Abstract
Background:
Women with chest pain and no obstructive coronary artery disease often have coronary microvascular dysfunction (CMD), diagnosed by invasive coronary reactivity testing (CRT). The relationship between CMD and diastolic function measured by cardiac magnetic resonance imaging (CMR) is not well described.
Methods:
41 women with suspected CMD underwent CRT and CMR. Left ventricular end-diastolic pressure (LVEDP), coronary flow reserve (CFR) and coronary blood flow (CBF) were measured invasively. Resting CMR of these women and 20 reference controls was assessed for LV mass, septal wall thickness, ejection fraction (LVEF), end-diastolic volume (EDV), peak filling rate (PFR) and time-to-peak-filling rate (tPFR). Pearson correlations and linear regression models were made.
Results:
Mean age was 55±9, all had LVEF≥50%, and 16/41(40%) had LVEDP>15 mmHg. CMD (CFR<2.5 or CBF<50%) was present in 34/41(83%) women. tPFR (mean 178±110 msec) and PFR (mean 3.2±0.64 EDV/sec) were not significantly different in women with or without CMD. tPFR increased with age (r=0.37, p=0.017) and septal wall thickness (r=0.47, p=0.002), while PFR decreased with age (r=−0.45, p=0.003). There was an inverse relationship between CFR and tPFR (r=−0.3, p=0.058). Increasing mass was associated with decreasing CBF (p=0.02). Compared to controls, cases had lower LVEF (p=0.049) and lower EDV (p=0.0002).
Conclusion:
In women with signs and symptoms of ischemia but no obstructive coronary artery disease, CMD and elevated LVEDP are prevalent. While non-endothelial dependent CMD may be related to diastolic dysfunction, further investigation is needed regarding links between CMD, diastolic dysfunction and the development of heart failure with preserved LVEF.
Keywords: microvascular coronary dysfunction, diastolic dysfunction, women’s heart disease
Introduction
Sex-specific differences in ischemic heart disease presentation and pathophysiology are increasingly described. Ischemic heart disease is the leading cause of mortality among women in the United States and the world1,2. Chest pain and acute coronary syndromes are less likely to be associated with obstructive epicardial coronary artery disease (CAD) in women than in men3. Approximately 50% of women with signs and symptoms of ischemia but no obstructive CAD have evidence of coronary microvascular dysfunction (CMD)4,5. CMD is diagnosed by invasive coronary reactivity testing (CRT) using vasoactive substances to test endothelial and non-endothelial vascular function6,7.
Diastolic dysfunction appears to be a primary mechanism in approximately 40% of all patients with heart failure8 and is an independent predictor of mortality9. Women are more likely to be diagnosed with heart failure with preserved LV ejection fraction (HFpEF)10-12 than men. Diastolic heart failure, which is associated with an annual mortality rate of 5-8% comparable to systolic heart failure, has been the focus of recent heart failure investigations9,13-16. While diastolic dysfunction does not always lead to HFpEF, the relationship between diastolic dysfunction and CMD has been uncertain and may offer therapeutic implications. Invasive measurement of left ventricular (LV) end-diastolic pressure is a marker of LV function and compliance. Elevated LV end-diastolic pressure (LVEDP) has been shown to be an independent predictor of mortality in ischemic heart disease, independent of LV systolic function17,18.
There is little known regarding the relationship between CMD, LVEDP, and parameters of diastolic function in women. Cardiac magnetic resonance imaging (CMR) is an emerging modality for not only non-invasive evaluation of myocardial ischemia secondary obstructive CAD but also evaluation of ischemia from CMD, which is a more diffuse pattern of hypoperfusion with an abnormal myocardial perfusion reserve index19,20. Furthermore, myocardial systolic and diastolic function can also be assessed via CMR21,22. Diastolic function has recently been found to be impaired in women with signs and symptoms of ischemia in the absence of obstructive CAD, but there was no assessment of the potential relationship between invasive measures of CMD and the diastolic parameters23.
The objective of this study is to examine the association between CMD, LVEDP, and diastolic dysfunction measured by invasive CRT and noninvasive CMR in women with signs and symptoms of ischemia but no obstructive CAD.
Methods
Patient Population:
Women with signs and symptoms of myocardial ischemia but no obstructive CAD at diagnostic invasive coronary angiography who underwent clinically indicated CRT and CMR as part of ongoing care related to persistent signs and symptoms of ischemia were enrolled into a registry at the Cedars-Sinai Women’s Heart Center. Inclusion criteria included patients with signs and symptoms of myocardial ischemia (chest pain, abnormal stress testing, abnormal noninvasive testing) in the absence of obstructive CAD (<50% luminal obstruction in one or more epicardial coronary arteries on angiography) and preserved ejection fraction. Patients were excluded from the registry if they had life expectancy less than 6 months, age<21 years, valvular heart disease, history of congestive heart failure, and structural heart disease (such as LV hypertrophy). All study participants gave written informed consent before undergoing evaluation and the study protocol was approved by the Institutional Review Board at Cedars-Sinai Medical Center (CSMC). Demographic data were recorded with standardized questionnaires. CRT and CMR data were read on-site at the CSMC Core Laboratories.
