Abstract
Shockwave intravascular lithotripsy for the treatment of peripheral arterial disease is routinely performed via femoral artery access. In patients with complex femoropopliteal disease, this traditional approach is not feasible. To address this situation, we used an exclusive pedal access approach along with other adjunctive treatments, resulting in a successful outcome. (Level of Difficulty: Advanced.)
Key Words: claudication, peripheral vascular disease, treatment
Abbreviations and Acronyms: DP, dorsalis pedis; IVL, intravascular lithotripsy; IVUS, intravascular ultrasound; PAD, peripheral arterial disease; SFA, superficial femoral artery
Graphical abstract

Shockwave intravascular lithotripsy for the treatment of peripheral arterial disease is routinely performed via femoral artery access. In patients…
Shockwave intravascular lithotripsy (IVL) (Shockwave Medical, Inc., Santa Clara, California) for peripheral arterial disease (PAD) has shown promise in the last few years. Traditional access is usually via the femoral artery. An exclusive pedal access approach for Shockwave IVL, to the best of the authors knowledge, has not been previously described in the literature.
Learning Objectives
-
•
To exemplify the alternative pedal access for treating patients with complicated femoropopliteal PAD.
-
•
To use adjunctive peripheral interventional therapies, including IVL (Shockwave IVL) and atherectomy via pedal only access.
History of Presentation
A 66-year-old white man presented with progressively worsening, severe bilateral calf pain significantly affecting his activities of daily living (Rutherford category 3 claudication). Physical examination revealed no palpable pulses on the left popliteal, dorsalis pedis (DP), posterior tibial, right DP, and posterior tibial (grade 0); however, they were monophasic per Doppler analysis. The patient’s creatinine level at the initial visit was 1.8 mg/dl, and his glomerular filtration rate was 46 ml/min/1.73 m2.
Medical History
The patient had a history of hypertension, chronic kidney disease stage 3, diabetes mellitus, and known PAD with multiple previous endovascular interventions. These interventions included several prior iliac and superficial femoral endovascular interventions with numerous balloon angioplasties and stenting along with a previous surgical femoral–femoral bypass for a chronically occluded right iliac arterial system.
Investigations
In an attempt to limit contrast exposure and yield a definitive anatomical assessment, an invasive diagnostic-only abdominal aortography with bilateral lower-extremity runoff was performed by using the left radial artery approach. Prior noninvasive imaging was deferred given the patient’s severe symptoms and high pre-test probability for significant lower-extremity PAD. The initial angiogram revealed occluded right iliac arteries, patent left iliac with moderate to severe disease (Figure 1A), patent right superficial femoral artery (SFA) and prior femoral-femoral bypass (Figure 1B), and severe heavily calcified SFA disease bilaterally (Figure 1C). Severe PAD below the knee was also noted (Figure 1D) with the exception of a single vessel runoff via bilateral patent anterior tibial arteries. One week after observation of stable kidney function, the patient underwent a series of staged bilateral peripheral interventions for his severe SFA disease.
Figure 1.
Pre-Intervention Images of the Right and Left Lower Extremities
(A) Pre-intervention image of the left external iliac artery. (B) Pre-intervention image of the aortoiliac femorofemoral bypass. (C) Pre-intervention image of the left superficial femoral artery image. (D) Pre-intervention of the right and left popliteal images.
Management
Using ultrasound guidance, a 6-F, 10-cm Slender sheath (Terumo, Elkton, Maryland) was inserted into the right DP. Through the sheath, intravenous heparin was used for anticoagulation during the procedure, and intra-arterial nicardipine and nitroglycerin were used to prevent vasospasm. A 2.6-F CXI 150-cm straight microcatheter (Cook, Bloomington, Indiana) and a ViperWire (Cardiovascular Systems, Inc., St. Paul, Minnesota) were used to cross the 80% diffuse, severely calcified right SFA stenosis and a 70% mid to proximal right popliteal stenosis. Intravascular ultrasound (IVUS) was then used with a 0.014 PV Visions catheter (Philips, Rancho Cordova, California), which established a proximal and distal reference segment in the right SFA and popliteal artery of 6.0 mm while confirming severe circumferential calcium diffusely throughout the vessel. Orbital atherectomy with a Diamondback 2.0 mm solid crown (Cardiovascular Systems, Inc.) was performed with a single pass on low speed in the popliteal artery and multiple passes on low, medium, and high speeds throughout the distal right SFA.
