Abstract
Background:
Periodontal disease has been linked to the development of Alzheimer’s disease in people. It is theorized that the chronic inflammatory condition characteristic of oral dysbiosis in patients with periodontal disease leads to disruption of the blood–brain barrier, cytotoxin- and pathogen-induced brain damage, and accumulation of neurotoxic β-amyloid. In this inflammatory theory of Alzheimer’s disease, β-amyloid—a known antimicrobial protein—accumulates in response to oral pathogens. Canine cognitive dysfunction (CCD) is considered a naturally occurring animal model of human Alzheimer’s disease. Like humans, periodontal disease is quite common in dogs; however, a link between periodontal disease and cognitive dysfunction has not been identified in this species.
Aim:
The purpose of this prospective investigation was to compare visual periodontal scores (from digital oral photographs) with numerical (0–54) cognitive assessment questionnaire forms in aging dogs with and without a clinical diagnosis of CCD.
Methods:
A visual analogue scale (0–4) was used to score the severity of periodontal disease in 21 aging dogs: 11 dogs with a clinical diagnosis of presumptive CCD and 10 dogs without a clinical history of cognitive decline. Individuals scoring the dental photographs were blinded to all case information, including cognitive assessment scores. Cognitive assessment scores were compared with periodontal disease scores for all dogs.
Results:
There was a significant (p < 0.05) association between periodontal and cognitive scores, with higher cognitive impairment scores being more likely in dogs with more severe periodontal disease and vice versa. No associations were identified between age and either periodontal disease or cognitive impairment.
Conclusion:
Although a cause-and-effect relationship between periodontal disease and cognitive impairment cannot be ascertained from this preliminary study, we established a link between these two disorders that warrants further investigation using more stringent criteria for evaluating both periodontal disease and cognitive dysfunction.
Keywords: Dog, Periodontal, Cognitive, Amyloid, Alzheimer’s
Introduction
Periodontal disease, a common chronic inflammatory disease of dogs and humans, increases in incidence and severity with advancing age (Singhrao et al., 2015; Wallis et al., 2019). Many factors contribute to periodontal disease, most notably colonization of the mouth with pathogenic Gram-negative bacteria. The altered oral microbiome (dysbiosis) in periodontal disease leads to tissue destruction and disruption of the normal gingival barrier, ultimately allowing bacteria and their toxins entry into the systemic circulation. Various researches have implicated systemic inflammation and bacteremia stemming from periodontal disease in several disorders (Kouki et al., 2013; Polkowska et al., 2014; Bui et al., 2019; Wallis et al., 2019), and with heart, liver, and kidney damage (Kouki et al., 2013; Bui et al., 2019; Wallis et al., 2019). Furthermore, periodontal disease has been hypothesized to be linked to the development of Alzheimer’s disease in people (Singhrao et al., 2015).
Comprehensive evaluation of periodontal disease in dogs requires anesthesia, probing of gingiva and dental radiographs (Kouki et al., 2013; Polkowska et al., 2014; Bauer et al., 2018; Wallis et al., 2019). However, one study provided evidence that a visual examination of the teeth correlated moderately with more comprehensive evaluations and could allow stratification of dogs into those requiring immediate attention and those with milder periodontal disease (Bauer et al., 2018).
There is evidence that pathogenic oral bacteria in people with chronic periodontal disease can eventually cause disruption of the blood–brain barrier and incite inflammation of the central nervous system. This inflammation, and the subsequent response to it, has been implicated as contributory and/or causal factors in the pathogenesis of Alzheimer’s disease. Deposition of neurotoxic β-amyloid protein in brain parenchyma might represent an immune response to microbial pathogens and/or their constituents rather than a primary disease process (Gaur and Agnihotri, 2015; Singhrao et al., 2015; Ide et al., 2016; Aragon et al., 2018; Maurer et al., 2018; Bui et al., 2019; Choi et al., 2019; Dioguardi et al., 2020).
Canine cognitive dysfunction (CCD) is the canine analog of human Alzheimer’s disease having remarkably similar clinical and pathological features (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019). As with periodontal disease, the likelihood of a dog developing cognitive dysfunction increases with age. To the authors’ knowledge, an association between periodontal disease and CCD has not been identified in aging dogs.