A reference control group of 20 asymptomatic healthy women were recruited at CSMC based on their age and hormone use-status to match the cases in the registry. Inclusion criteria included the absence of signs or symptoms of myocardial ischemia and absence of cardiac risk factors by Framingham/NCEP criteria. Exclusion criteria included contraindications to CMR and inability to perform treadmill exercise. All women underwent maximum symptom-limited exercise treadmill testing and stress-rest CMR as previously published24. All women had no evidence of ischemia by exercise treadmill testing and CMR perfusion. This reference control group did not undergo any invasive coronary angiography or CRT.
Coronary Reactivity Testing:
Coronary angiography was performed to confirm the absence of obstructive CAD. LVEDP was measured, and diastolic dysfunction was defined as LVEDP > 15 mm Hg. Coronary microvascular and macrovascular endothelial and non-endothelial dependent function were then measured, using a standard protocol25. Using a Doppler flow wire (FloWire Volcano, San Diego, CA) in the proximal left anterior descending artery, coronary flow reserve (CFR) was measured by graded intracoronary adenosine injections of 18 mcg and 36 mcg, to create maximal hyperemia. Graded doses of intracoronary acetylcholine (0.182 and 18.2 mcg/ml) were infused for 2 minutes to measure average peak velocity and calculate coronary blood flow (CBF) as previously described25. Adenosine tests non-endothelial-dependent microvascular function, while acetylcholine tests endothelial dependent function. Non-endothelial-dependent CMD was defined as CFR < 2.5. Endothelial-dependent CMD was defined as CBF change < 50%.
CMR:
CMR scans were performed in a supine position on a 1.5 Tesla magnet (Siemens Sonata, Erlangen, Germany) with ECG-gating and a phase-array surface coil (CP Body Array Flex, Siemens Medical Systems, Erlangen, Germany). Resting cine CMR images were obtained in consecutive short-axis planes to encompass the entire left ventricle from the base to the apex. Standard Siemens Medical Imaging sequence was used for the studies: retrospectively ECG-gated steady-state free-precession cine, with repetition time 1 x RR interval [60 msec, dependent on heart rate], echo time 1.4 msec, slice thickness 8 mm, FOV 300x206 mm, image matrix 132 x 256, number of phases=25, bandwidth 830 Hz . All CMR scans were performed by the same operator.
Endocardial contours were traced manually from end-systole to end-diastole using Argus software (Siemens Medical) and used to measure end-systolic volume (ESV) and end-diastolic volume (EDV). Contours were applied by a single experienced observer. Frame-by-frame and slice-by-slice evaluation of cine images from end-systole to end-diastole was performed to ensure exclusion of epicardial fat, as well as the quality of endocardial and epicardial contours. Papillary muscles were included in the LV blood volume measurement and not included in the myocardial mass measurement; since papillary muscle volume is constant throughout the cardiac cycle, inclusion of papillary muscle volume would not significantly affect filling rates, which is the principal parameter determined in this study26, although they will affect stroke volume and EDV used to normalize filling data27. Septal wall thickness and LV mass were calculated from the end-diastolic frames. Stroke volume was calculated as the difference between EDV and ESV, and ejection fraction was calculated as stroke volume divided by EDV. Heart rate and blood pressure were measured and recorded.
