Abstract
A 79-year-old woman presented in cardiogenic shock with a flail bioprosthetic mitral valve leaflet and Staphylococcus aureus endocarditis. In the absence of other viable options, transfemoral valve-in-valve transcatheter mitral valve replacement was performed with a novel embolic protection device, resulting in trace mitral regurgitation and no neurologic complications. (Level of Difficulty: Advanced.)
Key Words: endocarditis, mitral valve, treatment, valve replacement
Abbreviations and Acronyms: EPD, embolic protection device; MVR, mitral valve replacement; MR, mitral regurgitation; TAVR, transcatheter aortic valve replacement; TMVR, transcatheter mitral valve replacement; VIV, valve in valve
Graphical abstract

A 79-year-old woman presented in cardiogenic shock with a flail bioprosthetic mitral valve leaflet and Staphylococcus aureus endocarditis…
History of Presentation
Thirteen years after mitral valve replacement (MVR) with a 33-mm Mosaic valve for severe degenerative mitral regurgitation (MR), a 79-year-old woman presented to a peripheral hospital in cardiogenic shock after a 1-day history of fever, sudden dyspnea, and a new pansystolic grade IV murmur loudest at the apex. Methicillin-sensitive Staphylococcus aureus grew from 1 blood culture, and she developed necrotic areas to left second and third toes, likely due to septic emboli. The patient was admitted to the intensive care unit for inotropic support and subsequently transferred to our center for further investigation and management.
Learning Objectives
-
•
In patients with bioprosthetic endocarditis and a high likelihood of suppression of infection with long-term antibiotic therapy, transcatheter VIV treatment may be a viable treatment option.
-
•
EPDs may be used in cases where embolization of valve tissue and vegetations is a concern.
Medical History
The patient underwent a 33-mm Mosaic bioprosthetic MVR for severe degenerative MR in 2006. She was diagnosed with multiple sclerosis in 2013 that mainly affected her mobility and required the use of a walking frame. She was also anticoagulated with dabigatran for permanent atrial fibrillation.
Differential Diagnosis
Given her history of a bioprosthetic MVR, new murmur, positive blood culture, and septic emboli, the main differential diagnosis was bioprosthetic valve infective endocarditis. Other differentials included bacteremia from another source, such as pneumonia.
Investigations
Transesophageal echocardiogram showed a flail mitral valve leaflet and severe MR (Figure 1) with a small vegetation (Figure 2). Cardiac computed tomography defined the neo– left ventricular outflow tract as having a diameter of 6 to 7 mm and an area of 2.8 cm2 (Figure 3). It also showed a hypoattenuating mass associated with the posterior leaflet, most likely a vegetation (Figure 4).
Figure 1.
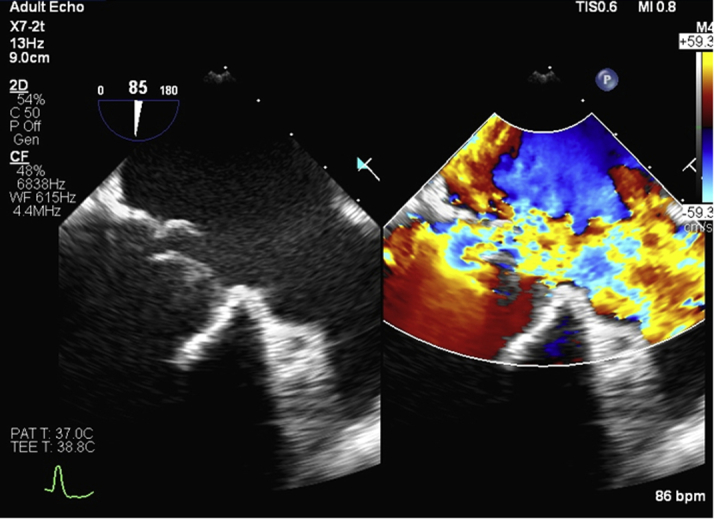
Pre-Procedure Transesophageal Echocardiogram
Flail bioprosthetic mitral valve leaflet with severe mitral regurgitation.
Figure 2.

Transesophageal Echocardiogram Showing Infective Endocarditis
Small mobile mass measuring ∼4 mm consistent with vegetation (arrow).
Figure 3.
Pre-Procedure Cardiac Computed Tomography
Cardiac computed tomography defined the neo–left ventricular outflow tract as a diameter of 6 to 7 mm and an area of 2.8 cm2.
Figure 4.
Vegetation Shown on Cardiac Computed Tomography
Cardiac computed tomography shows the vegetation (arrows) associated with the posterior bioprosthetic mitral valve leaflet.
Management
Because of the patient’s frailty related to multiple sclerosis and previous sternotomy, emergency surgical MVR was believed to be an extreme risk. In the absence of other viable options, the heart team recommended transfemoral valve-in-valve (VIV) transcatheter mitral valve replacement (TMVR) with an embolic protection device (EPD), followed by long-term suppressive oral antibiotic therapy.
Because of concern about embolization of the vegetation, an Emboliner (Emboline, Santa Cruz, California) EPD was deployed across the aortic arch through 10-F femoral access. Under general anesthesia and transesophageal echocardiogram guidance, through transfemoral venous access, following transseptal puncture and septostomy, a 29-mm Sapien 3 valve (Edwards Lifesciences, Irvine, California) was deployed in good position (Figure 5). The EPD was retrieved and captured significant debris, consistent with valve tissue (Figure 6). The debris was subsequently sent for analysis, but no infective material was identified.
Figure 5.