IVL and balloon angioplasty were then performed with a Shockwave balloon 6 × 60 mm (Shockwave Medical Inc.) with treatment of 180 pulses throughout the distal right SFA and the right popliteal artery (6 rounds of 30 pulses each). A drug-coated balloon angioplasty was then performed with overlapping 6 × 120 mm and 6 × 100 mm Stellarex drug-coated balloons (Philips, Fremont, California) from the distal right SFA into the right popliteal artery. IVUS exhibited a markedly improved lumen gain with <30% residual stenosis in the SFA (Figure 2A) and in the popliteal arteries on the right that correlated with improvement in functional status.
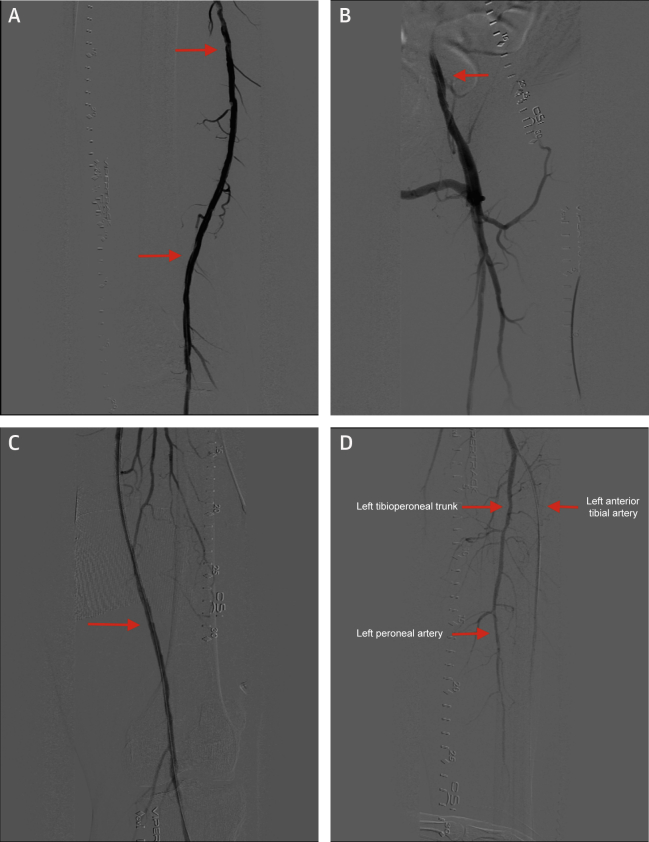
Figure 2.
Post-Intervention Images of the Right and Left Lower Extremities
(A) Post-intervention image of the right superficial femoral artery. (B) Post-intervention image of the left external iliac artery. (C) Post-intervention image of the left superficial femoral artery. (D) Post-intervention image of the left anterior tibial artery, left tibio-peroneal trunk and left peroneal artery.
A few weeks later, the patient was treated similarly for the left SFA and popliteal artery disease via left DP access exclusively. Again, orbital atherectomy was used, this time with a Diamondback solid 1.5 mm crown with 1 pass on low speed and 2 passes on medium speed in the distal left SFA into the popliteal artery; this was followed by more proximal treatments with multiple passes on low, medium, and high speeds in the proximal SFA. The previously stented segments were avoided for orbital atherectomy. Shockwave IVL was performed again with two 5.5 × 60 mm Shockwave balloons for a total of 180 pulses for each balloon in the proximal left SFA as well as the distal left SFA into the left popliteal artery. Pacific Plus (Medtronic, Minneapolis, Minnesota) and Angiosculpt balloons (Philips, Fremont, California) were then used for 2 inflations in the previous in-stent segment.
