There is a lot of interest in a safe, nonexpensive drug that prevents bleeding or stops ongoing bleeds in patients without underlying hemostatic disorders. Especially since the positive results from CRASH-2 trial in trauma patients with extracranial bleeding in 20101 and the WOMAN trial in postpartum hemorrhage in 2017,2 there is increasing interest in tranexamic acid (TXA) to fulfill this role. The subsequent CRASH-3 trial in patients with traumatic brain injury (TBI) in 2019 demonstrated no mortality benefit in acute trauma patients (18.5% versus 19.8%, P = 0.94).3 In the past few months, several randomized clinical trials have been published in high ranked journals on the effects of TXA in several circumstances (Figure 1). Their results are univocally disappointing.
Figure 1.
Recent randomized clinical trials with TXA as a hemostastic agent. TXA = tranexamic acid.
Recent trials in trauma and surgery
Acute gastrointestinal bleeding (HALT-IT; Lancet 2020)
This was a prospective, double-blind, randomized, placebo-controlled trial, where 11,952 patients with upper or lower gastrointestinal bleeding and at risk of bleeding to death were enrolled.4 Patients received either a loading dose of 1 g TXA, followed by a maintenance dose of 3 g TXA for 24 hours or placebo. The primary outcome was death due to bleeding within 5 days. There was no difference on the rate of death between patients treated with TXA or placebo: 4% versus 4%, respectively (risk ratio [RR] 0.99, 95% confidence interval [CI] 0.82–1.18). Instead, venous thromboembolic events were higher in the TXA group than in the placebo group (0.8% versus 0.4%; RR 1.85; 95% CI 1.15–2.98), although still infrequent.
Traumatic brain injury (prehospital TXA for TBI trial; JAMA 2020)
This was a prospective, double-blind, randomized, placebo-controlled trial, where 966 patients with moderate or severe TBI who were not in shock were treated with out-of-hospital TXA (1 or 2 g) or placebo within 2 hours of injury.5 The primary outcome was functional neurologic outcome measured 6 months after injury using the Glasgow Outcome Scale-Extended score. There was no difference in favorable Glasgow Outcome Scale-Extended score (>4) between the combined TXA group and the placebo group (65% versus 62%; P = 0.16). Mortality rates were 14% versus 17%, respectively (P = 0.26). This trial did therefore not show a benefit of TXA in TBI.
Trauma (STAAMP; JAMA Surg 2020)
This was a double-blind, placebo-controlled, randomized clinical trial that compared outcomes in 927 trauma patients at risk for hemorrhage receiving 1 g TXA before hospitalization administered during air medical or ground transport.6 The primary outcome for the trial was 30-day mortality. It was 8% for TXA and 10% for placebo (P = 0.17). When comparing TXA dosing regimens in patients, mortality rates were 10.0% for placebo, 9.3% for 1 bolus, 7.8% for 2, and 7.3% for 3 bolus TXA groups. Among the prespecified comparisons of each TXA regimen to placebo, the repeat bolus regimen had lower 30-day mortality after adjusting for site (7.3% versus 10.0%; difference, P = 0.04). Therefore, this trial did not show an overall benefit for TXA, but an effect was seen in patients with repeated dosages.
Intracranial hemorrhage (STOP-AUST; Lancet Neurol 2020)
This was a prospective, double-blind, randomized, placebo-controlled trial, where 100 patients with a nontraumatic intracerebral hemorrhage (ICH) were randomized within 4.5 hours of symptom onset.7 The intervention consisted of infusion of intravenous TXA 1 g in 100 mL 0·9% NaCl over 10 minutes followed by 1 g in 500 mL 0·9% NaCl infusion over 8 hours or placebo with the same administration schedule. The primary outcome measure was the presence of ICH growth by 24 hours after the start of study drug administration. It was 52% in patients on placebo and 42% in patients treated with TXA (P = 0.41). Mortality rates were 16% versus 26%, respectively (P = 0.19). This trial did therefore not show a benefit of TXA in ICH.
Subarachnoidal hemorrhage (ULTRA; Lancet 2021)
This was a prospective, randomized, controlled, open-label trial where 955 patients with spontaneous CT-proven subarachnoid hemorrhage (SAH) randomized to receive care as usual or TXA (immediately after diagnosis; 1 g bolus, followed by continuous infusion of 1 g every 8 h for 24 h).8 The primary endpoint was clinical outcome at 6 months, assessed by the modified Rankin Scale, dichotomized into a good or poor clinical outcome. Good clinical outcome was observed in 60% in the TXA group and 64% in the control group (odds ratio 0.86, 95% CI 0.66–1.12). This trial did therefore not show a benefit of TXA in SAH.