Valve Deployment
Post-deployment of 29-mm Sapien 3 valve in the 33-mm Mosaic mitral valve.
Figure 6.
Debris Captured by the Novel Cerebral Protection Device
A transthoracic echocardiogram performed the next day showed the TMVR in stable position, with a 5 mm Hg mean gradient and trace MR (Figure 7).
Figure 7.

Post-Procedure Transthoracic Echocardiogram
Post-procedure transthoracic echocardiogram showing the 29-mm Sapien valve in a stable position with a mean gradient of 5 mm Hg.
Discussion
Transfemoral trans-septal VIV TMVR has emerged as a viable therapeutic option for patients with severe bioprosthetic mitral dysfunction and high surgical risk (1, 2, 3, 4, 5). Although the presence of active infective endocarditis is generally considered a contraindication to VIV therapy, transcatheter aortic valve replacement (TAVR) has been described in a patient with active endocarditis and severe homograft aortic stenosis (6).
In this case, the patient’s blood cultures became sterile immediately after commencing antibiotics and the infectious diseases team believed there was a high probability for successful suppression with lifelong oral antibiotics.
Given the presence of a vegetation and previous septic embolism, the patient was at high risk of further septic embolization related to the procedure. This was mitigated by the use of a novel EPD, the Emboliner. The device was being evaluated in a TAVR early-feasibility trial, and a compassionate-use exemption was granted for this patient, as there were no commercially available alternatives.
The Emboliner has a circumferential design, made of dual-layer nitinol mesh, and is mounted on a 6-French catheter which allows guidewires and diagnostic catheters to be introduced through the Emboliner during the procedure (Figure 8). The Emboliner is initially housed in a delivery sheath and is positioned under fluoroscopic guidance from the ascending to proximal descending aorta. It is deployed by withdrawing the delivery sheath, allowing it to expand and provide full circumferential coverage of the aortic arch, protecting all the arch vessels, as well as the descending aorta. At the end of the procedure, the Emboliner is retrieved by withdrawing it back into the delivery sheath, with the captured debris, and the entire system is removed from the body.
Figure 8.

Emboliner (Emboline, Santa Cruz, California)
The filter of the Emboliner is deployed across the aortic arch to prevent embolic debris generated during the procedure from reaching the cerebral circulation and other vulnerable areas of the body.
Cerebral embolic protection devices may minimize the risk of periprocedural ischemic stroke during TAVR (7) and, although their use has been described for the prevention of stroke in transcatheter mitral procedures (8), we are not aware of EPD use to prevent embolization in VIV TMVR for mitral endocarditis.
Follow-Up
The patient made an uneventful recovery with no neurologic complications and was discharged home on the postoperative day 9. She completed 6 weeks of intravenous benzylpenicillin, and is now taking lifelong oral amoxicillin. Four months post-procedure she was asymptomatic and living independently.
Conclusions
In extreme-risk patients with bioprosthetic endocarditis and high likelihood of suppression of infection, transcatheter VIV treatment with embolic protection, followed by long-term antibiotic therapy, may be a viable treatment option.
Footnotes
Dr. Webster and the Department of Cardiology, Green Lane Cardiovascular Service, Auckland City Hospital, Auckland, New Zealand, have received research funding from Edwards Lifesciences and Emboline. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Putrino A., Scalia G., Natani S. Percutaneous transvenous mitral valve-in-valve implantation using commercially available transcatheter valve. First Australian experience. Heart Lung Circ. 2018;27:e42–e45. doi: 10.1016/j.hlc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Eleid M.F., Cabalka A.K., Williams M.R. Percutaneous transvenous transseptal transcatheter valve implantation in failed bioprosthetic mitral valves, ring annuloplasty, and severe mitral annular calcification. J Am Coll Cardiol Intv. 2016;9:1161–1174. [Google Scholar]
- 3.Attizzani G.F., Cheung Tam C., Markowitz A. Transcatheter mitral valve-in-ring implantation in prohibitive surgical risk patients: single center initial experience in the United States. Catheter Cardiovasc Interv. 2016;88:E233–E238. doi: 10.1002/ccd.26134. [DOI] [PubMed] [Google Scholar]
- 4.Descoutures F., Himbert D., Maisano F. Transcatheter valve-in-ring implantation after failure of surgical mitral repair. Eur J Cardiothorac Surg. 2013;44:e8–e15. doi: 10.1093/ejcts/ezt155. [DOI] [PubMed] [Google Scholar]
- 5.Latib A., Ruparelia N., Bijuklic K. First-in-man transcatheter mitral valve-in-ring implantation with a repositionable and retrievable aortic valve prosthesis. EuroIntervention. 2016;11:1148–1152. doi: 10.4244/EIJY15M11_02. [DOI] [PubMed] [Google Scholar]
- 6.Albu C., Swaans M.J., ten Berg J.M. With the back against the wall: TAVI in a patient with endocarditis. Cather Cardiovasc Interv. 2013;82:E595–E597. doi: 10.1002/ccd.24772. [DOI] [PubMed] [Google Scholar]
- 7.Demir O.M., Iannopollo G., Mangieri A. The role of cerebral embolic protection devices during transcatheter aortic valve replacement. Front Cardiovasc Med. 2018;5:150. doi: 10.3389/fcvm.2018.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagnesi M., Regazzoli D., Ancona M.B. Cerebral embolic risk during transcatheter mitral valve interventions: an unaddressed and unmet clinical need? J Am Coll Cardiol Intv. 2018;11:517–528. doi: 10.1016/j.jcin.2017.12.018. [DOI] [PubMed] [Google Scholar]





