Abstract
This case reports on an 8-year-old boy with homozygous familial hypercholesterolemia with large tuberous xanthomas over his hands, elbows, buttocks, knees, and feet. Lomitapide 40 mg daily (steadily increased) was added to his classical lipid-lowering therapy. A 50% reduction in the thickness, hardness, size, and color intensity of xanthomas was reported after 2 years of treatment. (Level of Difficulty: Intermediate.)
Key Words: colesevelam, ezetimibe, homozygous familial hypercholesterolemia, lomitapide, statins
Abbreviations and Acronyms: CVD, cardiovascular disease; HoFH, homozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; LL, lipid-lowering
Graphical abstract

This case reports on an 8-year-old boy with homozygous familial hypercholesterolemia with large tuberous xanthomas over his hands, elbows, buttocks, knees, and feet…
Untreated patients with homozygous familial hypercholesterolemia (HoFH) may experience a first cardiovascular disease (CVD) event as early as the first decade of life. Generally, patients with HoFH phenotype may present xanthomas, aortic valve disease, and CVD before 20 years of age (1). In patients with HoFH, the established lipid-lowering (LL) therapy usually cannot adequately reduce low-density lipoprotein cholesterol (LDL-C) levels, and new pharmacology agents such as lomitapide, a microsomal transfer protein inhibitor, are required.
History of Presentation
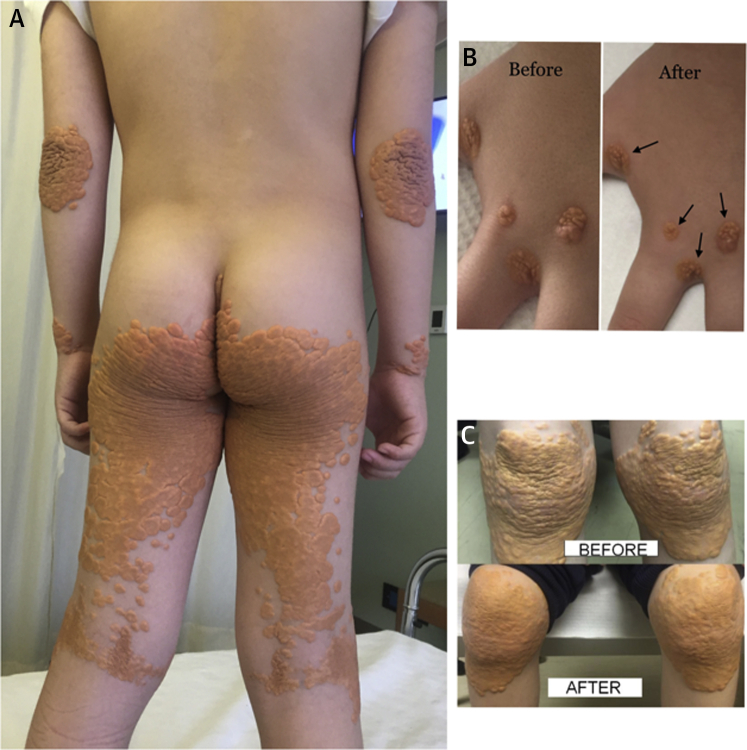
The authors report a case of an 8-year old boy who was referred to our lipid clinic 3 years ago with severe hypercholesterolemia. Physical examination revealed tuberous xanthomas over his hands, elbows, buttocks, knees, and feet (Figure 1A). Clinical examination revealed a grade 3/6 systolic murmur radiating to both carotid arteries. The transthoracic echocardiographic evaluation revealed a stenotic aortic valve with a peak pressure gradient of 68 mm Hg and a mean of 37 mm Hg. The coronary arteries (according to computed tomography scanning) were normal, and a hypodense atheromatous plaque was detected at the wall of the proximal descending aorta.
Figure 1.
Skin Xanthomas Before and After Lomitapide Treatment
(A) Skin lesions presented in an 8-year-old boy with homozygous familial hypercholesterolemia phenotype. Extended nodular xanthomas of hands, elbows, and buttocks. (B) Reduction in the size (indicated by arrows) of xanthomas of the hand compared with conventional treatment (stated before) and 1 year later with use of lomitapide (stated after). (C) Extended nodular xanthomas of knees compared with conventional treatment (stated before) and 1 year later with use of lomitapide (stated after). There has been enormous reduction in the thickness and hardness of xanthomas and, in some places, in their size.
The patient’s LDL-C level before any treatment was 1,050 mg/dl (26 mmol/l). Sequence analysis of the LDLR gene revealed a compound heterozygote mutation LDLR c666C>A and c1646C>A. Family history included a sister with HoFH undergoing LL therapy and lipoprotein apheresis, both parents with heterozygous familial hypercholesterolemia, and a grandfather with CVD.
Management
The boy was treated with LL therapy (rosuvastatin 40 mg + ezetimibe 10 mg + colesevelam 2.5 g daily). No lipoprotein apheresis was performed due to venous access issues and reluctance by the parents. The patient’s LDL-C level with conventional treatment was 866 mg/dl (22.2 mmol/l). The boy initiated treatment with lomitapide 2.5 mg/day, which was steadily increased to 40 mg daily. The nearly 2-year treatment with lomitapide reduced his LDL-C level to 390 mg/dl (10 mmol/l). A remarkable reduction in the thickness, hardness, and size of xanthomas (Figures 1B and 1C) was observed. The patient has not experienced any side effects while taking lomitapide.
Discussion
The authors reported a regression of xanthomas in a case of pediatric HoFH. Young children with HoFH are usually asymptomatic and are recognized when xanthomas are present. The management of HoFH should involve a combination of lifestyle changes, statin treatment (with or without ezetimibe), started as early as possible, and lipoprotein apheresis. Lomitapide and mipomersen may be considered as additional treatments (2). The proprotein convertase subtilisin kexin type 9 inhibitors are also an option but in patients with HoFH, LL response is variable because these inhibitors work mainly through the LDL receptors, opposite of lomitapide and mipomersen. The microsomal transfer protein is expressed in the hepatocytes and enterocytes, and it mediates the transfer of triglycerides to very-low-density lipoprotein and chylomicrons. Thus, the inhibition of this reaction leads to a decreased formation of very-low-density lipoprotein and, as a consequence, lowers LDL-C levels. The adverse events observed in patients who used lomitapide come from the gastrointestinal tract. An increase in liver enzyme levels and hepatic fat accumulation may be observed. Until now, the patient tolerated lomitapide well. Chacra et al. (3) reported on the efficacy and safety of a 49-month compassionate use of lomitapide in a child with HoFH, with no side effects reported.
Conclusions
Lomitapide in this pediatric case of HoFH provided a 63% reduction in LDL-C levels (along with use of statin, ezetimibe, and colesevelam therapy) and regression of xanthomas. Whether lomitapide initiation at a younger age could have delayed or avoided CVD in this child is unclear.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Raal F.J., Hovingh G.K., Catapano A.L. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis. 2018;277:483–492. doi: 10.1016/j.atherosclerosis.2018.06.859. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Omran T., Masana L., Kolovou G. Real-world outcomes with lomitapide use in paediatric patients with homozygous familial hypercholesterolaemia. Adv Ther. 2019;36:1786–1811. doi: 10.1007/s12325-019-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chacra A.P.M., Ferrari M.C., Rocha V.Z., Santos R.D. Case report: the efficacy and safety of lomitapide in a homozygous familial hypercholesterolemic child. J Clin Lipidol. 2019;13:397–401. doi: 10.1016/j.jacl.2019.03.001. [DOI] [PubMed] [Google Scholar]