We hypothesized that dogs diagnosed with cognitive dysfunction would have significantly worse periodontal disease, based on visual evaluation of dentition from digital photographs, than similarly aged dogs without obvious cognitive impairment, which we based on numerical cognitive dysfunction owner questionnaires.
Materials and Methods
We conducted a prospective case-control study. We recruited aging dogs (>9 years) with a clinical diagnosis of cognitive dysfunction. We based the diagnosis of cognitive dysfunction on historical complaints and neurologic examination findings. A board-certified neurologist (CD) conducted all neurologic examinations. We also recruited similarly aged dogs presenting for disorders not associated with cognitive dysfunction (e.g., joint disease, disk extrusion/protrusion) to serve as controls. Clients consented to allowing their pets’ dental photographs and cognitive scores to be used for the study. All dogs were fully awake for dental photography (no chemical restraint was used). Each client filled out a cognitive dysfunction questionnaire used in a previously published prospective research investigation (Table 1) (Pan et al., 2018). Briefly, this short questionnaire examines six variables: Disorientation, Interactions-social, Sleep/wake cycles, House soiling/learning/memory, Activity, and Anxiety (DISHAA) to produce a cumulative score that determines the degree of cognitive impairment or dysfunction (Table 1). We then obtained digital photographs of the dentition in each dog, consisting of frontal, right lateral, and left lateral views. One investigator (CD) also carried out a visual oral examination and photographed the dentition at the same time. We excluded dogs that received regular dental prophylactic care (at least yearly). A cohort of 11 general practice veterinarians and one co-author (MR), unassociated with the cases, evaluated the sets of dental photographs without any additional information. Each observer scored the photographic periodontal dental disease with values between 0 and 4; this scale was based on a previously published visual scoring system (Table 2) (Bauer et al., 2018).
Table 1. DISHAA (Disorientation; Interactions-social; Sleep/wake cycle; House soiling, learning and memory; Activity; and Anxiety) scoring system used to assess cognitive ability in dogs.
| Disorientation |
| Gets stuck, difficulty getting around objects, goes to hinge side of door |
| Stares blankly at walls, floor, or into space |
| Does not recognize familiar people/familiar pets |
| Gets lost in home or yard |
| Less reactive to visual (sights) or auditory (sounds) stimuli |
| Interactions (social) |
| More irritable/fearful/aggressive with visitors, family, or other animals |
| Decreased interest in approaching, greeting, or affection/petting |
| Sleep/wake cycles |
| Pacing/restless/sleeps less/waking at night |
| Vocalization at night |
| House soiling, learning and memory |
| Less able to learn new tasks or respond to previously learned commands/name/work |
| Indoor soiling of urine or stool/decreased signaling to go out |
| Difficulty getting dog’s attention/increased distraction/decreased focus |
| Activity |
| Decrease in exploration or play with toys, family members, other pets |
| Increased activity including aimless pacing or wandering |
| Repetitive behaviors, e.g., circling, chewing, licking, stargazing |
| Anxiety |
| Increased anxiety when separated from owners |
| More reactive/fearful to visual (sights) or auditory (sounds) stimuli |
| Increased fear of places/locations (e.g., new environments/going outdoors) |
Each subcategory (bullet point) is scored as 0 = none, 1 = mild, 2 = moderate, and 3 = severe. Total scores are assessed as 4–15 = mild impairment, 16–33 = moderate impairment, and >33 = severe impairment (Landsberg et al., 2012).
Table 2. Scoring system for periodontal disease based on dental photographs.
| Value | Definition |
|---|---|
| 0 | No plaque or calculus. Gums are normal |
| 1 | Mild amount of plaque. Gums can be mildly red. |
| 2 | Moderate amount of plaque. There is likely redness and swelling of the gums. |
| 3 | Tartar is present. Gums are inflamed, receding or hyperplastic. |
| 4 | Heavy tartar is present. Gums are severely inflamed. There may be evidence of infection or tooth loss. |
Statistical analyses
First, we examined the agreement between observers in rating the periodontal disease using Fleiss’ kappa (Real Statistics Using Excel Add-in for Microsoft Excel; https://www.real-statistics.com/). We did this in two ways – by examining the actual scores provided by each observer, and then by grouping periodontal disease into “less than moderate” (periodontal disease scores of 2 or less) versus “moderate to severe” (periodontal disease scores greater than 2).
