Abstract
Background.
Early allograft dysfunction (EAD) after liver transplantation has been associated with long-term reduced graft and patient survival.
Methods.
In this single-center cohort study, we aimed to compare incidence, risk factors, and outcomes in liver transplant recipients who developed EAD. Patients who received donation after circulatory death (DCD) or donation after brain death (DBD) grafts between January 2007 and December 2017 were included. EAD was defined as bilirubin of ≥10 mg/dL (171 μmol/L) or an international normalized ratio of ≥1.6 on postoperative day 7 or transaminases >2000 U\L in the first-week posttransplantation as previously described.
Results.
In our cohort of 1068 patients, incidence of EAD was 44%. EAD occurred more frequently in the DCD versus DBD group (71% versus 41%, P < 0.01). Overall, recipients who developed EAD showed a significantly lower graft and patient survival at 1, 3, and 5 y after transplantation (all P < 0.05). This was also the case for recipients of DBD grafts. However, for recipients of DCD grafts, patient and graft survival were not affected by the presence of EAD. For recipients of DBD grafts, donor age, body mass index (BMI) and gender, recipient BMI and model for end-stage liver disease score and warm and cold ischemia time were associated with EAD. For DCD recipients, donor BMI and cold ischemia time were associated with EAD.
Conclusions.
In our cohort study, EAD resulted in reduced long-term patient and graft survival only for DBD recipients but not for DCD recipients. Predictive markers for EAD were dependent on the donor type.
INTRODUCTION
Liver transplantation (LT) is the standard of care for patients with acute and end-stage liver disease and hepatocellular carcinoma (HCC). The success of LT has resulted in a dramatic increase of organ shortage with a considerable mortality on the waiting list. To overcome the imbalance between organ demand and availability, over the last years, more and more extended donor criteria grafts have been utilized. This has decreased the mortality on the waiting list,1 but it has also lead to an increased number of complications, such as graft dysfunction and failure.2 One major concern after LT is early allograft dysfunction (EAD), which is believed to impact long-term patient and graft survival.3,4 Several criteria have been proposed for the definition of EAD, but at the moment, it is still not fully understood which of them plays an essential role. One of the most accepted definitions was proposed by Olthoff and colleagues in 2010 and relies on 1 of the 3 criteria: total serum bilirubin ≥ 10 mg/dL on postoperative day (POD) 7, an international normalized ratio (INR) ≥ 1.6 on POD 7, an alanine (ALT) or aspartate (AST) aminotransferase >2000 U/L within the first 7 POD,5 and a few studies have validated these criteria.6-9
It remains unclear if risk factors for developing EAD depend on the donor type. In addition, the impact of EAD on the outcomes of LT with different donor types is not well defined. Therefore, we analyzed EAD risk factors and impact on the outcomes of LT in a large single-center cohort of patients who received a deceased donor graft either from donation after circulatory death (DCD) or donation after brain death (DBD). We determined donor-type specific criteria associated with EAD and the impact of EAD on the outcome of DCD and DBD LT.
PATIENTS AND METHODS
Study Design
This retrospective study included 1068 cases from a prospectively collected database from the University of Toronto. The study population was composed of adult patients who received a first full graft deceased donor LT between January 2007 and December 2017 at the University of Toronto. Patients with acute liver failure, retransplantation, split LT, or multiorgan transplantation were excluded. Patients were followed until December 2018. For analysis, the study population was divided into 2 groups, depending on the type of donor: DCD group and DBD group.
Approval for the study was obtained from the ethical committee of the Toronto General Hospital.
DCD Grafts
Currently, all DCD grafts used at our hospital are Maastricht category 3 DCD donors. Recipient allocation is based on the Model for end-stage liver disease (MELD) score, except for the patients who are listed for HCC, who receive exceptional points. Recipients with complicated hepatectomies, such as retransplantation or need for vascular reconstruction, and diagnosed portal vein thrombosis are avoided for DCD grafts to maintain the cold ischemia time (CIT) under 8 h.10 The protocol for organ procurement for DCD grafts at the University of Toronto has been described previously.10,11 The maximum accepted warm ischemia time (WIT) is 30 min. At our center, WIT is defined as the time between withdrawal of life support of the donor and organ perfusion, irrespective of the mean arterial pressure or the Po2 levels. Recipients of DCD grafts are administered 100 µg/kg (donor weight) tissue plasminogen activator through the hepatic artery 5–10 min before portal reperfusion.11,12
Statistical Analysis
Descriptive statistics were calculated (mean, median, frequencies), and comparison tests were conducted (ANOVA, Kruskal–Wallis, Fisher) to compare variables between the study groups and subgroups. For continuous variables, when a normal distribution of data was identified, data were expressed as mean ± SD, and ANOVA test was used for comparing groups; in the case of a nonnormal distribution, data were expressed as median (range), and Kruskal-Wallis test was used for analysis. In the case of categorical variables, Fisher’s test was used for examining the significance of the association between groups. As a nonparametric statistic, univariate logistic regression with odds ratio (OR) estimates was used to determine the influence of different variables on developing EAD and the influence of EAD on different postoperative parameters. Subsequently, a multivariate logistic regression of the same recipient, donor, and intraoperative factors was performed. The association of variables was analyzed by calculating OR together with confidence intervals (CI). The Kaplan–Meier estimator was used to calculate survival rates, and log-rank tests were used to compare survival rates. Patient and graft survival were calculated from the time of LT. P < 0.05 was considered statistically significant. All statistical analyses were performed with RStudio software (version 1.1.463).