Cesarean section (TRAAP2; NEJM 2021)
This was a prospective, double-blind, randomized, placebo-controlled trial, where 4153 patients that underwent a cesarean section were recruited.9 Women were randomly assigned to receive a uterotonic agent plus either 1 g TXA or placebo immediately after delivery. The primary outcome was postpartum hemorrhage, defined as a calculated estimated blood loss greater than 1000 ml or receipt of a red-cell transfusion within 2 days after delivery. It was 31.6% in the placebo group and 26.7% in the TXA group (P = 0.003). This difference was primarily driven by less blood loss; there was no difference in red blood cell transfusion. Although the primary endpoint was better for TXA, TXA did not result in lower incidences of clinical secondary outcomes related to blood loss than placebo. The overall calculated blood loss was 680 versus 787 mL in favor of TXA (P < 0.001). Therefore, although a significant outcome was achieved, the real clinical benefit of TXA is questionable.
Why TXA is failing?
After the initial encouraging results of the CRASH-2 and WOMAN trials, TXA was postulated to be a safe and effective hemostatic agent in clinical situations in which hyperfibrinolysis was implicated. However, subsequent randomized clinical trials universally were negative, which is reminiscent of the negative trials of recombinant factor VIIa that was proposed as a “universal hemostatic agent” in the early 2000s.10
As TXA was effective in large RCTs in trauma and postpartum bleeding, and is widely used with the aim to reduce bleeding during cardiac surgery and liver transplantation, efficacy in other clinical conditions was perhaps rightfully expected. What could be reasons for failure of TXA in the above-mentioned trials?
Bleeding type
TXA will only be effective in treating bleeding related to hemostatic failure. Although TXA is often proposed to be used in patients with a hyperfibrinolytic state, its efficacy in patients without overt hyperfibrinolysis (eg, patients with von Willebrand disease) suggests that TXA also provides efficient clot protection in the absence of excessive fibrinolytic activity. TXA, however, will likely be ineffective in preventing or treating bleeding that is not a direct consequence of hemostatic failure. For example, variceal bleeding is a consequence of portal hypertension and local vascular abnormalities.11 Variceal bleeding does not appear to have a hemostatic component as severity and outcome of the bleed was independent of the use of anticoagulant drugs at the time of the bleed. Indeed, the treatment of variceal bleeding is directed at reducing portal pressure in combination with local (endoscopic) measures, with no added values of TXA or other prohemostatic interventions such as recombinant factor VIIa.3,12 TXA will also be ineffective in surgical bleeds, in which mechanical injury to larger vessels requires suturing or cauterization.
Dosing
TXA dosing and dosing duration are unclear. Analyses of over 40,000 patients included in randomized trials indicated that TXA is most effective when given as early as possible after onset of bleeding.13 TXA is no longer beneficial when given >3 hours after the onset of bleeding. Although the reasons for this striking time-dependent effect of TXA are poorly understood, these data clearly identify that timing of TXA administration is crucial. These data might explain why TXA failed in the HALT-IT trial, in which patients received TXA hours after the onset of variceal bleeding. Following intravenous administration, the apparent elimination half-life is approximately 2 hours and the mean terminal half-life is approximately 11 hours. Based on in vivo and in vitro data, the effective therapeutic plasma concentration of TXA for inhibiting fibrinolysis has been reported to be around 10 mg/L.14 Intravenous administration of a single 1 g dose achieved plasma concentrations ≥10 mg/L for up to 5–6 hours.15 Dosing regimens have varied across studies and plasma TXA concentrations have not been systematically verified in the various trials. The relatively short TXA half-life and a possible dilution effect in severely bleeding patients that may receive generous amounts of fluids and blood products provides uncertainty on the plasma concentrations achieved.
Overpowering the message
The CRASH2 and WOMAN trial results may be less spectacular than communicated. They have been communicated as game changing studies that proved a cheap drug could reduce death due to bleeding. However, both studies have been criticized. The CRASH2 study, for example, only included 5% of patients that died due to bleeding, and the effect size of TXA for bleeding reduction was small. Importantly, TXA did not reduce blood transfusion requirements in this study. In addition, the study has been criticized for its approach to randomization in which the treating physician needed to be “substantially uncertain” as to whether to use an antifibrinolytic in the trauma patient. The WOMAN trial has been criticized because the primary endpoint (a composite of mortality and hysterectomy) was negative and for a modest effect size.
Paradoxal effects of TXA
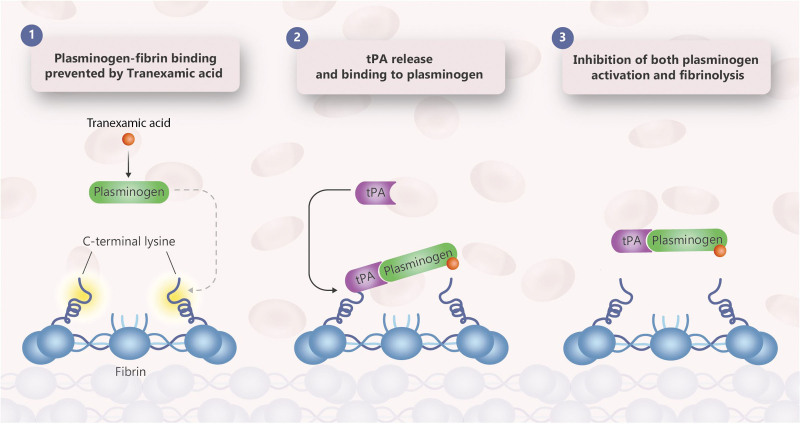
In Figure 1, a simplified overview is given on the mechanism of fibrinolysis. Plasminogen binds to fibrin C-terminal lysine residues. Subsequently, it is activated by tissue-plasminogen activator (t-PA) to form plasmin. In turn, plasmin cleaves fibrin, inducing new C-terminal lysine binding sites.