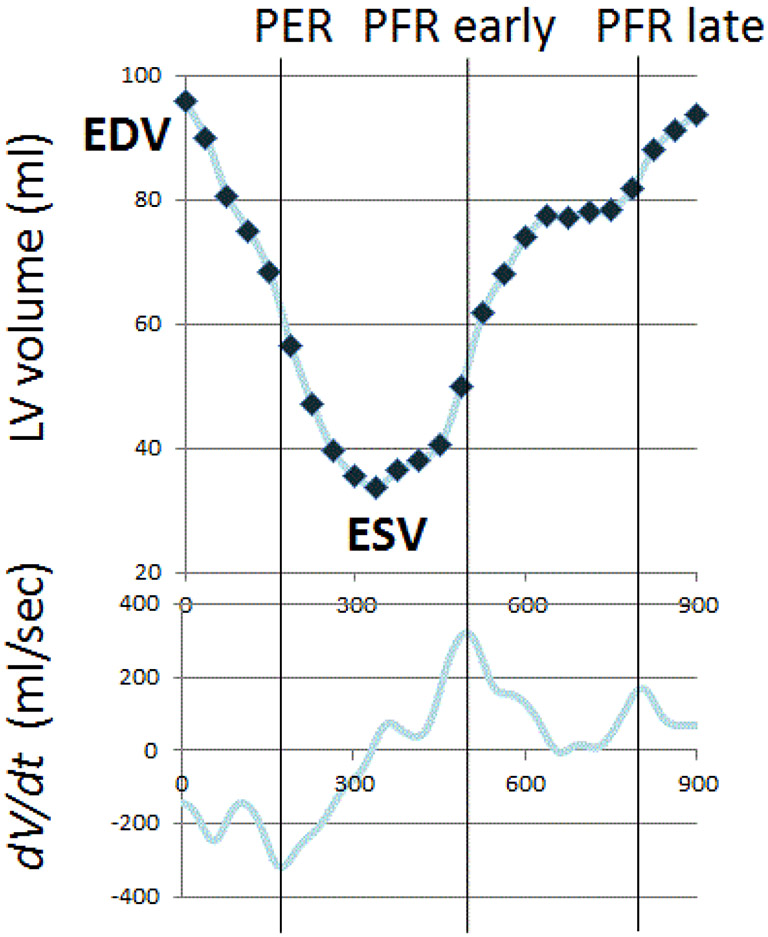
Volume-time curves were used to derive indices of diastolic dysfunction, including early peak filling rate (PFR) and time-to-peak-filling rate (tPFR) (Figure 1). Similar to peak early mitral inflow velocity (E) by echocardiography, PFR decreases during the progression from normal diastolic function to grade 1 diastolic dysfunction, and PFR normalized for EDV has been demonstrated to be a useful index for evaluation diastolic function28,29. Similar to deceleration time by echocardiography, tPFR is expected to prolong with grade 1 diastolic dysfunction30. From grade 1 diastolic dysfunction to grade 3 diastolic dysfunction, PFR increases while tPFR shortens30. Means, standard deviation and 95% confidence intervals have been reported for functional and geometric parameters of the normal LV in both men and women24,31.
Figure 1: Time-Volume Curve.
The LV time-volume curve and its first derivative were used to determine the peak filling rate and the time to peak filling rate from end systole. LV=left ventricular, dV/dt= filling rate curve, EDV= end-diastolic volume, ESV=end-systolic volume, PER=peak ejection rate, PFR=peak filling rate
Statistical Methods:
Values of the continuous variables are presented as mean and standard deviations, unless otherwise specified. The relationships among demographic variables and measurements from CRT and CMR were examined using the Pearson product moment correlation. Fisher’s z transformation was applied to test correlation coefficients significantly different from zero, and Fisher’s exact test was used to evaluate categorical data. Linear regression models used logarithmic transformation on CFR and on CBF+100 to adjusted for skewed distributions. A standard stepwise procedure was used for model building with the CMR variables as explanatory covariates. SAS 9.1.3 was used for all statistical analyses. All probabilities were two-tailed and a p value of less than 0.05 was considered statistically significant.
Results:
Demographics
Baseline demographics are depicted in Table 1. Almost all women were Caucasian, and many of the cases had risk factors for coronary artery disease, however, less than a third of the cases were taking antihypertensive or anti-anginal agents. Five cases were taking loop or thiazide diuretics. All cases had normal LV ejection fraction and no evidence of obstructive coronary artery disease on angiography. Angina symptoms (location, duration, and triggering/alleviating factors) did not differ between cases with and without CMD, although there was a trend towards lower frequency of chest pain episodes in the cases with CMD vs the cases without CMD (p=0.08). Mean time difference between the CRT and CMRICMR in 95% of women was 40 ± 13 days. Two women had their CRT and CMRICMR one year apart.
Table 1.
Baseline Demographics and Clinical Variables
| Variables | Total Cases N=41 |
With CMD N=34 |
Without CMD N=7 |
Reference Controls N=20 |
p- Value* |
|---|---|---|---|---|---|
| Age | 55 ± 9 | 55 ± 8 | 53 ± 8 | 54 ± 9 | 0.41 |
| Body Mass Index | 26 ± 4.3 | 26 ± 4.7 | 27 ± 3.9 | 25 ± 4 | 0.62 |
| Race (non-Caucasian) | 2 (4.9%) | 2 | 0 | 2 (10%) | 1.00 |
| Hypertension | 20 (49%) | 15 | 3 | 0 | 1.00 |
| Hyperlipidemia | 20 (49%) | 18 | 2 | 0 | 0.41 |
| History of Smoking | 18 (44%) | 15 | 3 | 8 (40%) | 1.00 |
| Diabetes | 1 (2%) | 0 | 1 | 0 | 0.17 |
| Systolic BP (mmHg) | 138 ± 20 | 138 ± 21 | 134 ± 16 | 121 ± 24 | 0.58 |
| Diastolic BP (mmHg) | 71 ± 9.5 | 70 ± 9.1 | 77 ± 10 | 73 ± 11 | 0.13 |
| Beta – Blockers | 12 (29%) | 12 | 0 | 0 | 0.08 |
| Calcium Channel Blocker | 9 (22%) | 8 | 1 | 0 | 1.00 |
| ACE Inhibitors | 7 (17%) | 7 | 0 | 0 | 0.32 |
| Angiotensin receptor blocker | 5 (12%) | 4 | 1 | 0 | 1.00 |
| Diuretics | 5 (12%) | 4 | 1 | 0 | 1.00 |
| Nitrates | 7 (17%) | 6 | 1 | 0 | 1.00 |
| Statins | 19 (46%) | 17 | 2 | 0 | 0.42 |
Values expressed as mean ± SD, or N (%).