We then examined the associations between several variables by Rank Sum tests (Hamme r et al., 2001). We used the modal periodontal disease score for these comparisons when comparing periodontal disease scores – analysis of the scores showed that the modal values and the median values in every case but one were identical. First, we examined the differences in DISHAA scores between dogs with “less than moderate” and “moderate to severe” periodontal disease. Next, we compared the periodontal disease scores between dogs diagnosed with CCD and dogs without the diagnosis. Third, we compared DISHAA scores between dogs diagnosed with CCD and dogs without the diagnosis. Finally, we examined the association between DISHAA scores and periodontal disease scores using Kendall’s Tau.
We created the dot plots using a free online software (Potsma and Goedhart, 2019).
Results
Our study included 21 aging dogs: 10 control dogs and 11 dogs with a presumptive diagnosis of CCD. The two groups did not differ in age (p = 0.08) or body weight (p = 0.31). Breeds represented in the control group included mixed breed (4) and one each of Brittany Spaniel, Standard Poodle, Rat Terrier, Cavalier King Charles Spaniel, Golden Retriever, and Labrador Retriever. There were five female spayed and five male castrated control dogs. Breeds represented in the group of dogs with presumptive CCD included mixed breed (4), Shih Tzu (2), Beagle (2), and one each of Toy Poodle, Lhasa Apso, and Coonhound. There were three female spayed dogs and eight castrated male dogs with CCD.
Observers ranking periodontal disease showed moderate agreement for the 21 cases (kappa = 0.39; 95% CI = 0.36–0.42). When cases were dichotomized into “less than moderate” and “moderate to severe”, observers showed better agreement (kappa = 0.56, 95% CI = 0.51–0.61). The cases showing most disagreement were those that observers scored as either “mild” or “moderate” – i.e., the marginal cases. Cases considered “severe” showed substantial agreement (kappa = 0.7).
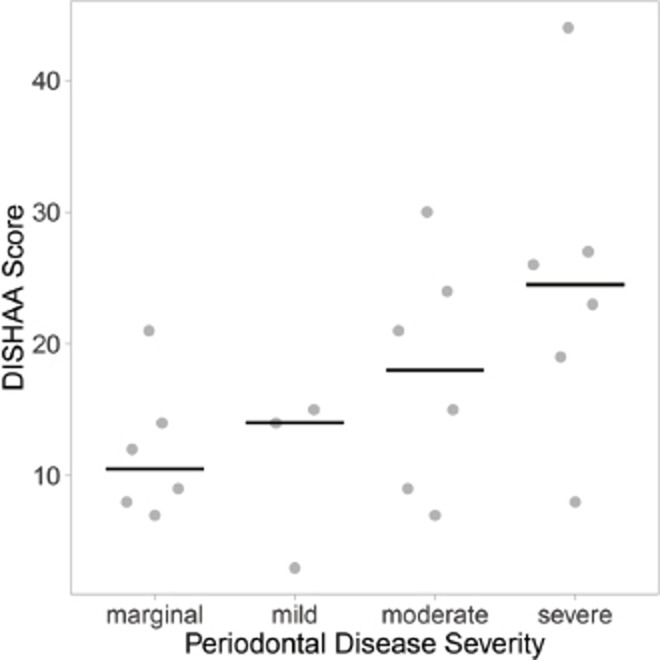
Dogs with “less than moderate” periodontal disease had lower DISHAA scores than dogs with “moderate to severe” periodontal disease (Fig. 1, p = 0.027). Dogs with a diagnosis of CCD had worse periodontal disease (Fig. 2, p = 0.001) and higher DISHAA scores (Fig. 3, p = 0.012) than dogs without CCD. DISHAA scores showed a modest correlation with periodontal scores (Fig. 4, tau = 0.42, p = 0.008).
