned beneath the azygos vein confluence. Controlled top-down deployment of the stent was executed, and valve functionality was tested via angiography before the final stent release. Intraprosthetic and intra-atrial angiography confirmed good placement and absence of major endoleaks. Invasive measurements of central venous and RA pressures showed an inversely correlated decrease of mean central venous pressure from 28 mm Hg to 21 mm Hg and increase of mean RA pressure from 18 to 24 mm Hg after implantation (Table 1). A significant increase in the V wave of the right atrial curve was observed during hemodynamic evaluation after implantation (Figure 5). Endovascular closure of the right femoral vein was performed using 2 ProGlide vascular closure systems (Abbott).
Online Video 1.
Fluoroscopic View of the Tricento Caval Valve Implantation
Procedural steps.
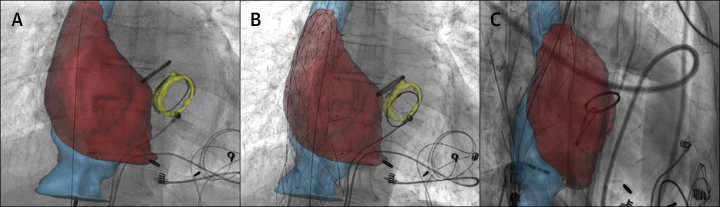
Figure 4.
Intraprocedural Fusion Imaging
Intra-procedural fusion imaging with projection of 3-dimensional reconstructions and landmarks within fluoroscopic live imaging (Video 1); (A) anteroposterior view before implantation; (B) anteroposterior view after implantation; and (C) lateral view after implantation.
Table 1.
Pre- and Peri-Interventional Hemodynamic Measurements
| Pre-Implant | Post-Implant | |
|---|---|---|
| Inferior vena cava pressure (A/V/M waves), mm Hg | 28 (CVP) | (27/27/1) |
| Right atrial pressure (A/V/M waves), mm Hg | (20/22/18) | (38/33/24) |
| Cardiac output, l/min | 3.39 | NA |
| Pulmonary vascular resistance, dynes/s/cm−5 | 449.65 | NA |
CVP = central venous pressure; IVC = inferior vena cava; NA = not assessed.
Figure 5.
Peri-Interventional Hemodynamic Curves
Peri-interventional hemodynamic curves of the (A) right atrial pressure before implantation; (B) right atrial pressure after implantation; and (C) inferior vena cava pressure after implantation.
Discussion
Severe symptomatic TR constitutes a growing burden of disease with major impact on morbidity and mortality, irrespective of parameters such as ventricular function and pulmonary hypertension (1). Patients undergoing surgical TV repair do experience a high perioperative mortality. Current transcatheter options provide a good safety profile; however, long-term data on patient outcomes are still missing (1).
Transcatheter caval valve implantation is an interesting treatment option for patients with symptomatic TR who are unfavorable candidates for open surgery and other structural interventions. The rationale behind this therapeutic approach is the reduction of hepatic, abdominal, and systemic venous congestion and associated symptoms such as ascites and peripheral edema. Currently, several devices are under clinical investigation: 1) the Sapien 3 (Edwards Lifesciences, Irvine, California) has been used for intracaval implantation, however, it can only be positioned in caval position after preceding stent implantation; 2) the TricValve (P & F Products & Features, Wessling, Germany) is a system with 2 nonidentical self-expandable tissue valves on a nitinol stent positioned in the SVC and IVC (2); and 3) the Tricento valve is a self-expanding stent with landing zones in the SVC and IVC, and a bicuspid valve opening in the lower atrial segment of the stent (3). In the largest caval valve cohort (n = 25) (4), a procedural success rate of 96% with implantation of either the Sapien 3 or the TricValve with a complete reversal of hepatic vein flow in all patients with implants was reported. Thirty-day and in-hospital mortality in this group was 12% and 24%, respectively. However, the patients presented with a Society of Thoracic Surgeons predicted risk of mortality score of 14.0 ± 12.7% and with various comorbidities, including end-stage renal failure (44%) and malignant disease (28%).