ACE= Angiotensin converting enzyme, BP= blood pressure, CMD = coronary microvascular dysfunction defined by abnormal coronary flow reserve to adenosine and/or abnormal coronary blood flow change to acetylcholine
cases with CMD vs cases without CMD
CRT measures of CMD
Mean LVEDP was mildly elevated at 15 ± 4.5 (range 5-24) mmHg (Table 2). 53% of cases had an LVEDP > 12 mm Hg, and 40% of cases had an LVEDP > 15 mm Hg. Mean CFR (2.8 ± 0.8) and CBF (53 ± 92%) were normal, but CMD (CFR<2.5 and/or CBF<50%) was present in 34/41 (83%) women. LVEDP did not correlate with CFR or CBF. CFR correlated with increasing age (r −0.45, p=0.0028)
Table 2.
Coronary Reactivity Testing Results
| Variables | Total Cases N=41 |
With CMD N=34 |
No CMD N=7 |
Controls N=20 |
p- Value* |
|---|---|---|---|---|---|
| LV End Diastolic Pressure | 15 ± 4.5 | 15 ± 4.5 | 14 ± 6.1 | n/a | 0.62 |
| Coronary Flow Reserve | 2.8 ± 0.8 | 2.7 ± 0.9 | 3.1 ± 0.5 | n/a | 0.37 |
| Δ Coronary Blood Flow | 53 ± 92 | 29 ± 72 | 168 ± 94 | n/a | <0.0001 |
Values expressed as mean ± SD.
CMD= coronary microvascular dysfunction defined by abnormal coronary flow reserve to adenosine and/or abnormal coronary blood flow change to acetylcholine, LV= left ventricular, Δ = change in coronary blood flow compared to baseline
cases with CMD vs cases without CMD
CMR measures of LV structure and function
For all cases, LV mass index and septal wall thickness index were within normal range (Table 3). Overall case group mean tPFR was 178±110 msec and PFR was 3.2±0.64 end-diastolic volume/sec, with no significant differences between cases with CMD and cases without CMD. There were also no significant differences in tPFR and PFR in the cases with abnormal CFR vs abnormal CBF (Table 4).
Table 3.
CMR measures of left ventricular structure and function
| Variables | Total Cases N=41 |
With CMD N=34 |
No CMD N=7 |
Controls N=20 |
p- Value* |
|---|---|---|---|---|---|
| LV Ejection Fraction (%) | 64 ± 13% | 67 ± 7.6% | 62 ± 6.8 | 70 ± 3.9 | 0.15 |
| LV Mass, index** (g/m2) | 44 ± 9.2 | 43 ± 8.1 | 38 ± 2.6 | 48 ± 6.4 | 0.17 |
| Septal WT, index** (mm/m2) | 4.6 ± 0.7 | 4.6 ± 0.7 | 4.3 ± 0.5 | 4.6 ± 0.6 | 0.27 |
| EDV, index** (mL/m2) | 59 ± 12 | 58 ± 11 | 56 ± 6.5 | 73 ± 14.5 | 0.64 |
| PFR (EDV/sec) | 3.2 ± 0.6 | 3.2 ± 0.6 | 3.1 ± 0.7 | 2.9 ± 0.4 | 0.68 |
| Time to PFR (msec) | 178 ± 110 | 186 ± 116 | 137 ± 32 | 200 ± 20 | 0.27 |
Values expressed as mean ± SD.
CMD = coronary microvascular dysfunction defined by abnormal coronary flow reserve to adenosine and/or abnormal coronary blood flow change to acetylcholine, EDV= end-diastolic volume, LV =left ventricle, PFR= peak filling rate, WT= wall thickness.
cases with CMD vs cases without CMD
indexed to body surface area
Table 4.