Fig. 1. Scatterplots with medians (bars) comparing DISHAA scores in dogs with less than moderate and moderate to severe periodontal disease. DISHAA scores (y axis) are plotted against periodontal disease scores (x axis). DISHAA = Disorientation; Interactions-social; Sleep/wake cycle; House soiling, learning and memory; Activity; and Anxiety.
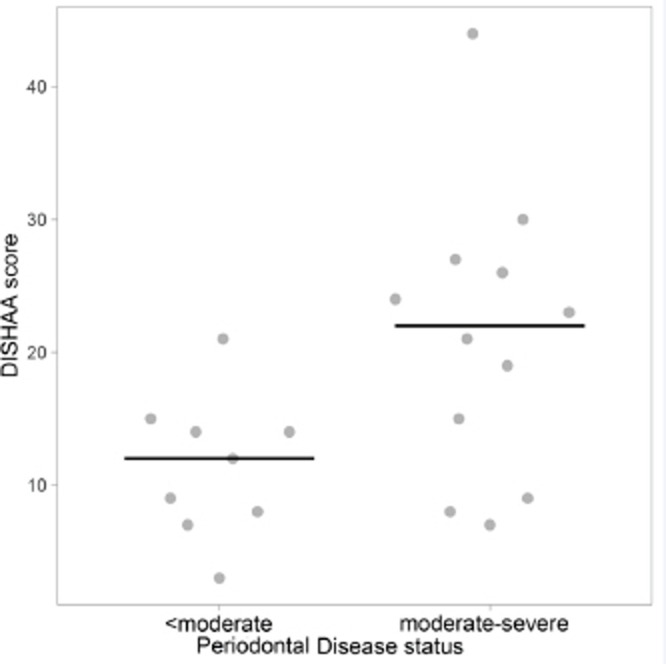
Fig. 2. Scatterplots with medians (bars) comparing periodontal disease scores in CCD dogs versus control dogs. Median periodontal disease score (y axis) is plotted against CCD or control status (x axis). CCD = canine cognitive dysfunction.
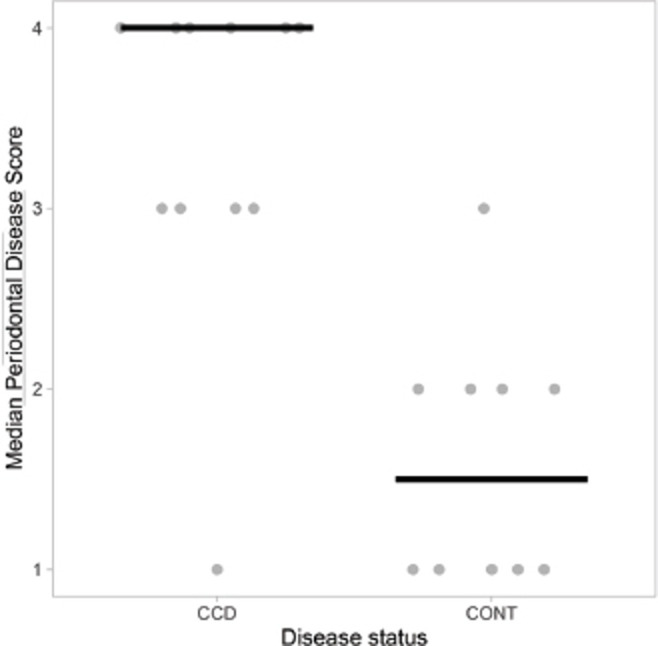
Fig. 3. Scatterplots with medians (bars) comparing DISHAA scores in CCD and control dogs. DISHAA scores (y axis) are plotted against CCD dogs and control dogs (x axis). CCD = canine cognitive dysfunction; DISHAA = Disorientation; Interactions-social; Sleep/wake cycle; House soiling, learning and memory; Activity; and Anxiety.