RESULTS
Recipient Characteristics
From January 2007 to December 2017, 1136 patients fulfilled the criteria for inclusion in our study. Of these, 30 were excluded due to insufficient data to calculate the EAD score (none of these patients died within the first week), 18 were excluded due to primary nonfunction, which was considered a different entity, and 20 were excluded because the liver was subjected to normothermic ex situ liver perfusion before transplantation, which can potentially influence the rate of EAD among these patients. In this cohort of 1068 patients, 103 patients received a DCD graft (DCD group), and 965 received a DBD graft (DBD group).
According to Olthoff et al criteria,5 465 patients developed EAD (44%). EAD incidence was significantly higher in the DCD group (71%) compared with the DBD group (41%).
Factors Influencing EAD Development
Univariate analysis was performed for several donor, recipient, and intraoperative factors (Table 1). From all the recipient factors, recipient body mass index (BMI) was significantly different between groups (P < 0.01). From the donor factors, donor age, BMI, and gender were also significantly different between groups (P < 0.01, P < 0.001, respectively, P = 0.01). From the intraoperative factors, recipient WIT, estimated blood loss (EBL), vasopressor necessity at 1 h and the end of transplant, and the amount of fresh frozen plasma (FFP) that was administered were significantly different between groups (P < 0.001, P < 0.01, P < 0.01, P = 0.03 and P = 0.045, respectively).
TABLE 1.
Donor, recipient, and intraoperative factors were compared between recipients who developed EAD and those who did not develop EAD
| Factors | EAD = 0 | EAD = 1 | P | |
|---|---|---|---|---|
| (N = 603) | (N = 465) | |||
| Recipient factors | ||||
| Age | 58.3 (52.3–62.0) | 57.3 (50.6–62.3) | 0.24 | |
| BMI | 26.3 (23.6–30.3) | 27.6 (24.3–31.0) | <0.01 | |
| Gender | Female | 156 (25.9%) | 121 (26.0%) | 1.000 |
| Male | 447 (74.1%) | 344 (74.0%) | ||
| MELD | 18.0 (11.0–24.0) | 16.0 (9.00–25.0) | 0.056 | |
| HCV | 0 | 384 (63.7%) | 320 (68.8%) | 0.09 |
| 1 | 219 (36.3%) | 145 (31.2%) | ||
| HCC | 0 | 347 (57.5%) | 253 (54.4%) | 0.34 |
| 1 | 256 (42.5%) | 212 (45.6%) | ||
| PBC_PSC | 0 | 543 (90.0%) | 431 (92.7%) | 0.16 |
| 1 | 60 (9.95%) | 34 (7.31%) | ||
| Na-MELD | 21.1 (13.2–28.0) | 20.0 (11.3–29.0) | 0.27 | |
| Location | Home | 399 (66.2%) | 311 (66.9%) | 0.41 |
| Hospital | 176 (29.2%) | 125 (26.9%) | ||
| ICU | 28 (4.64%) | 29 (6.24%) | ||
| Alcohol | 0 | 486 (80.6%) | 380 (81.7%) | 0.69 |
| 1 | 117 (19.4%) | 85 (18.3%) | ||
| Dialysisa | No | 494 (81.9%) | 390 (83.9%) | 0.65 |
| Yes | 49 (8.13%) | 36 (7.74%) | ||
| Donor factors | ||||
| Donor type | DCD | 30 (4.98%) | 73 (15.7%) | <0.001 |
| DBD | 573 (95.0%) | 392 (84.3%) | ||
| Age | 48.0 (32.0–60.0) | 51.0 (38.0–61.0) | <0.01 | |
| BMI | 25.6 (22.5–28.7) | 26.4 (23.6–30.1) | <0.001 | |
| Gender | Female | 254 (42.1%) | 161 (34.6%) | 0.01 |
| Male | 348 (57.7%) | 300 (64.5%) | ||
| Length of ICU stay, d | 1.00 (0.00–3.00) | 2.00 (0.00–3.00) | 0.10 | |
| Operative factors | ||||
| CIT | 422 (334–519) | 430 (345–519) | 0.39 | |
| WIT | 48.0 (41.0–56.0) | 53.0 (44.0–61.0) | <0.001 | |
| PRBC | 3.00 (1.00–6.00) | 4.00 (1.00–7.00) | 0.09 | |
| FFP | 5.00 (2.00–8.00) | 6.00 (2.00–10.0) | 0.045 | |
| EBL | 2 (1.2–3.5) | 2.5 (1.2–5) | <0.01 | |
| PLATE | 1.00 (0.00–4.50) | 1.00 (0.00–4.00) | 0.85 | |
| CA_1hb | 0.03 (0.05) | 0.04 (0.07) | <0.01 | |
| CA_finalb | 0.01 (0.04) | 0.02 (0.05) | 0.03 | |
| Postoperative factors | ||||
| Length of ICU stay, db | 3.57 (7.9) | 4.50 (9.14) | 0.09 | |
| Cr_2wks | 215 (36%) | 226 (49%) | <0.001 | |
| Length of hospital stay, d | 11.0 (8.00–21.8) | 13.0 (8.00–23.0) | 0.11 | |
| Rej_1 mo | 0 | 519 (86.1%) | 407 (87.5%) | 0.54 |
| 1 | 84 (13.9%) | 58 (12.5%) | ||
| Rej_after 1 mo | 0 | 491 (81.4%) | 391 (84.1%) | 0.29 |
| 1 | 112 (18.6%) | 74 (15.9%) | ||
Data are given as n (%) or median (interquartile range) unless otherwise noted.