The antifibrinolytic effects of TXA are mediated by reversible interactions at multiple binding sites within plasminogen. Native human plasminogen contains 4–5 lysine binding sites with low affinity for TXA and 1 with high affinity. The high affinity lysine site of plasminogen is involved in its binding to fibrin. Saturation of the high affinity binding site with TXA displaces plasminogen from the surface of fibrin and inhibits plasmin formation (Figure 2). Although plasmin may be formed by conformational changes in plasminogen, binding to and dissolution of the fibrin matrix is inhibited. In contrast to direct plasmin inhibitors such as aprotinin, TXA blocks binding of plasmin to fibrin, but not plasmin itself. In addition, TXA may paradoxically activate fibrinolysis. In vitro experiments have demonstrated that TXA under certain experimental conditions can stimulate rather than inhibit plasmin formation, specifically by stimulating uPA activity.16 In addition, TXA delays plasmin inhibition by antiplasmin. In a study in humans receiving TXA, a paradoxical increase in plasmin-antiplasmin (PAP) complexes has been observed, which may align with these in vitro findings.17 An alternative explanation for increases in PAP complexes in TXA treated patients is an effect of TXA on clearance of PAP complexes. It has been proposed that combining TXA with a direct plasmin inhibitor such as aprotinin might be beneficial.
Figure 2.
Schematic simplified overview of fibrinolysis. tPA = tissue-plasminogen activator.
Conclusion
TXA as a universal hemostatic agent has no clinical significant benefit in recent randomised trials in patients with cerebral and subarachnoid hemorrhage, gastrointestinal bleeds, trauma or cesarean section. There may be clinical situations in which TXA has a more pronounced clinical benefit, especially in conditions of hyperfibrinolysis.
Figure 3.
Mode of action of TXA. TXA = tranexamic acid.
References
- 1.Shakur H, Roberts I, Bautista R, et al. ; CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 2.WOMAN trial collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CRASH-3 trial collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394:1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HALT-IT Trial Collaborators. Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial. Lancet. 2020;395:1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowell SE, Meier EN, McKnight B, et al. Effect of out-of-hospital tranexamic Acid vs Placebo on 6-month functional neurologic outcomes in patients with moderate or severe traumatic brain injury. JAMA. 2020;324:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyette FX, Brown JB, Zenati MS, et al. Tranexamic acid during prehospital transport in patients at risk for hemorrhage after injury: a double-blind, placebo-controlled, randomized clinical trial. JAMA. 2020;156:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meretoja A, Yassi N, Wu TY, et al. Tranexamic acid in patients with intracerebral haemorrhage (STOP-AUST): a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2020;19:980–987. [DOI] [PubMed] [Google Scholar]
- 8.Post R, Germans MR, Tjerkstra MA, et al. ; ULTRA Investigators. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021;397:112–118. [DOI] [PubMed] [Google Scholar]
- 9.Sentilhes L, Sénat MV, Le Lous M, et al. ; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic acid for the prevention of blood loss after cesarean delivery. N Engl J Med. 2021;384:1623–1634. [DOI] [PubMed] [Google Scholar]
- 10.Hedner U. NovoSeven as a universal haemostatic agent. Blood Coagul Fibrinolysis. 2000;11(suppl 1):S107–S111. [DOI] [PubMed] [Google Scholar]
- 11.Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366–413. [DOI] [PubMed] [Google Scholar]
- 12.Bosch J, Thabut D, Bendtsen F, et al. ; European Study Group on rFVIIa in UGI Haemorrhage. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. [DOI] [PubMed] [Google Scholar]
- 13.Gayet-Ageron A, Prieto-Merino D, Ker K, et al. ; Antifibrinolytic Trials Collaboration. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40 138 bleeding patients. Lancet. 2018;391:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Picetti R, Shakur-Still H, Medcalf RL, et al. What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis. 2019;30:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormack PL. Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs. 2012;72:585–617. [DOI] [PubMed] [Google Scholar]
- 16.Longstaff C, Locke M. Increased urokinase and consumption of α2 -antiplasmin as an explanation for the loss of benefit of tranexamic acid after treatment delay. J Thromb Haemost. 2019;17:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draxler DF, Zahra S, Goncalves I, et al. Tranexamic acid rapidly inhibits fibrinolysis, yet transiently enhances plasmin generation in vivo. Blood Coagul Fibrinolysis. 2021;32:172–179. [DOI] [PubMed] [Google Scholar]