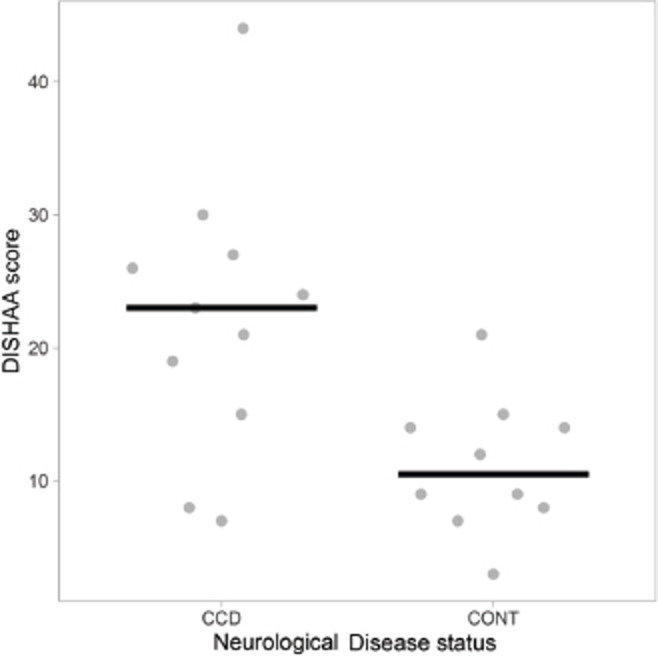
Fig. 4. Scatterplots with medians (bars) comparing DISHAA scores in dogs with marginal, mild, moderate, and severe periodontal disease, based on the median periodontal disease scores ranging from 1 and 4. DISHAA scores (y axis) plotted against periodontal disease severity (x axis). DISHAA = Disorientation; Interactions-social; Sleep/wake cycle; House soiling, learning and memory; Activity; and Anxiety.
Discussion
Our study suggests that older dogs with CCD tend to exhibit worse periodontal disease, as assessed by examination of dental photographs, than similarly aged dogs without cognitive dysfunction. Scores of cognitive dysfunction (DISHAA) tended to correlate positively, but modestly, with periodontal disease. Unsurprisingly, DISHAA scores correlated, albeit imperfectly, with a diagnosis of CCD. It is important to interpret our findings in light of the limitations of the study design and not necessarily indicative of a cause-and-effect relationship. Nonetheless, the establishment of this association between the two disorders warrants further investigation of the existence and potential nature of such a relationship.
The relationship between periodontal disease and Alzheimer’s disease in humans is well established, with periodontal disease being recognized as a risk factor for the development of Alzheimer’s disease. Whether periodontal disease is causal versus contributory in human Alzheimer’s disease remains unknown, but evidence suggests that gastrointestinal dysbiosis might play a role in the pathogenesis of Alzheimer’s disease (Cerajewska et al., 2015; Gaur and Agnihotri, 2015; Singhrao et al., 2015; Ide et al., 2016; Teixeira et al., 2017; Aragon et al., 2018; Maurer et al., 2018; Bui et al., 2019; Choi et al., 2019; Kowalski and Mulak, 2019; Liu et al., 2019; Sochocka et al., 2019; Dioguardi et al., 2020; Sun et al., 2020). The gut–brain axis describes an anatomic and physiologic communication pathway between the gastrointestinal system and the central nervous system that is mediated via the systemic circulation, peripheral nerve pathways, and the immune system. The oral cavity is one contributor to the gut–brain axis (Ambrosini et al., 2019; Kowalski and Mulak, 2019; Liu et al., 2019; Sochocka et al., 2019; Sun et al., 2020). Oral pathogens commonly implicated in severe human periodontal disease include Porphyromonas gingivalis, Treponema denticola, and Tanerella forsythia, among others (Cerajewska , et al., 2015; Gaur and Agnihotri, 2015; Singhrao et al., 2015; Harding et al., 2017; Teixeira et al., 2017; Bui et al., 2019; Dioguardi et al., 2020). Dogs with periodontal disease harbor similar oral bacteria (Polkowska et al., 2014; Golynska et al., 2017; Nomura et al., 2020). Some of these pathogens have been directly or indirectly linked to the development of Alzheimer’s disease (Cerajewska et al., 2015; Gaur and Agnihotri, 2015; Singhrao et al., 2015; Harding et al., 2017; Teixeira et al., 2017; Bui et al., 2019; Dioguardi et al., 2020). It is theorized that the pathogens themselves, some of their constituents (e.g., lipopolysaccharide) and/or the inflammation induced by the presence of these factors induces and promulgates central nervous system inflammation. The subsequent inflammation leads to direct parenchymal damage as well as promotion of neurotoxic β-amyloid deposition (Poole et al., 2013; Noble et al., 2014; Cerajewska et al., 2015; Gaur and Agnihotri, 2015; Singhrao et al., 2015; Ide et al., 2016; Teixeira et al., 2017; Maurer et al., 2018; Bui et al., 2019; Kowalski and Mulak, 2019; Sochocka et al., 2019; Dioguardi et al., 2020; Sun et al., 2020).