Diastolic Function in women with CMD
| Abnormal Coronary Flow Reserve (<2.5) (n=15) |
Abnormal Δ Coronary Blood Flow (<50%) (n=27) |
p-Value | |
|---|---|---|---|
| PFR (EDV/sec) | 3.1±0.7 | 2.9±0.9 | 0.46 |
| Time to PFR (msec) | 238±160 | 187±112 | 0.23 |
PFR= peak filling rate, EDV= end-diastolic volume, Δ = change in coronary blood flow compared to baseline
Compared to the reference control group, the case group had lower LV ejection fraction (64±13% vs 70±3.9%, p= 0.049), lower EDV indexed to body surface area (59±12 vs 73±14 mL/m2, p= 0.0002), and higher PFR indexed to EDV (3.2±0.6 vs 2.9±0.4, p=0.048). LV mass index was not quite statistically significant (44±9.2 vs 48±6.4, p= 0.086). Septal wall thickness and tPFR were not significantly different (p=0.38, p=1.0; respectively).
A trend for an inverse relationship was seen between CFR and tPFR (r= −0.3, p= 0.058) and between CBF and PFR (r= −0.28, p= 0.08). tPFR increased with age (r= 0.37, p= 0.017) and septal wall thickness (r= 0.47, p= 0.002) (Figure 2). We also found that PFR decreased with age (r= −0.45, p= 0.003). There was no statistically significant relationship noted between elevated LVEDP and CMR diastolic parameters.
Figure 2: Correlation Graphs.
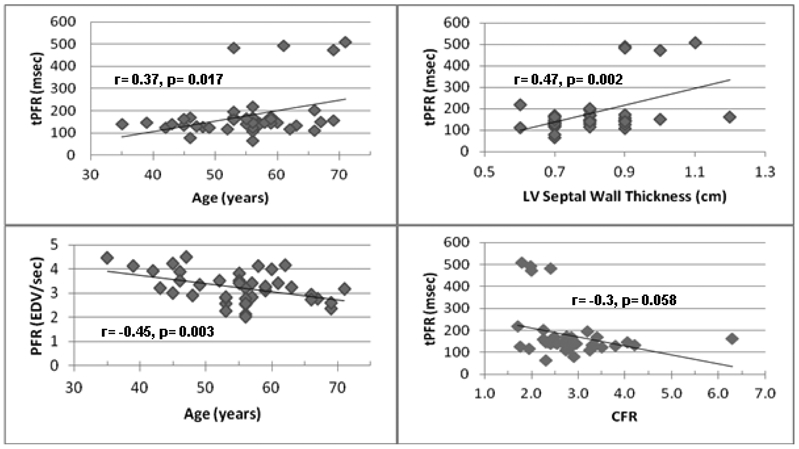
Relationships between age, time to peak filling rate (tPFR), peak filling rate (PFR), left ventricular (LV) septal wall thickness, and coronary flow reserve (CFR) were evaluated. TOP: tPFR increased with age and septal wall thickness. BOTTOM: There was an inverse relationship between PFR and age and between CFR and tPFR.
Regression models were also made using the CMD parameters as continuous outcome to evaluate their relationship to CMR and clinical variables. BMI and LV mass were in a multiple linear regression with the transformed CBF as the outcome (model F test p=0.04, R-squared=0.20). LV mass had a significant negative association (increasing LV mass was associated with decreasing CBF) with transformed CBF (p=0.02), and BMI had a positive association that was not quite significant (p=0.08). The model with log CFR used all 41 subjects and time to peak filling as the only explanatory factor (model F test p=0.01, R-squared=0.15). TPFR had a negative association with log CFR (p=0.01).
Discussion:
We report that a high percentage of women with signs and symptoms of ischemia but no obstructive CAD have an elevated invasively measured LVEDP. These women have lower LV ejection fraction and smaller EDV compared to reference controls. We found that these women had higher PFR than the age-matched reference controls, suggesting that the women may be progressing towards grade 2 diastolic dysfunction as opposed to grade 1 diastolic dysfunction30. Interestingly, diastolic function as measured by TPFR appears to be inversely related to adenosine CFR, a non-endothelial dependent pathway of CMD both in our correlation and regression analyses. However, CMR indices of diastolic dysfunction defined by tPFR and PFR were not significantly different between our patients with and without CMD. To our knowledge, this is the first study reporting abnormal filling pressures in women with a definitive diagnosis of CMD by invasive CRT, many of whom have resting diastolic dysfunction despite normal ventricular size and mass23,32,33.
There appeared to be a trend for an inverse relationship between change in CBF to acetylcholine (a function of endothelial dependent pathways) and PFR in this study, which suggests that worsening CBF may be related to higher PFR. CBF also appeared to have an inverse relationship with LV mass, ie increasing LV mass was associated with worsening CBF. These observations have not been previously reported in CMR literature in women with CMD. Mendoza et al have reported that PFR measured by CMRICMR decreases during the progression from normal diastolic function to grade 1 diastolic dysfunction, and then increases during the progression from grade 1 diastolic dysfunction to grade 3 diastolic dysfunction30. However, this is consistent with echocardiography literature, as Lerman et al34 found that abnormal CBF change to acetylcholine was associated with diastolic dysfunction by tissue Doppler echocardiography assessment.
