Abstract
This case demonstrates the feasibility and procedural success of a novel supra-annular transcatheter mitral valve, the AltaValve via transapical approach in a patient with severe symptomatic mitral regurgitation who was a prohibitive surgical risk candidate. (Level of Difficulty: Advanced.)
Key Words: AltaValve, mitral regurgitation, supra-annular, transcatheter mitral valve therapy
Abbreviations and Acronyms: AS, aortic stenosis; CT, computed tomography; LV, left ventricular; LVOT, left ventricular outflow tract; TEE, transesophageal echocardiography; TMVT, transcatheter mitral valve therapy
Graphical abstract

This case demonstrates the feasibility and procedural success of a novel supra-annular transcatheter mitral valve, the AltaValve via transapical…
Transcatheter mitral valve therapy (TMVT) represents the next frontier in the burgeoning field of structural heart interventions. A well-recognized limitation of transcatheter mitral valve replacement is the potential for left ventricular (LV) outflow tract (LVOT) obstruction after placement of the new prosthetic valve. The AltaValve (4C Medical Technologies, Minneapolis, Minnesota) is a novel supra-annular TMVT system. The AltaValve consists of a spherical nitinol frame that is sized to fit the left atrium in a compliant fashion. The frame contains a 27-mm trileaflet bovine pericardium valve and a fabric skirt on the bottom at the level of the annular ring to prevent paravalvular regurgitation and enhance tissue growth. There will eventually be 2 delivery systems: the current system is implanted through a transapical approach using a 32-F delivery system, and is repositionable and partially retrievable; a transseptal system is currently in development. The first-in-human experience has been reported recently (1). The authors report the first experience in the United States with the AltaValve System, using 3-dimensional transesophageal echocardiography (TEE) imaging guidance and fusion with live fluoroscopy.
Learning Objective
-
•
The AltaValve is a novel transcatheter mitral valve therapy that can be implanted via left ventricular apical approach in a supra-annular fashion with low risk for left ventricular outflow tract obstruction and warrants further study.
History of Presentation
An 89-year-old active and functional man with history of prior percutaneous coronary interventions, coronary artery bypass grafting surgery, paroxysmal atrial fibrillation, and chronic kidney disease presented with worsening symptoms of dyspnea on exertion (New York Heart Association [NYHA] functional class III) in addition to 2 hospitalizations for decompensated congestive heart failure within the past 2 months.
Investigations
Echocardiography demonstrated severe mitral regurgitation with calcification of the posterior leaflet and the posterior mitral annulus (Figures 1A and 1B). His STS-PROM (Society of Thoracic Surgery Predicted Risk of Mortality) score was 11.25%, and he was deemed to be a prohibitive surgical risk candidate. Furthermore, his mitral valve anatomy was not suitable for transcatheter edge-to-edge repair with MitraClip (Abbott Vascular, Abbott Park, Illinois) due to the calcification of the posterior leaflet and annulus. Off-label use of commercially available transcatheter aortic valves was not an option due to lack of annular calcium other than the focal area of the posterior annulus.
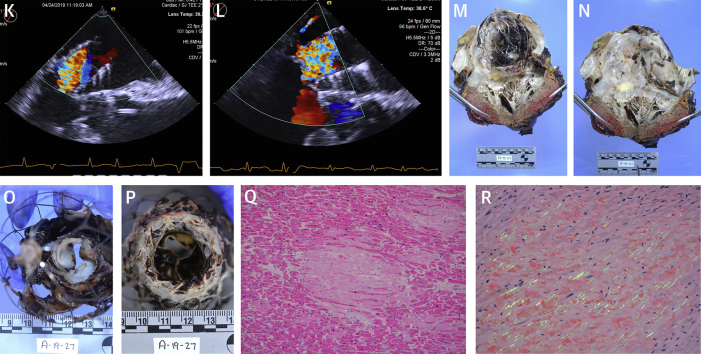
Figure 1.
Pre-, Intra-, and Post-Procedural Imaging
(A) Pre-procedural transthoracic echocardiography demonstrating severe mitral regurgitation. (B) Pre-procedural transesophageal echocardiography demonstrating severe mitral regurgitation. (C) AltaValve. (D) AltaValve leaflets. (E, F, G) CT images demonstrating mitral annulus. (H, I) Fluoroscopy images of valve deployment with TrueFusion guidance; the 4 markers with ring depict the mitral annular plane identified by transesophageal echocardiography. (J, K, L) Post AltaValve deployment transesophageal echocardiography images demonstrating supra-annular position, no valvular regurgitation, and mild posterior paravalvular regurgitation (red arrow). (M, N, O, P) Post-mortem images demonstrating accurate supra annular position of the AltaValve with good apposition (imprint [red arrow]) and intact leaflets (yellow arrows). (Q, R) Histopathological images demonstrating amyloidosis appearing as light-pink hyaline extracellular deposits (black arrow) displacing cardiac myocytes (yellow arrow) and apple-green birefringence under polarized light (white arrow) on Congo red–stained sections.