We examined the association between periodontal disease and cognitive dysfunction scores for all dogs and compared results between dogs diagnosed with cognitive dysfunction and those considered cognitively normal by their owners and showing no strong evidence of cognitive dysfunction on a neurological evaluation (control dogs). Based on the questionnaire scores, only one of the control dogs achieved a score indicating no cognitive impairment, and one scored as moderately cognitively impaired; the remainder scored as mildly cognitively impaired, despite owner assessments that their dogs did not exhibit cognitive dysfunction. This phenomenon likely reflects a degree of imprecision of the DISHAA questionnaire. Owners might attribute some signs of mild cognitive impairment as aspects of normal aging or certain findings on the DISHAA questionnaire might actually reflect normal aging – without additional examination of the questionnaire, we cannot determine why most of our control dogs had scores consistent with mild cognitive dysfunction. On the other hand, none of the dogs with cognitive dysfunction scored as not cognitively impaired and only three scored as mildly impaired on the client questionnaire; the remainder scored as moderately or severely impaired.
There are several limitations to this small, prospective, case-control study, most of which relate to the methods of quantifying both periodontal disease and severity of cognitive dysfunction. The clinical standard of scoring the severity of canine periodontal disease involves full dental radiographs and a full oral examination under general anesthesia that incorporates gingival probing (to assess depths of gingival pockets) (Kouki et al., 2013; Polkowska et al., 2014; Bauer et al., 2018; Wallis et al., 2019). A visual assessment scale as used in our study is a crude approximation of this standard. Nonetheless, one study demonstrated that visual assessment scores agree – albeit moderately – with more stringent periodontal disease severity assessments that include dental radiographs and full oral examination with patients under anesthesia (Bauer et al., 2018). Also, in that study, it was found that disagreement was rarely extended by more than one level of disease and did not show bias with the visual method (i.e., the visual examination did not consistently over- or underestimate the severity of the periodontal disease). The authors commented that a visual examination might overcome some of the requirements of comprehensive evaluations when screening dogs for periodontal disease. We found similar degrees of agreement between observers in evaluating periodontal disease from photographs as did the previous study, given that Fleiss’ Kappa produces more conservative estimates of agreement than a simple Cohen’s Kappa. Furthermore, regardless of the method of periodontal evaluation, our study identified a difference in periodontal disease between dogs presenting with or without cognitive dysfunction. We used a more crude measure of periodontal disease, ultimately classifying dogs as “less than moderate” or “at least moderate” for purposes of analysis. Importantly, none of the dogs in our study presented primarily for evaluation of their dentition – therefore, our study was not biased by pre-existing knowledge or suspicion of dental disease. Whether similar results to ours would be obtained with more comprehensive periodontal evaluation remains undetermined.
Similarly, although prior investigators have successfully used the same questionnaire we used for investigating cognitive dysfunction (Pan et al., 2018), the questionnaire is subject to owner-dependent variability. More stringent and non-biased methods of cognitive status would entail objective behavioral testing of cognitive ability such as delayed nonmatching to positioning memory tasks, attention tasks, food searching tasks, and problem-solving tasks (Landsberg et al., 2012; Gonzalez-Martinez et al., 2013; Chapagain et al., 2018).