Diastolic heart failure, increasingly termed HFpEF, is a prevalent and a significant problem in women10. Ischemic heart disease without obstructive CAD may be a mechanistic pathway in the pathogenesis of HFpEF. Our current data demonstrate that low CFR may be related to early evidence of diastolic dysfunction based on CMR indices. Although LVEDP was only modestly elevated in these women, we may be identifying preclinical diastolic dysfunction, which has been shown to progress to symptomatic HFpEF35. Diastolic dysfunction can be determined by CMR21,36,37, and PFR and tPFR can be simply evaluated at the time of a routine CMR, and although they may not be as accurate in defining diastolic function as tagged imaging CMR38,39, they are less time consuming to analyze. Automated processing of CMR can now generate LV filling curves within minutes, can measure PFR and tPFR with high reproducibility, and can be used to recognize graded severity of diastolic dysfunction similar to echocardiography30.
The women in our study all had normal LV ejection fraction; 53% had an LVEDP >12 mm Hg and 40% had an LVEDP > 15 mm Hg. Mildy elevated LVEDP in the setting of preserved ejection fraction may contribute to exertional dyspnea and chest pain in women with CMD. Prior reports have demonstrated that increased LV stiffness impairs exercise tolerance in patients with HFpEF 40. Our findings are consistent with a study by Elhabyan et al41 who found a strong association between elevated LVEDP at cardiac catheterization and ischemia noted by perfusion defect on nuclear scan in 210 patients with no obstructive CAD at angiography. They concluded that elevated LVEDP may be considered one of the factors contributing to the pathogenesis of signs and symptoms of ischemia in this group of patients41. These findings are consistent with other myocardial perfusion studies that show that diastolic filling parameters with PFR and tPFR may correlate with LVEDP42.
Several factors and clinical conditions are known to influence LVEDP. Impairment of myocardial contractility due to concentric hypertrophy from hypertension or valvular stenosis, restrictive or infiltrative cardiomyopathy, as well as myocardial ischemia due to obstructive CAD all lead to rise in compensatory preload as the left ventricle tries to maintain stroke volume43. CMD may lead to a compensatory elevated filling pressure because increased microvascular resistance causes worsening myocardial tissue perfusion and leads to an ischemic left ventricle44,45. CMD may also lead to progressive heart failure through oxidative stress and reduction in nitric oxide bioavailability16. Our results demonstrating normal LV mass and absence of LV hypertrophy using CMR suggest that the elevated LVEDP in our patients was not secondary to these known causes.
An elevated LVEDP is one of the first hemodynamic abnormalities seen in diastolic dysfunction46. Accordingly, our results suggest that CMD may be mechanistic pathway and pathophysiologic explanation of elevated filling pressures. Chronic pressure overload may lead to structural microvascular abnormalities which lead to myocardial hypoperfusion, which becomes obvious at times of increased demand, manifesting as exercise intolerance47. A relationship between arterial stiffness and diastolic dysfunction has been described, and women differ from men in central arterial stiffness48. A recent study reports that LV diastolic function measured by doppler echocardiography correlates significantly to arterial stiffness in women49. Since older women tend to develop HFpEF, it is interesting that in our cohort of symptomatic middle-aged women, we already find borderline elevations of LVEDP; we may be detecting an early form of cardiomyopathy, which if left untreated over the next several decades of a woman’s life, may lead to the syndrome of overt diastolic heart failure.
Study Limitations:
Absolute values for PFR and tPFR at one time-point in this cross-sectional study do not always accurately indicate the stage of diastolic function, similar to echo-derived early mitral inflow velocity (E) and deceleration time. Our study excluded patients with clinically diagnosed heart failure, however both PFR and tPFR have wide confidence intervals and thus are far from perfect for sensitivity to detect diastolic dysfunction as confirmed by echocardiographic or invasive methods. Given that loading conditions may vary and affect diastolic measurements, we obtained CMR on average within 2 months of the invasive CRT. We cannot make any conclusions regarding causality of diastolic dysfunction in this study. CMD may be a consequence of diastolic dysfunction or vice versa. Invasive diastolic measurements of impaired relaxation (i.e. peak –dP/dt or measurement of passive stiffness) was not assessed. Research based techniques such as CMR tissue tagging to evaluate diastolic function was not used in this study, since this study evaluated clinically ordered CMR.
Another limitation of this study is the lack of adjustment for heart rate, which influences the loading condition of the heart. We performed averaging of the volume-time curves without adjustment for different cycle lengths, because the period of early diastole varies within a narrow range50. Despite not adjusting for heart rate, we were able to show that tPFR increases with age and increasing wall thickness, which is consistent with prior studies showing that worsening diastolic function with age and LV hypertrophy and helps to validate the methods46,51,52. Our measurements of both LVEDP and diastolic dysfunction by CMR were conducted at rest and therefore may be an underestimate of functional diastolic dysfunction. Our exclusion of papillary muscles from the LV mass and inclusion in the LV volumes also must be considered, as we adjusted the PFR for EDV, although our methods were consistent with our published reference control values. Finally, our high rate of CMD determined by clinically indicated invasive testing limits study specificity. Future work in larger sample sizes in more heterogenous populations is needed.