The proximal to mid-left SFA disease was treated with a IN.PACT Admiral 6 × 250 mm drug-coated balloon (Medtronic, Minneapolis, Minnesota), and the disease extending from the left SFA into the left popliteal artery was treated with a Stellarex 6.0 × 120 mm balloon. A 7 × 22 mm iCAST covered balloon-expandable stent (Getinge, Merrimack, New Hampshire) was then deployed at the distal stent margin of the prior existing stent in the left external iliac artery given the distal edge in-stent restenosis and calcified vasculature, which had a >25 mm Hg gradient across the iliac stenosis (Figure 2B). As a result, there was <20% residual stenosis and a marked improvement in flow (Figures 2C and 2D) with at least 2-vessel runoff below the left knee. A total of 10 ml of Isovue-250 contrast was used for the first procedure, and 50 ml was used for the second intervention. IVUS was used as a guide for lesion characterization and optimal device sizing of the vessel, which also greatly assisted in limiting the overall contrast used. Patent hemostasis was achieved each time with VascBands XXL (Teleflex, Morrisville, North Carolina) only. No complications were observed after the 2 procedures, and the patient was eventually discharged on the same day each time with aspirin and clopidogrel.
Discussion
DISRUPT PAD (Shockwave Medical Peripheral Lithoplasty System Study for PAD) I and II studies (1,2) have reported the efficacy of IVL in treating PAD, predominantly via the femoral access approach. In patients with complicated PAD, this approach may not always be feasible, however. Previous studies have shown the successful use of atherectomy via the tibio-pedal approach (3), although there are no reported cases of using Shockwave IVL with pedal access. Based on previous literature that assessed successful outcomes with the pedal-first approach (4,5) for intervening on the femoropopliteal PAD, the authors report the utility of exclusive pedal access with the novelty of using Shockwave IVL in conjunction with atherectomy and other adjunctive treatment modalities.
Follow-Up
The patient was followed up as an outpatient in the weeks immediately after the procedures and >3 months beyond. His claudication symptoms completely resolved and remained so all while his renal function remained stable.
Conclusions
The end results revealed significantly improved angiographic outcomes that translated into marked clinical improvement. This case using Shockwave IVL with the primary pedal access approach reported clinical efficacy along with safety in terms of avoidance of potential renal and vascular access complications. In patients with complex femoral popliteal disease, which precludes the femoral access option for Shockwave IVL, using pedal arteries to intervene may be reasonable and efficacious.
Footnotes
Both authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
References
- 1.Brodmann M., Werner M., Britton T. Safety and performance of lithoplasty for treatment of calcified peripheral artery lesions. J Am Coll Cardiol. 2017;70:908–909. doi: 10.1016/j.jacc.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 2.Brodmann M., Werner M., Holden A. Primary outcomes and mechanism of action of intravascular lithotripsy in calcified, femoropopliteal lesions: results of Disrupt PAD II. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2019;93:335–342. doi: 10.1002/ccd.27943. [DOI] [PubMed] [Google Scholar]
- 3.Mustapha J.A., Saab F., McGoff T. Tibio-pedal arterial minimally invasive retrograde revascularization in patients with advanced peripheral vascular disease: the TAMI technique, original case series. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2014;83:987–994. doi: 10.1002/ccd.25227. [DOI] [PubMed] [Google Scholar]
- 4.Sanghvi K.A., Kusick J., Krathen C. Retrograde tibio-pedal access for revascularization of lower-extremity peripheral artery disease using a 6 Fr Slender Sheath: the “Pedal-First” Pilot project. J Invasive Cardiol. 2018;30:334–340. [PubMed] [Google Scholar]
- 5.El-Sayed H., Bennett M.E., Loh T.M., Davies M.G. Retrograde pedal access and endovascular revascularization: a safe and effective technique for high-risk patients with complex tibial vessel disease. Ann Vasc Surg. 2016;31:91–98. doi: 10.1016/j.avsg.2015.09.015. [DOI] [PubMed] [Google Scholar]