aData regarding dialysis need before transplantation was missing for some subjects; therefore, the sum of patients that had dialysis and those who did not receive dialysis before transplantation does not add to 100%. The level of missingness was around 10%.
bData given as mean ± SD.
BMI, body mass index; CA_1h, catecholamine amount at 1 h after the start of the transplant; CA_final, catecholamine administration at the end of the transplant; CIT, cold ischemia time, minutes; Cr_2 wks, elevated creatinine > 150 μmol/L at 2 wk posttransplantation; DBD, donation after brain death; DCD, donation after circulatory death; EAD, early allograft dysfunction; EBL, estimated blood loss, L; FFP, freshly frozen plasma packs; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; ICU, intensive care unit; MELD, model for end-stage liver disease; Na-MELD, sodium MELD; PBC, primary biliary cirrhosis; PLATE, platelet units; PRBC, red blood cell packs; PSC, primary sclerosing cholangitis; Rej_after 1 mo, rejection after 1 mo from the transplantation; Rej_1mo, rejection in the first month posttransplantation; WIT, warm ischemia time of recipient, min.
The cohort was then split into 2 groups, DCD group and DBD group, and univariate analysis of the same factors was again run for the 2 groups, for the EAD and no-EAD subgroups (Table 2).
TABLE 2.
Donor, recipient, and intraoperative factors were compared between recipients who developed EAD and those who did not develop EAD separately for the DCD group and DBD group
| Factors | DCD group | DBD group | |||||
|---|---|---|---|---|---|---|---|
| EAD = 0 (N = 30) | EAD = 1 (N = 73) | P | EAD = 0 (N = 573) | EAD = 1 (N = 392) | P | ||
| Recipient factors | |||||||
| BMI | 24.4 (22.1–28.4) | 27.0 (23.3–29.9) | 0.13 | 26.4 (23.7–30.3) | 27.8 (24.7–31.0) | <0.01 | |
| MELD | 16.0 (11.2–25.2) | 18.0 (12.0–27.5) | 0.55 | 18.0 (11.0–24.0) | 15.0 (9.00–24.0) | 0.01 | |
| HCV | 0 | 17 (56.7%) | 45 (61.6%) | 0.8 | 367 (64.0%) | 275 (70.2%) | 0.057 |
| 1 | 13 (43.3%) | 28 (38.4%) | 206 (36.0%) | 117 (29.8%) | |||
| Na-MELD | 21.0 (13.9–27.3) | 20.0 (13.0–31.9) | 0.75 | 21.1 (13.2–28.0) | 19.8 (11.1–28.5) | 0.16 | |
| Donor factors | |||||||
| Age | 40.0 (24.0–49.0) | 39.5 (25.5–55.0) | 0.64 | 49.0 (32.0–61.0) | 53.0 (42.0–63.0) | <0.001 | |
| BMI | 22.9 (21.3–25.6) | 25.3 (22.6–27.6) | 0.03 | 25.8 (8–42) | 26.8 (16–49) | <0.001 | |
| Gender | Female | 9 (30.0%) | 17 (23.3%) | 0.43 | 245 (42.8%) | 144 (36.7%) | 0.02 |
| Male | 20 (66.7%) | 55 (75.3%) | 328 (57.2%) | 245 (62.5%) | |||
| Operative factors | |||||||
| CIT | 301 (276–336) | 342 (298–382) | 0.01 | 430 (344–522) | 452 (358–540) | 0.02 | |
| WIT | 53.5 (41.5–59.5) | 54.0 (45.0–60.5) | 0.6 | 48.0 (41.0–55.0) | 53.0 (44.0–61.2) | <0.001 | |
| EBL | 2.3 (1.5–4.5) | 3.5 (2–5.5) | 0.10 | 2 (1.2–3.5) | 2.5 (1–4.5) | 0.057 | |
| CA_1ha | 0.03 (0.04) | 0.06 (0.06) | 0.06 | 0.03 (0.05) | 0.04 (0.07) | 0.059 | |
| CA_finala | 0.01 (0.03) | 0.03 (0.05) | 0.053 | 0.01 (0.04) | 0.02 (0.05) | 0.12 | |
| Postoperative factors | |||||||
| Cr_2wks | 9 (30.0%) | 39 (53%) | 0.03 | 206 (36.0%) | 187 (48.0%) | <0.001 | |
All the factors presented in Table 1 were analyzed. Above are presented only the factors which reached significance. Data are given as n (%) or median (range) unless otherwise noted.
aData given as mean ± SD.