Conclusions:
There is a high prevalence of elevated filling pressures in symptomatic women with no obstructive CAD, who are suspected to have CMD. Prior investigation has demonstrated that these women also have diastolic dysfunction by CMR23. We now report that abnormal non-endothelial dependent CMD appears to be related to abnormal diastolic function. Further investigation is needed regarding links between CMD, diastolic dysfunction and the development of HFpEF.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles and The Society for Women’s Health Research (SWHR), Washington, D.C., and the Linda Joy Pollin Women’s Heart Health Program.
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation 2007;115:1481–501. [DOI] [PubMed] [Google Scholar]
- 2.Heron M Deaths: leading causes for 2008. Natl Vital Stat Rep 2012;60:1–94. [PubMed] [Google Scholar]
- 3.Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation 2007;115:823–6. [DOI] [PubMed] [Google Scholar]
- 4.Hasdai D, Holmes DR Jr., Higano ST, Burnett JC Jr., Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clinic proceedings Mayo Clinic 1998;73:1133–40. [DOI] [PubMed] [Google Scholar]
- 5.Reis SE, Holubkov R, Conrad Smith AJ, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. American heart journal 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 6.Hasdai D, Gibbons RJ, Holmes DR Jr., Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation 1997;96:3390–5. [DOI] [PubMed] [Google Scholar]
- 7.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–5. [DOI] [PubMed] [Google Scholar]
- 8.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA : the journal of the American Medical Association 2006;296:2209–16. [DOI] [PubMed] [Google Scholar]
- 9.Aljaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation 2012;125:782–8. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. The New England journal of medicine 2006;355:260–9. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Roman MJ, Liu JE, et al. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol 2000;86:1090–6. [DOI] [PubMed] [Google Scholar]
- 12.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine 2006;355:251–9. [DOI] [PubMed] [Google Scholar]
- 13.Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: a population-based study. Circ Heart Fail 2012;5:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Archives of internal medicine 2011;171:1082–7. [DOI] [PubMed] [Google Scholar]
- 15.Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. The New England journal of medicine 2004;351:1097–105. [DOI] [PubMed] [Google Scholar]
- 16.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. Journal of the American College of Cardiology 2012;60:1455–69. [DOI] [PubMed] [Google Scholar]
- 17.Li YY, Bush CA, Orsini A, Mi Z, Leier CV. Predictors of inpatient outcomes in hospitalized patients after left heart catheterization. Am J Cardiol 2009;103:486–90. [DOI] [PubMed] [Google Scholar]
- 18.Salem R, Denault AY, Couture P, et al. Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction. Br J Anaesth 2006;97:292–7. [DOI] [PubMed] [Google Scholar]
- 19.Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011;4:514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Geest RJ, Reiber JH. Quantification in cardiac MRI. Journal of magnetic resonance imaging : JMRI 1999;10:602–8. [DOI] [PubMed] [Google Scholar]
- 22.Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN. Diastolic dysfunction: improved understanding using emerging imaging techniques. American heart journal 2010;160:394–404. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MD, Szczepaniak LS, Wei J, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014;7:510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakir M, Wei J, Nelson MD, et al. Cardiac magnetic resonance imaging for myocardial perfusion and diastolic function-reference control values for women. Cardiovasc Diagn Ther 2016;6:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei J, Mehta PK, Johnson BD, et al. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng W, Nagaraj H, Gupta H, et al. A dual propagation contours technique for semi-automated assessment of systolic and diastolic cardiac function by CMR. J Cardiovasc Magn Reson 2009;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papavassiliu T, Kühl HP, Schröder M, et al. Effect of Endocardial Trabeculae on Left Ventricular Measurements and Measurement Reproducibility at Cardiovascular MR Imaging. Radiology 2005;236:57–64. [DOI] [PubMed] [Google Scholar]
- 28.Daneshvar D, Wei J, Tolstrup K, Thomson LE, Shufelt C, Merz CN. Diastolic dysfunction: improved understanding using emerging imaging techniques. Am Heart J 2010;160:394–404. [DOI] [PubMed] [Google Scholar]
- 29.Bowman LK, Lee FA, Jaffe CC, Mattera J, Wackers FJ, Zaret BL. Peak filling rate normalized to mitral stroke volume: a new Doppler echocardiographic filling index validated by radionuclide angiographic techniques. Journal of the American College of Cardiology 1988;12:937–43. [DOI] [PubMed] [Google Scholar]
