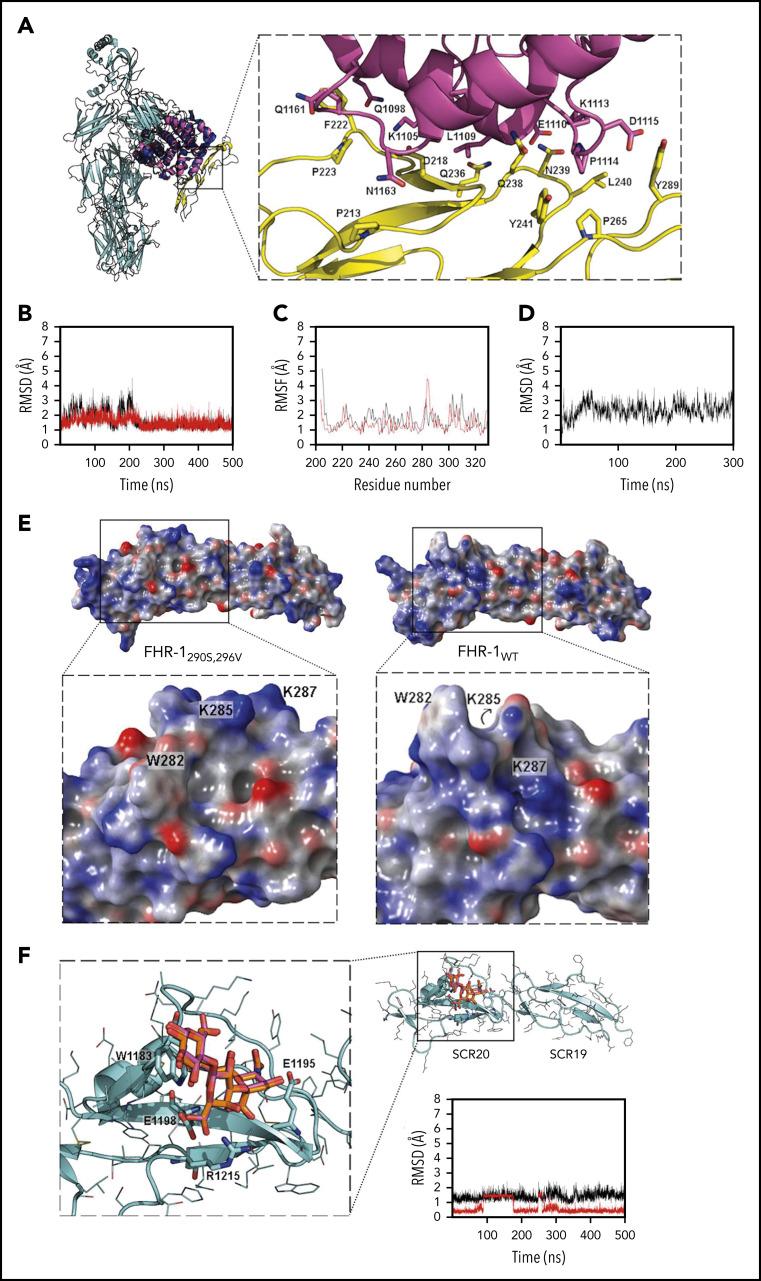
Figure 2.
Structural aspects of FHR-1 interaction with C3 TED and sialic acids. (A) Superimposition of C3 (cyan; Protein Data Bank [PDB] identifier 2A73; TED domain is shown in dark blue) and FHR-1 (yellow)/C3d (pink) complex from MD simulation (100 ns). Key residues are shown in sticks. (B) Root-mean-square deviation (RMSD; calculated with backbone heavy atoms) of FHR-1WT (red) and FHR-1L290S,A296V (black) during 500 ns of MD simulation. (C) Root-mean-square fluctuation (RMSF; calculated by residue) of FHR-1WT (red) and FHR-1L290S,A296V (black) during 500 ns of MD simulation. (D) RMSD (calculated with backbone heavy atoms) of FHR-1/C3dg complex during 500 ns of MD simulation. (E) Electrostatic potential surface of FHR-1L290S,A296V (left) and FHR-1WT (right). The sialic acid–binding sites in SCR-5 of FHR-1L290S,A296V and FHR-1WT are framed by squares that are enlarged below. (F) FH/3′-SL complex (cyan/orange) from docking calculations superimposed to 3′-SL (magenta) from x-ray structure (PDB identifier 4ONT). Key residues are shown in sticks. RMSD of the FH/3′-SL complex (calculated with heavy atoms for protein and with carbons for 3′-SL) from the MD simulation is also depicted (complex in black; 3′-SL in red).