BMI, body mass index; CA_1h, catecholamine amount at 1 h after the start of the transplant; CA_final, catecholamine administration at the end of the transplant; CIT, cold ischemia time, min; Cr_2 wks, elevated creatinine > 150 μmol/L at 2 wk posttransplantation; DBD, donation after brain death; DCD, donation after circulatory death; EAD, early allograft dysfunction; EBL, estimated blood loss, L; HCV, hepatitis C virus; MELD, model for end-stage liver disease; Na-MELD, sodium MELD; WIT, warm ischemia time of recipient, min.
In the DBD group, recipient BMI (P < 0.01) and MELD were associated with EAD (P = 0.01). Also, donor age, BMI, and gender (P < 0.001, P < 0.001, and P = 0.02, respectively) and recipient CIT and WIT (P = 0.02 and P < 0.001, respectively) were significantly different between EAD versus no-EAD recipients of DBD grafts.
In the DCD group, no recipient factors were associated with the development of EAD. Predictive of EAD were donor BMI (P = 0.03) and CIT (P = 0.01).
Patients who received a DCD graft had a higher risk of developing EAD than those who received a DBD graft, with an OR of 3.56 (95% CI, 2.28-5.55; P < 0.0001).
Influence of EAD Development on Postoperative Outcomes
The impact of EAD on the postoperative course was analyzed. Univariate analysis showed that in the overall cohort, the percentage of patients with an elevated serum creatinine (Cr, creatinine > 150 μmol/L) level at 2 wks’ posttransplantation was significantly higher in the EAD group versus no-EAD group (49% versus 36%, P < 0.001). However, postoperative intensive care unit (ICU) and hospital stay as well as the rejection rate posttransplantation were similar in both groups (Table 1).
Similar results were found when the cohort was split in DCD and DBD recipients. The percentage of patients with an elevated serum creatinine at 2 wks’ posttransplantation was significantly higher in the EAD groups versus no-EAD groups (DCD group—P = 0.03 and DBD group—P < 0.001). However, postoperative hospital and ICU stay and rejection rate posttransplantation were similar in the EAD and no-EAD groups, both for the DCD and DBD recipients.
Multivariate Analysis
For the multivariate model, all variables used in the previously discussed univariate analyses were included. The multivariable forward logistic regression model was adjusted for recipient, donor, and intraoperative factors that may impact the development of EAD.
First, the entire cohort was analyzed. The multivariate analysis of recipient factors identified 2 independent predictors: recipient age, with a P value of 0.045 and hepatitis C virus (HCV) infection with a P value of 0.03 (OR, 0.7; 95% CI, 0.51-0.96) (Table 3). HCV positivity as well as recipient age showed a protective effect for the development of EAD. From all donor variables, donor age, BMI, and donor gender male (all P < 0.01) were found to be associated with the development of EAD (Table 3). A multivariate analysis for the operative factors demonstrated an association between WIT and EBL (both P < 0.01) and the development of EAD (Table 3). There was no correlation between EAD and the other factors (data not shown).
TABLE 3.
Multivariate analysis of risk factors for early allograft dysfunction
| Factors | OR | 95% CI | P |
|---|---|---|---|
| Entire cohort | |||
| Recipient factors | |||
| Age | 0.98 | (0.97–0.99) | 0.045 |
| HCV positive | 0.7 | (0.51–0.96) | 0.04 |
| Donor factors | |||
| Age | 1.02 | (1.00–1.01) | 0.009 |
| Male gender | 1.41 | (1.09–1.82) | 0.009 |
| BMI | 1.05 | (1.03–1.08) | <0.001 |
| Intraoperative factors | |||
| WIT | 1.03 | (1.02–1.05) | <0.001 |
| EBL | 1.00 | (1.00–1.00) | 0.007 |
| DBD group | |||
| Recipient factors | |||
| Age | 0.98 | (0.96–0.99) | 0.04 |
| BMI | 1.02 | (1.00–1.05) | 0.04 |
| HCV positive | 0.7 | (0.49–0.96) | 0.03 |
| Donor factors | |||
| Age | 1.01 | (0.99–1.03) | 0.034 |
| DCD group | |||
| Donor factors | |||
| BMI | 1.22 | (1.05–1.46) | 0.02 |
BMI, body mass index; DBD, donation after brain death; DCD, donation after circulatory death; EBL, estimated blood loss, L; HCV, hepatitis C virus; OR, odds ratio; WIT, warm ischemia time of recipient, min.