Our study is the first to identify an association between visually categorized periodontal disease and CCD. Future investigation into the link between periodontal disease and cognitive dysfunction in dogs should include more detailed and objective dental evaluations and cognitive testing. Because such investigations can be both time-consuming and expensive, our initial study results justify moving forward with more extensive research into this apparent association.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contribution
Drs. Dewey and Rishniw were involved in the study’s inception and design. Dr. Dewey procured all digital photographs and questionnaire results. Dr. Rishniw carried out statistical analyses. Both Dr. Dewey and Dr. Rishniw were involved in manuscript preparation.
References
- Ambrosini Y.M, Borcherding D, Kanthasamy A, Kim H.J, Willette A.A, Jergens A, Allenspach K, Mochel J.P. The gut-brain axis in neurodegenerative diseases and relevance of the canine model: a review. Front. Aging. Neurosci. 2019;11:1–14. doi: 10.3389/fnagi.2019.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon F, Zea-Sevilla M.A, Montero J, Sancho P, Corral R, Tejedor C, Frades-Payo B, Paredes-Gallardo V, Albaladejo A. Oral health in Alzheimer’s disease: a multicenter case-control study. Clin. Oral Investig. 2018;22:3061–3070. doi: 10.1007/s00784-018-2396-z. [DOI] [PubMed] [Google Scholar]
- Bauer A.E, Stella J, Lemmons M, Croney C.C. Evaluating the validity and reliability of a visual dental scale for detection of periodontal disease (PD) in non-anesthetized dogs (Canis familiaris) PLoS One. 2018;13:e0203930. doi: 10.1371/journal.pone.0203930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui F.Q, Almeida-da-Silva C.L.C, Huynh B, Trinh A, Liu J, Woodward J, Asadi H, Ojcius D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerajewska T.L, Davies M, West N.X. Periodontitis: a potential risk factor for Alzheimer’s disease. Br. Dent. J. 2015;218:29–34. doi: 10.1038/sj.bdj.2014.1137. [DOI] [PubMed] [Google Scholar]
- Chapagain D, Range F, Huber L, Viranyi Z. Cognitive aging in dogs. Gerontology. 2018;64(2):165–171. doi: 10.1159/000481621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Kim K, Chang J, Kim S.M, Kim S.J, Cho H.J, Park SM. Association of chronic periodontitis on Alzheimer’s disease or vascular dementia. J. Am. Geriatr. Soc. 2019;67:1234–1239. doi: 10.1111/jgs.15828. [DOI] [PubMed] [Google Scholar]
- Dewey C.W, Davies E.S, Xie H, Wakshlag J.J. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. North Am. Small Anim. Pract. 2019;49:477–499. doi: 10.1016/j.cvsm.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Dioguardi M, Criincoli V, Laino L, Alovisi M, Sovereto D, Mastrangelo F, Lo Russo L, Lo Muzio L. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer’s disease: a systematic review. J. Clin. Med. 2020;9(495):1–21. doi: 10.3390/jcm9020495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S, Agnihotri R. Alzheimer’s disease and chronic periodontitis: is there an association? Geriatr. Gerontol. Int. 2015;15:391–404. doi: 10.1111/ggi.12425. [DOI] [PubMed] [Google Scholar]
- Golynska M, Polkowska I, Bartoszce-Tomaszewska M, Sobczynska-Rak A, Matuszewski L. Molecular-level evaluation of selected periodontal pathogens from subgingival regions in canines and humans with periodontal disease. J. Vet. Sci. 2017;18:51–58. doi: 10.4142/jvs.2017.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Martinez A, Rosado B, Pesini P, Garcia-Belenguer S, Palacio J, Villegas A, Suarez M.L, Santamarina G, Sarasa M. Effect of age and severity of cognitive dysfunction on two simple tasks in pet dogs. Vet. J. 2013;198(1):176–181. doi: 10.1016/j.tvjl.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Hammer Ø, Harper D.A.T, Ryan P.D. PAST: paleontological statistics software for education and data analysis. Palaeontologia Electron. 2001;4:9. Available via http://palaeo-electronica.org/2001_1/past/issue1_01.htm . [Google Scholar]
- Harding A, Robinson S, Crean S, Singhrao S.K. Can better management of periodontal disease delay the onset and progression of Alzheimer’s disease? J. Alzheimers Dis. 2017;58:337–348. doi: 10.3233/JAD-170046. [DOI] [PubMed] [Google Scholar]
- Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, Fuller J, Ibbett P, Raybould R, Thomas R, Puenter U, Teeling J, Perry H, Holmes C. Periodontitis and cognitive decline in Alzheimer’s disease. PloS One. 2016;11(3):1–9. doi: 10.1371/journal.pone.0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouki M.I, Papadimitriou S.A, Kazakos G.M, Savas I, Bitchava D. Periodontal disease as a potential factor for systemic inflammatory response in the dog. J. Vet. Dent. 2013;30(1):26–29. doi: 10.1177/089875641303000103. [DOI] [PubMed] [Google Scholar]
- Kowalski K, Mulak A. Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil. 2019;25(1):48–60. doi: 10.5056/jnm18087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg G.M, Nichol J, Araujo J.A. Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet. Clin. North Am. Small Anim. Pract. 2012;42:749–768. doi: 10.1016/j.cvsm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, Zhang L, Jia L, Yue S, Zhou K, Li L, Luo B. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Maurer K, Rahming S, Prvulovic D. Dental health in advanced age and Alzheimer’s disease: a possible link with bacterial toxins. Psychiatry Res. Neuroimaging. 2018;282:132–133. doi: 10.1016/j.pscychresns.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Noble J.M, Scarmeas N, Celenti R.S, Elkind M.S.V, Wright C.B, Schupf N, Papanou P.N. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 2014;9:1–14. doi: 10.1371/journal.pone.0114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R, Inaba H, Yasuda H, Shirai M, Kato Y, Murakami M, Iwashita N, Shirahata S, Yoshida S, Matayoshi S, Yasuda J, Arai N, Asai F, Matsumoto-Nakano M, Nakano K. Inhibition of Porphyromonas gulae and periodontal disease in dogs by a combination of clindamycin and interferon alpha. Sci. Rep. 2020;10:3113. doi: 10.1038/s41598-020-59730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Landsberg G, Mougeot I, Kelly S, Xu H, Bhatnagar S, Gardner C.L, Milgram N.W. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): a prospective double blinded placebo controlled clinical study. Front. Nutr. 2018;12(5):1–10. doi: 10.3389/fnut.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkowska I, Sobczynska-Rak A, Golynska M. Analysis of gingival pocket microflora and biochemical blood parameters in dogs suffering from periodontal disease. In Vivo. 2014;28:1085–1090. [PubMed] [Google Scholar]
- Poole S, Singhrao S.K, Kesavalu L, Curtis M.A, Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013;36:665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- Potsma M, Goedhart J. PlotsOfData—A web app for visualizing data together with their summaries. PLoS Biol. 2019;17:e3000202. doi: 10.1371/journal.pbio.3000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhrao S.K, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm. 2015;2015:1–10. doi: 10.1155/2015/137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochocka M, Donskow-Lysoniewska K, Diniz B.S, Kurpas D, Brzozowska E, Leszek J. The gut microbiome alterations and inflammation-driven pathogenesis of Alzheimer’s disease-a critical review. Mol. Neurobiol. 2019;55:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Ma K, Wen J, Wang G, Zhang C, Li Q, Bao Z, Wang H. A review of the brain-gut microbiome axis and the potential role of microbiota in Alzheimer’s disease. J. Alzheimers Dis. 2020;73:849–865. doi: 10.3233/JAD-190872. [DOI] [PubMed] [Google Scholar]
- Teixeira F.B, Saito M.T, Matheus F.C, Prediger R.D, Yamada E.S, Maia C.S.F, Lima R.R. Periodontitis and Alzheimer’s disease: a possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front. Aging. Neurosci. 2017;9:1–9. doi: 10.3389/fnagi.2017.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C, Pesci I, Colyer A, Milella L, Southerden P, Holcombe L.J, Desforges N. A longitudinal assessment of periodontal disease in Yorkshire terriers. BMC Vet. Res. 2019;15(207):1–11. doi: 10.1186/s12917-019-1923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