Management
The patient was not willing to travel to a center where he could potentially be considered for enrollment in ongoing studies evaluating other dedicated transcatheter mitral valve replacement devices such as Intrepid (Medtronic, Redwood City, California), Tendyne (Abbott Structural, Santa Clara, California) or CardiaQ (Edwards Lifesciences, Irvine, California). After discussion with the multidisciplinary heart team, it was decided to proceed with TMVT with the AltaValve system (Figures 1C and 1D) after obtaining institutional review board approval. On the basis of computed tomography (CT) measurements, an AltaValve with a 75-mm frame height, 70-mm frame width, and 46-mm annular ring was selected and manufactured. CT images of the mitral annulus are shown in Figures 1E to 1G. With these measurements, the implant would be oversized in the left atrial height, width, intercommissural dimension, and anteroposterior dimension by 15%, 18%, 10%, and 10%, respectively.
The procedure was performed in the hybrid catheterization laboratory with the patient under general anesthesia. The location of the apical puncture was guided by CT imaging to allow coaxial alignment of the delivery system with the mitral valve. After exposure of the LV apex and placement of purse-string sutures, the LV apex was punctured. The mitral valve was crossed with a balloon-tipped catheter to avoid entanglement with the chordal apparatus, and the large caliber delivery sheath was then inserted. The mitral annulus was identified by 3-dimensional TEE, and the image was fused with live fluoroscopy (TrueFusion; Siemens Healthineers, Erlangen, Germany) (Figures 1H and 1I). Under TEE guidance, the delivery system was then advanced up to the roof of the left atrium, which was identified by contrast angiography through the system’s side port. The valve frame was released by retrieval of the delivery system using 3-dimensional TEE and fluoroscopy with fusion imaging guidance to keep the delivery system and the frame supra-annular. The time from puncture of the LV apex to deployment of the valve frame was 26 min. During this time, there was significant bleeding at the LV apex due to poor tissue quality, requiring constant suction as well as vasopressor support to maintain hemodynamic stability. Initially, the TEE showed at least moderate posterior paravalvular mitral regurgitation. The valve position was adjusted by gently “pulling” ventricular using the 3 anchors in the delivery system; and after seating the valve in the annulus, the TEE showed mild posterior paravalvular regurgitation (Figures 1J to 1L). There was no LVOT obstruction, and the prosthetic leaflets appeared to be functioning well without any valvular regurgitation. The mean gradient across the TMVT was 1 mm Hg. The valve was then released, and the LV apex purse string suture tied in usual fashion with good hemostasis. The patient was transiently supported with intravenous vasopressor infusion post-procedure. On post-operative day 2, vasopressors were weaned off. Follow-up transthoracic echocardiogram showed persistent mild posterior paravalvular mitral regurgitation and normal prosthetic mitral leaflet function. On post-operative day 4, the patient was weaned off the ventilator and extubated. Low-dose inotrope support was maintained. On post-operative day 5, the patient developed a severe coughing episode resulting in bradycardia followed by pulseless electrical activity cardiac arrest. Cardiopulmonary resuscitation was initiated promptly; however, the patient died despite aggressive efforts. With the family’s consent, a limited autopsy was performed that showed that the AltaValve was well positioned in the supra-annular position in the left atrium abutting the native mitral valve, with “imprint” of the valve frame on the left atrial tissue implying good apposition and expansion (Figures 1M to 1P). The valve leaflets were intact and appeared normal. The LV apex and surrounding tissue did not reveal any active bleeding. The heart weighed 680 g, and histological examination revealed widespread myocardial amyloid deposition in a nodular and pericellular pattern (Figures 1Q and 1R) with characteristic apple-green birefringence under polarized light on Congo red–stained sections.
Discussion
The present report describes the feasibility and procedural success of TMVT with a novel supra-annular AltaValve system with no LVOT obstruction. The patient was a very elderly, prohibitive surgical risk candidate with significant comorbidities who had significant LV apical bleeding during the procedure, and unfortunately died on post-operative day 5 despite successful implantation. Histopathology revealed evidence of cardiac amyloidosis. There were no clinical markers pre-procedure to suggest or raise suspicion for amyloidosis. Cardiac amyloidosis has been detected in 6% of patients undergoing surgical aortic valve replacement for severe aortic stenosis (AS) and is associated with worse outcomes (2). Recent data have shown that the prevalence of amyloid transthyretin cardiac amyloidosis in patients with AS undergoing transcatheter aortic valve replacement is 16% as determined prospectively by technetium-99m pyrophosphate cardiac scintigraphy (3). In addition, such patients with evidence of amyloid transthyretin cardiac amyloidosis had low-flow low-gradient AS with reduced LV ejection fraction, severe diastolic dysfunction, and reduced myocardial systolic mechanics as determined by echocardiography. It is possible that cardiac amyloidosis contributed to the poor tissue quality, increased bleeding, and overall poor short-term outcome in our patient.
Conclusions
The AltaValve is a unique and promising new TMVT system. Due to its supra-annular position, it has the potential benefit of reducing the incidence of post-implantation LVOT obstruction. The current iteration is limited to a transapical system, although a transseptal delivery system is currently being developed. This technology will be further evaluated during an early feasibility study that will begin enrollment later this year.
Footnotes
Dr. Goel has served on the Speakers Bureau for Abbott Structural Heart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Konstantinos Charitakis, MD, served as Guest Associate Editor for this article.
Informed consent was obtained for this case.
References
- 1.Nunes Ferreira-Neto A., Dagenais F., Bernier M., Dumont E., Freitas-Ferraz A.B., Rodes-Cabau J. Transcatheter mitral valve replacement with a new supra-annular valve: first-in-human experience with the AltaValve system. J Am Coll Cardiol Intv. 2019;12:208–209. doi: 10.1016/j.jcin.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Treibel T.A., Fontana M., Gilbertson J.A. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 3.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]