Next, a multivariable logistic regression of the same recipient, donor, and intraoperative factors was run separately for the DBD and DCD group.
For recipients of DBD grafts, recipient factors age, BMI, and HCV infection were found to be associated with the development of EAD (P = 0.04; P = 0.04, respectively, P = 0.03). Of these, HCV positivity and recipient age were associated with a decreased risk of EAD. From all donor variables, donor age was found to be associated with the development of EAD (P = 0.034) (Table 3).
For recipients of DCD grafts, no recipient or intraoperative factors were found to be associated with the development of EAD. From the donor factors, BMI was predictive for the development of EAD (P = 0.02), with an OR of 1.22 (95% CI, 1.05-1.46) (Table 3).
Graft and Patient Survival in the EAD Versus No-EAD Groups
There was a significant difference in the entire study cohort in 1-, 3-, and 5-y graft survival for patients with EAD 89%, 81%, and 76% versus without EAD 93%, 87%, and 82% (P = 0.01, Figure 1A). Similar, the 1-, 3-, and 5-y patient survival was 90%, 82%, and 78% in the EAD group versus 94%, 88%, and 83% in the no EAD (P = 0.02, Figure 1B).
FIGURE 1.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD (marked in black) vs patients who did not develop EAD (marked in gray). Patients at risk are shown in the table below the graph. EAD, early allograft dysfunction.
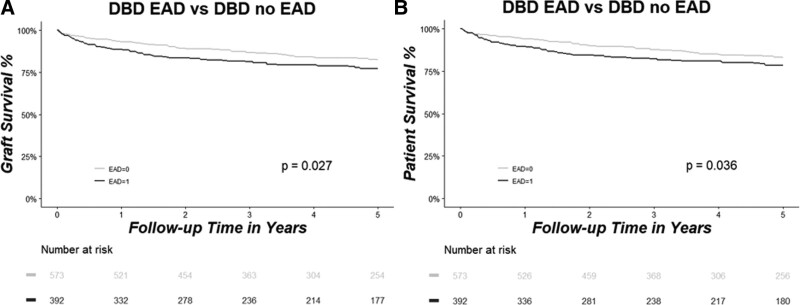
Next, we assessed the impact of EAD versus no EAD on graft and patient survival separately for the DBD and DCD groups. In the DBD group, graft survival was significantly reduced in the EAD versus no-EAD group at 1, 3, and 5 y (EAD 89%, 81%, and 77% versus no EAD 93%, 87%, and 82%, P = 0.03, Figure 2A). Similarly, patient survival with DBD grafts at 1, 3, and 5 y was lower in the EAD versus no-EAD group (EAD 90%, 82%, and 79% versus no EAD 94%, 88%, and 83%, P = 0.04, Figure 2B).
FIGURE 2.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD (marked in black) vs patients who did not develop EAD (marked in gray) in the DBD group. Patients at risk are shown in the table below the graph. DBD, donation after brain death; EAD, early allograft dysfunction.
Interestingly, for DCD grafts, no differences were found for EAD versus no-EAD recipients on graft and patient survival. For the DCD group, graft survival at 1, 3, and 5 y was 89%, 80%, and 70% in the EAD group versus 90%, 87%, and 75% in the no-EAD group (P = 0.44, Figure 3A). Similar, patient survival at 1, 3, and 5 y was 90%, 82%, and 72% in the EAD group versus 90%, 87%, and 75% in the no-EAD group (P = 0.54, Figure 3B).
FIGURE 3.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD (marked in black) vs patients who did not develop EAD (marked in gray) in the DCD group. Patients at risk are shown in the table below the graph. DCD, donation after circulatory death; EAD, early allograft dysfunction.
Importance of the Different EAD Criteria for DCD and DBD Grafts
To better understand which of the criteria proposed by Olthoff had most influence for the EAD definition, we decided to analyze each of the 3 criteria separately.
New groups were formed. EAD-AST was defined by an ALT or AST ≥ 2000 U/L within the first 7 POD. EAD-Bili was defined by a serum total bilirubin ≥ 10 mg/dL on POD 7. EAD-INR was defined by an INR ≥ 1.6 on POD 7.
In our cohort of patients, EAD-AST incidence was 66% in the DCD group and 33.1% in the DBD group. EAD-Bili incidence was 8.74% in the DCD group and 8.41% in the DBD group. EAD-INR incidence was 11.8% in the DCD group and 6.9% in the DBD group.
Univariate analysis of the same donor, recipient, and intraoperative factors that were presented in subsection 3.2 was run for the new subgroups, separately for the DBD and DCD cohorts. For the DBD recipients, EAD-AST versus no EAD-AST resulted in similar results as the comparison EAD versus no EAD (data not shown). Presence of EAD-AST was associated with poorer renal function at 2 wks’ posttransplantation (P = 0.001).