The Tricento valve is distinguishing itself from other current caval valves by its specific stent-based design with bicaval anchoring, which allows a controlled implantation process. The prosthesis is currently customized on the basis of patients’ anatomy of the landing zones by computed tomography, with specific attention to the azygos and hepatic vein confluence. There is an ongoing discussion whether implantation of 2 caval valves is superior to a single valve (IVC), and the Tricento valve allows only sealing of both caval veins (5). The associated increase of RV preload must be considered, especially in patients with reduced RVF.
To our knowledge, this case represents the first implantation of a Tricento valve in a patient with endocardial PM leads. In post-operative follow-up, only a nonsignificant endoleak was observed in the superior landing zone; however, presence of PM leads can lead to interaction with the prosthesis and significant endoleaks. Early hemodynamic measurements revealed an improvement, but long-term assessment is required for further analysis.
Follow-Up
The patient was discharged at the 10th post-operative day following prolonged medical diuretic therapy. Post-operative echocardiography confirmed normal opening of the implanted valve and no signs of major endoleakage. Left ventricular function and RVF were unchanged, as was the TR grade, without significant changes in TV regurgitant volume and the RA dimensions (anteroposterior diameter ∼5.5 cm). During the follow-up period, the patient underwent 2 rehospitalizations for hyponatremia. At 3- and 6-month follow-up visits, the patient presented with subjectively improved status (NYHA functional class I to II).
Conclusions
Caval valve implantation with the Tricento prosthesis appears to be a safe and feasible transcatheter treatment option for patients with symptomatic tricuspid disease and prohibitive surgical risk, when other options are contraindicated. Intraprocedural navigation and orientation is greatly facilitated by imaging fusion software, projecting pre-procedurally defined markers and structures within live fluoroscopic imaging.
Footnotes
Dr. Kocher has been a proctor and has received speaker fees from Edwards Lifesciences. Dr. Laufer has been an advisory board member and consultant for Edwards Lifesciences. Dr. Andreas has been a proctor for Edwards Lifesciences and Abbott; and an advisory board member for Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. This paper was accepted for presentation at the TCT 2019 meeting held September 25 to 29, 2019, in San Francisco, California.
Informed consent was obtained for this case.
Appendix
For a supplemental video, please see the online version of this paper.
References
- 1.Latib A., Grigioni F., Hahn R.T. Tricuspid regurgitation: what is the real clinical impact and how often should it be treated? EuroIntervention. 2018;14:AB101–A111. doi: 10.4244/EIJ-D-18-00533. [DOI] [PubMed] [Google Scholar]
- 2.Figulla H.R., Kiss K., Lauten A. Transcatheter interventions for tricuspid regurgitation - heterotopic technology: TricValve. EuroIntervention. 2016;12:Y116–Y118. doi: 10.4244/EIJV12SYA32. [DOI] [PubMed] [Google Scholar]
- 3.Toggweiler S., De Boeck B., Brinkert M. First-in-man implantation of the Tricento transcatheter heart valve for the treatment of severe tricuspid regurgitation. EuroIntervention. 2018;14:758–761. doi: 10.4244/EIJ-D-18-00440. [DOI] [PubMed] [Google Scholar]
- 4.Lauten A., Figulla H.R., Unbehaun A. Interventional treatment of severe tricuspid regurgitation: early clinical experience in a multicenter, observational, first-in-man study. Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.117.006061. [DOI] [PubMed] [Google Scholar]
- 5.O'Neill B.P. Caval valve implantation: are 2 valves better than 1? Circ Cardiovasc Interv. 2018;11 doi: 10.1161/CIRCINTERVENTIONS.118.006334. [DOI] [PubMed] [Google Scholar]