- 30.Mendoza DD, Codella NC, Wang Y, et al. Impact of diastolic dysfunction severity on global left ventricular volumetric filling - assessment by automated segmentation of routine cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. [DOI] [PubMed] [Google Scholar]
- 32.Cain PA, Ahl R, Hedstrom E, et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC medical imaging 2009;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larghat AM, Maredia N, Biglands J, et al. Reproducibility of first-pass cardiovascular magnetic resonance myocardial perfusion. Journal of magnetic resonance imaging : JMRI 2013. [DOI] [PubMed] [Google Scholar]
- 34.Elesber AA, Redfield MM, Rihal CS, et al. Coronary endothelial dysfunction and hyperlipidemia are independently associated with diastolic dysfunction in humans. American heart journal 2007;153:1081–7. [DOI] [PubMed] [Google Scholar]
- 35.Okura Y, Ohno Y, Ramadan MM, et al. Characterization of outpatients with isolated diastolic dysfunction and evaluation of the burden in a Japanese community: Sado Heart Failure Study. Circ J 2007;71:1013–21. [DOI] [PubMed] [Google Scholar]
- 36.Zeidan Z, Erbel R, Barkhausen J, Hunold P, Bartel T, Buck T. Analysis of global systolic and diastolic left ventricular performance using volume-time curves by real-time three-dimensional echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2003;16:29–37. [DOI] [PubMed] [Google Scholar]
- 37.Caudron J, Fares J, Bauer F, Dacher JN. Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics 2011;31:239–59. [DOI] [PubMed] [Google Scholar]
- 38.Gotte MJ, Germans T, Russel IK, et al. Myocardial strain and torsion quantified by cardiovascular magnetic resonance tissue tagging: studies in normal and impaired left ventricular function. Journal of the American College of Cardiology 2006;48:2002–11. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging 2008;1:366–76. [DOI] [PubMed] [Google Scholar]
- 40.Sinning D, Kasner M, Westermann D, Schulze K, Schultheiss HP, Tschope C. Increased left ventricular stiffness impairs exercise capacity in patients with heart failure symptoms despite normal left ventricular ejection fraction. Cardiol Res Pract 2011;2011:692862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elhabyan AK, Reyes BJ, Hallak O, et al. Subendocardial ischemia without coronary artery disease: is elevated left ventricular end diastolic pressure the culprit? Curr Med Res Opin 2004;20:773–7. [DOI] [PubMed] [Google Scholar]
- 42.Patel D, Robinson VJ, Arteaga RB, Thornton JW. Diastolic filling parameters derived from myocardial perfusion imaging can predict left ventricular end-diastolic pressure at subsequent cardiac catheterization. J Nucl Med 2008;49:746–51. [DOI] [PubMed] [Google Scholar]
- 43.de Marchi SF, Oswald P, Windecker S, Meier B, Seiler C. Reciprocal relationship between left ventricular filling pressure and the recruitable human coronary collateral circulation. Eur Heart J 2005;26:558–66. [DOI] [PubMed] [Google Scholar]
- 44.Sezer M, Umman B, Okcular I, Nisanci Y, Umman S. Relationship between microvascular resistance and perfusion in patients with reperfused acute myocardial infarction. Journal of interventional cardiology 2007;20:340–50. [DOI] [PubMed] [Google Scholar]
- 45.Kitabata H, Imanishi T, Kubo T, et al. Coronary microvascular resistance index immediately after primary percutaneous coronary intervention as a predictor of the transmural extent of infarction in patients with ST-segment elevation anterior acute myocardial infarction. JACC Cardiovasc Imaging 2009;2:263–72. [DOI] [PubMed] [Google Scholar]
- 46.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography 2009;22:107–33. [DOI] [PubMed] [Google Scholar]
- 47.Najjar SS. Heart failure with preserved ejection fraction failure to preserve, failure of reserve, and failure on the compliance curve. Journal of the American College of Cardiology 2009;54:419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. Journal of the American College of Cardiology 1997;30:1863–71. [DOI] [PubMed] [Google Scholar]
- 49.Shim CY, Park S, Choi D, et al. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. Journal of the American College of Cardiology 2011;57:1226–33. [DOI] [PubMed] [Google Scholar]
- 50.Garrido JM, Gerson MC, Hoit BD, Walsh RA. Load independence of early diastolic filling parameters in the anesthetized canine model. J Nucl Med 1993;34:1520–8. [PubMed] [Google Scholar]
- 51.Gardin JM, Arnold AM, Bild DE, et al. Left ventricular diastolic filling in the elderly: the cardiovascular health study. Am J Cardiol 1998;82:345–51. [DOI] [PubMed] [Google Scholar]
- 52.Masugata H, Senda S, Inukai M, et al. Differences in left ventricular diastolic dysfunction between eccentric and concentric left ventricular hypertrophy in hypertensive patients with preserved systolic function. The Journal of international medical research 2011;39:772–9. [DOI] [PubMed] [Google Scholar]