Although only a few patients fulfilled the criterion EAD-Bili (n = 81), univariate analysis showed that multiple recipient and intraoperative factors were associated with the development of EAD-Bili. Recipient age, pretransplant MELD and Na-MELD score, diagnosis of HCC, HCV, or primary sclerosing cholangitis/primary biliary cirrhosis and patient location before transplantation (home/hospital/ICU) were associated with EAD-Bili (all P < 0.05). Interestingly, no donor factors were associated with EAD-Bili. However, several intraoperative factors were associated with EAD-Bili: WIT, EBL, administration of red blood packs (PRBC), FFP, and platelets (all P < 0.05). Presence of EAD-Bili was associated with a longer stay on the ICU and in the hospital and a poorer renal function at 2 wks’ posttransplantation (all P < 0.01).
For the EAD-INR group, results were comparable to the EAD-Bili group. Only 66 patients were included in the EAD-INR group. Several recipients (pretransplant MELD and Na-MELD) and intraoperative factors (EBL, administration of PRBC and FFP and catecholamine [CA] necessity at 1 h and the end of transplant) were associated with the presence of EAD. Again, no donor factors were associated with EAD-INR. Also, the presence of EAD-INR was associated with a longer ICU and hospital stay (P < 0.001), as well as an increased rate of rejection in the first month posttransplantation (P = 0.04).
For the DCD recipients, EAD-AST versus no EAD-AST resulted in comparable results as the comparison EAD versus no EAD (data not shown). Presence of EAD-AST was predictive for a poorer renal function at 2 wks’ posttransplantation (P = 0.025).
Again, only a few patients fulfilled the criterion EAD-Bili (n = 9). Univariate analysis showed that recipient age, pretransplant MELD score, donor BMI, and WIT were associated with EAD-Bili (all P < 0.05). Presence of EAD-Bili was associated with a longer stay on the ICU and in the hospital (P < 0.05).
In the DCD cohort, 12 patients were included in the EAD-INR group. No recipient factors were associated with the presence of EAD-INR. From the donor factors, only the length of ICU stay was associated with EAD (P < 0.001). Also, several intraoperative factors were associated with EAD-INR: EBL, administration of PRBC, and catecholamine necessity the end of transplant (all P < 0.05). Presence of EAD-INR was associated with a longer ICU stay.
Graft and Patient Survival in the Subgroups EAD-AST/EAD-Bili/EAD-INR
First, the entire cohort was analyzed. For the EAD-AST group, graft (P = 0.28) and patient (P = 0.29) survival were similar with those in the no EAD-AST group. For the other 2 criteria, a significant difference was observed between groups. EAD-Bili group had a significantly lower graft and patient survival rate than the no EAD–Bili group, and this was also the case for the EAD-INR group versus no EAD-INR group (all P < 0.01).
The cohort was then again split in the DBD and DCD groups, and the influence of each factor was analyzed.
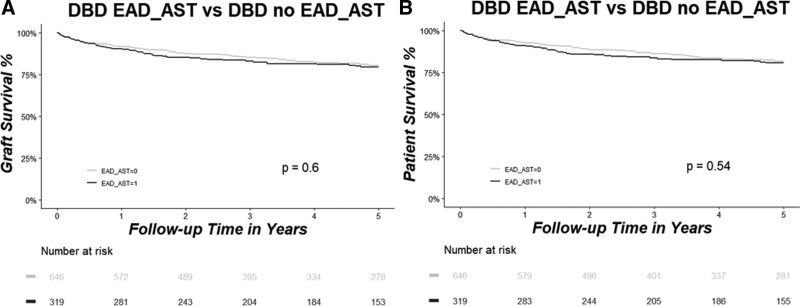
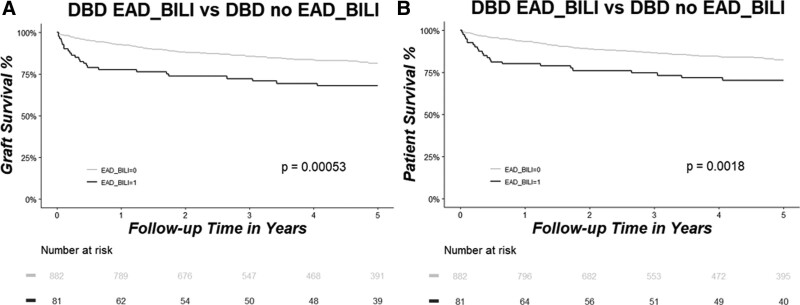
In the DBD group, results were similar to the whole cohort. When EAD was defined only based on the EAD-AST criterion, no differences in graft and patient survival were present (Figure 4A and B). However, when the EAD-Bili or EAD-INR criteria were used to define EAD, there was significantly lower patient and graft survival in the EAD group compared with the no-EAD group (all P < 0.01) (Figures 5A, B and 6A, B).
FIGURE 4.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-AST (marked in black) vs patients who did not develop EAD-AST (marked in gray) in the DBD group. Patients at risk are shown in the table below the graph. AST, aspartate aminotransferase; DBD, donation after brain death; EAD, early allograft dysfunction.
FIGURE 5.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-Bili (marked in black) vs patients who did not develop EAD-Bili (marked in gray) in the DBD group. Patients at risk are shown in the table below the graph. Bili, serum total bilirubin; DBD, donation after brain death; EAD, early allograft dysfunction.
FIGURE 6.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-INR (marked in black) vs patients who did not develop EAD-INR (marked in gray) in the DBD group. Patients at risk are shown in the table below the graph. DBD, donation after brain death; EAD, early allograft dysfunction; INR, international normalized ratio.
For the DCD group, only when EAD was defined by the EAD-Bili criterion, we found a significant difference in patient and graft survival between the EAD and no-EAD group (both P < 0.05) (Figures 7A, B, 8A, B, and 9A, B).
FIGURE 7.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-AST (marked in black) vs patients who did not develop EAD-AST (marked in gray) in the DCD group. Patients at risk are shown in the table below the graph. AST, aspartate aminotransferase; DCD, donation after circulatory death; EAD, early allograft dysfunction.
FIGURE 8.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-Bili (marked in black) vs patients who did not develop EAD-Bili (marked in gray) in the DCD group. Patients at risk are shown in the table below the graph. Bili, serum total bilirubin; DCD, donation after circulatory death; EAD, early allograft dysfunction.
FIGURE 9.
Graft (A) and patient (B) survival rates as analyzed by Kaplan–Meier estimation and compared between patients who developed EAD-INR (marked in black) vs patients who did not develop EAD-INR (marked in gray) in the DCD group. Patients at risk are shown in the table below the graph. DCD, donation after circulatory death; EAD, early allograft dysfunction; INR, international normalized ratio.
DISCUSSION
Our study investigated the impact of EAD on long-term graft and patient survival in LT with DBD and DCD grafts. In addition, we analyzed risk factors for EAD for both graft types. EAD was associated with a reduced graft survival in DBD grafts but not after DCD LT. For DBD grafts, EAD was associated with recipient age, BMI, and HCV positivity and donor age. In contrast, in DCD grafts, only donor BMI was associated with EAD. Interestingly, INR and bilirubin as EAD parameters had a stronger impact on graft outcome than AST.
Conflicting results have been reported in the past regarding the impact of EAD on graft survival. Olthoff et al reported a strong impact of EAD on graft survival (EAD group: 73.9% versus no-EAD group: 96.5%, P < 0.001).5 Olthoff and colleagues investigated the impact of EAD on graft and patient survival in a cohort of subjects who received both DBD and DCD grafts from 3 transplant centers. Taking into consideration which factors had been associated with graft outcome by previous publications, the authors decided to define EAD as 1 of the following 3 criteria: total serum bilirubin ≥ 10 mg/dL on POD 7 or INR ≥ 1.6 on POD 7 or ALT/AST > 2000 U/L within the first 7 POD. The study was limited by a relatively small sample size with 297 subjects. Also, the inclusion of patients irrespective of the donor type—DBD and DCD—could have confounded the results. In our study, the DCD cohort behaved very differently than the DBD cohort.
Similar to the findings of Olthoff, we observed a reduction in graft survival in patients with EAD, but the impact was smaller than in previous studies. Several possibilities exist for the differences between our findings and those of previous studies. Our study population included a larger single-center experience. Also, to assess the long-term outcomes of EAD, our subjects were followed for 5 y instead of only 6 mo. We excluded from our analysis patients who developed PNF, which was not the case in the Olthoff study.
Lee and colleagues also found decreased graft and patient survival in patients with EAD.8 In a large single-center retrospective study, the Mayo group followed the patients for 5 y and reported that the development of EAD influences long-term patient and graft survival. No separate analysis of the DCD and DBD groups was performed, also the importance of different criteria on the development of EAD was not investigated.
In our series of DBD transplants, the reason for being classified as EAD was important with INR and bilirubin having a larger effect than AST. Interestingly, when EAD was solely defined by EAD-Bili or EAD-INR criterion, graft and patient survival were significantly reduced in the EAD group. If the group was split by the EAD-AST criterion, no differences were found between groups.
In our DCD cohort, overall, there was no difference in graft and patient survival in patient with versus without EAD. In contrast, elevated bilirubin had a strong effect on the outcome, and when EAD was defined only by the EAD-Bili criterion, we did find a significantly lower patient and graft survival in the EAD group versus the no-EAD group.
Contrary to our findings, Lee and colleagues found in a DCD cohort that EAD has a significant influence on graft and patient survival.7 In a single-center retrospective study, they investigated the long-term patient and graft survival in patients with and without EAD. In this study, patients who developed PNF were not excluded from the analysis and this could have contributed to the increased graft and patient survival in the no-EAD cohort. However, similar to our findings, INR and bilirubin were more important than AST.
It is unclear if EAD in DCD grafts has the same mechanisms as in DBD LT. DCD donors are usually younger without steatosis. In most cases, graft injury in DCD grafts is limited to warm and cold ischemia without additional types of graft injury, such as advanced age, steatosis, and underlying preexisting liver disease. It is possible that different criteria have to be developed for different graft types, reflecting the specific mechanisms of injury resulting in delayed graft function.
Interestingly, in the DBD cohort, patients who developed EAD had a lower MELD score, likely because higher MELD patients receive better grafts. Also, in the multivariate analysis, HCV infection seemed to have a protective effect for the development of EAD. This is probably due to the donor selection for HCV patients.
The findings of our study have several important implications. First, EAD in DBD and DCD grafts should be investigated separately because different risk factors contribute to the development of graft dysfunction, and the impact of EAD on the outcome of the 2 graft types is different. Second, additional information about prognosis of grafts with EAD can be obtained if the contributing factors are investigated separately.
Predicting EAD in LT is important to guide clinical decision making but also to provide outcome parameters for the comparison of clinical trials. Ideally, risk factors for EAD should be available before transplantation to allow transplant physicians to select the right recipient for each graft. In our series, different parameters had to be considered for DBD and DCD graft types. The assessment of posttransplant graft function in livers is further complicated because injury to different cell types might present differently on outcome. For example, hepatocyte, biliary, and endothelial cell function might not respond similarly to different injury types and have varying impact on outcome. In addition, biliary injury might be severe but with good synthetic hepatocyte function, so reduced graft survival might only become apparent after prolonged follow-up when biliary drainage interventions have failed. Novel preservation techniques, such as normothermic ex vivo graft preservation, might allow us in the future to collect additional function parameters of marginal grafts that will allow us more precisely to determine the risk of EAD in our patients.
Our study has several limitations. The retrospective study design and selection bias due to the nonrandomized study design could confound the results. Moreover, this study is complicated, and the variables are interacting, indicating that any findings are associative and could be the result of practice bias. The small size of the DCD group might have not had sufficient power to evidence some findings. For DBD grafts, more donor selection risks are taken, such as age and steatosis, which could potentially affect long-term graft function and explain the high proportion of EAD in the DBD group.
In conclusion, our study demonstrates that the current definition for EAD is controversial and might not be applicable for all donor types. Depending on the donor type, different donor and recipient factors influence the outcome of LT, and robust predictors for poorer outcome are still a matter of debate. Novel graft assessment technologies may allow us to better predict posttransplant graft function before transplantation.
Footnotes
Published online 16 July, 2021.
This work has been supported by grants from the Deutsche Forschungsgemeinschaft (DFG) (MA 8516/1-1 to L.I.M.).
The authors declare no conflicts of interest.
L.I.M., S.K., A.G., L.L., T.W.R., Z.G., M.S.C., M.B., I.D.M., G.S., B.S., N.S., and M.S. participated in research design. L.I.M., S.K., T.W.R., A.G., N.S., and M.S. participated in writing the article. L.I.M., S.K., A.G., T.W.R., N.S., and M.S. participated in data analysis. N.S. and M.S. have a shared senior authorship.
REFERENCES
- 1.Barshes NR, Horwitz IB, Franzini L, et al. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7:1265–1270. [DOI] [PubMed] [Google Scholar]
- 2.Maluf DG, Edwards EB, Kauffman HM. Utilization of extended donor criteria liver allograft: is the elevated risk of failure independent of the model for end-stage liver disease score of the recipient? Transplantation. 2006;82:1653–1657. [DOI] [PubMed] [Google Scholar]
- 3.Deschênes M, Belle SH, Krom RA, et al. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Transplantation. 1998;66:302–310. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Z, Weiss-Schmilovitz H, Sulkes J, et al. Serum cholestasis markers as predictors of early outcome after liver transplantation. Clin Transplant. 2004;18:130–136. [DOI] [PubMed] [Google Scholar]
- 5.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. [DOI] [PubMed] [Google Scholar]
- 6.Croome KP, Wall W, Quan D, et al. Evaluation of the updated definition of early allograft dysfunction in donation after brain death and donation after cardiac death liver allografts. Hepatobiliary Pancreat Dis Int. 2012;11:372–376. [DOI] [PubMed] [Google Scholar]
- 7.Lee DD, Singh A, Burns JM, et al. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014;20:1447–1453. [DOI] [PubMed] [Google Scholar]
- 8.Lee DD, Croome KP, Shalev JA, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol. 2016;15:53–60. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer DP, Paul A, Gallinat A, et al. Donor information based prediction of early allograft dysfunction and outcome in liver transplantation. Liver Int. 2015;35:156–163. [DOI] [PubMed] [Google Scholar]
- 10.Kollmann D, Sapisochin G, Goldaracena N, et al. Expanding the donor pool: donation after circulatory death and living liver donation do not compromise the results of liver transplantation. Liver Transpl. 2018;24:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seal JB, Bohorquez H, Reichman T, et al. Thrombolytic protocol minimizes ischemic-type biliary complications in liver transplantation from donation after circulatory death donors. Liver Transpl. 2015;21:321–328. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto K, Eghtesad B, Gunasekaran G, et al. Use of tissue plasminogen activator in liver transplantation from donation after cardiac death donors. Am J Transplant. 2010;10:2665–2672. [DOI] [PubMed] [Google Scholar]