Abstract

The electrochemical performance of Ge, an alloying anode in the form of directly grown nanowires (NWs), in Li-ion full cells (vs LiCoO2) was analyzed over a wide temperature range (−40 to 40 °C). LiCoO2||Ge cells in a standard electrolyte exhibited specific capacities 30× and 50× those of LiCoO2||C cells at −20 and −40 °C, respectively. We further show that propylene carbonate addition further improved the low-temperature performance of LiCoO2||Ge cells, achieving a specific capacity of 1091 mA h g–1 after 400 cycles when charged/discharged at −20 °C. At 40 °C, an additive mixture of ethyl methyl carbonate and lithium bis(oxalato)borate stabilized the capacity fade from 0.22 to 0.07% cycle–1. Similar electrolyte additives in LiCoO2||C cells did not allow for any gains in performance. Interestingly, the capacity retention of LiCoO2||Ge improved at low temperatures due to delayed amorphization of crystalline NWs, suppressing complete lithiation and high-order Li15Ge4 phase formation. The results show that alloying anodes in suitably configured electrolytes can deliver high performance at the extremes of temperature ranges where electric vehicles operate, conditions that are currently not viable for commercial batteries without energy-inefficient temperature regulation.
Keywords: germanium nanowire, graphite, lithium-ion battery, full cell, wide temperature performance, temperature-controlled electrochemical amorphization
Introduction
The rate of displacement of conventional internal combustion engine (ICE) vehicles by electric vehicles (EVs) is strongly dependent on key performance gains in Li-ion batteries (LIBs), particularly regarding the range and fast charging. However, the adaptability of Li-ion cells to different climates in which ICE vehicles can operate is still a major issue that has yet to be adequately addressed. Typically, 18,650 cells operate well between 0 and 30 °C,1,2 but variations in cell temperature outside of this range trigger rapid capacity fade or a significant reduction in overall performance. The diminished performance of EVs in hot climates is also a key issue, with high-temperature cycling of the battery inducing rapid capacity fade and an overall reduction in power output.3 Similarly, EVs operating in cold climates experience a diminished range due to a combination of insufficient charging and a significant amount of power being redirected to heat the vehicle.4 The inability of EVs to operate under cold conditions also has important environmental implications considering that cold starts of ICE vehicles dramatically reduce fuel efficiency, leading to greater emissions.5,6 There is also a wider interest in enhancing the low-temperature performance of Li-ion cells for aerospace applications that require high storage capabilities over an extended period under severe environmental conditions.7,8 Extending the operable temperature range for Li-ion cells is thus of huge significance at high and low extremes.
The high-temperature performance of LIBs is limited by the instability of the liquid electrolyte and its susceptibility to degradation.9 The solid electrolyte interphase (SEI), formed during first cycle lithiation, plays a critical role in cell stability, removing direct contact at the electrode–electrolyte interface. Under normal conditions, electrolyte decomposition of the LiPF6 salt is a simple dissociation process, forming LiF and PF5, both of which aid SEI formation.10 However, high-temperature cycling promotes increased HF formation along with the buildup of internal pressure through gas evolution.11−13 High-temperature additives such as lithium bis(oxalato)borate (LiBOB) and ethyl methyl carbonate (EMC) have appeared as viable additives to suppress electrolyte decomposition reactions and in turn improve overall cell stability.14−16
Low temperatures dramatically increase cell impedance, increasing SEI layer resistance and charge transfer resistance (RCT) and suppressing Li ion transport through the cell. A combination of poor lithium diffusivity through the SEI layer and low conductivity of the graphite anode leads to a substantial drop in power output, as the LIB cells cannot sufficiently charge/discharge.17−20 Commercial cells lose the majority of their capacity below −10 °C, with 18,650 cells returning around 50% of their room temperature capacity when discharged at −20 °C, with a substantial drop to 5% below −40 °C.18 At low temperatures, cell output becomes strongly dependent on applied current density. At −10 °C, 18,650 cells lose the entirety of their energy when discharged at 1.5C.21 Electrochemical impedance spectroscopy (EIS) of graphite cells reveals that at low temperatures, cell impedance is dominated by a substantial increase in RCT.22 Consequently, the electrical characteristics of the anode material are critical in dictating the overall cell performance.
Recently, Li-ion alloying anodes in the form of group-IV Si and Ge materials have appeared as candidates to replace graphite intercalation anodes, owing to their high gravimetric capacities and high volumetric energy densities.23−30 Nanoscaling of the anode allows a significant increase in electronic and ionic conductivities, facilitating fast charging by increasing the electrode–electrolyte contact area and shortening the diffusion length for Li-ion transport.31,32 Moreover, shortened diffusion lengths and higher surface area/volume ratios can better overcome reduced conductivity at low temperatures. Ge nanowires (NWs) have been routinely shown to offer stable, high capacity cycling with a great propensity for high current densities.33−35 In this study, we show that an alloying anode (Ge NW-based) substantially outperforms conventional graphite anodes at high and low temperatures in full-cell configurations using “standard” electrolytes. Significant performance enhancements beyond this benchmark were obtained through careful electrolyte modifications, with the enhanced performance explained through ex situ analysis of morphological and SEI compositional changes. A comparison table to similar published work is provided in Table S1.
Results and Discussion
High- and low-temperature cycling of conventional graphite-based cells impose dramatic consequences on capacity output, cell stability, and rate capability. Figure 1a summarizes the major anode-specific issues of adverse temperatures on the cyclability of a LiCoO2||C cell. At low temperatures, graphite intercalation is predicated by huge increases in RCT as low temperatures suppress Li-ion desolvation at the interface and diffusion through the graphite layers and exacerbate the propensity for Li plating through electrode polarization and anodic overcharging.36−38 At elevated temperatures, the instability of the conventional LiPF6 electrolyte leads to HF/POF3 formation along with CO2 evolution.2,39 Subsequent HF etching of the electrode current collectors manifests as irreversible capacity loss and eventual cell failure.40,41 The performance impacts of these established temperature-specific issues were analyzed through galvanostatic cycling of LiCoO2||C cells between the temperature limits of −40 and 40 °C (Figure 1b,c). A 1 M LiPF6 in EC–DEC (1:1 v/v) + 3 wt % VC was chosen as the electrolyte and denoted as the “standard” or “std.” for all subsequent testing. From the discharge capacity profiles in Figure 1b, LiCoO2||C returned a capacity of 301 mA h g–1 @ 20 °C, dropping to 106 mA h g–1 @ 0 °C and to 39 mA h g–1 @ –20 °C, and finally tapering to a negligible 11 mA h g–1 @ –40 °C. Discharge capacity aging tests of LiCoO2||C between these limits highlighted the strong dependence of LiCoO2||C cell performance on cell temperature (Figure 1c). At 40 °C, LiCoO2||C exhibited rapid capacity fade, with the initial 306 mA h g–1 falling to 185 mA h g–1 after 100 cycles. At low temperatures, the initial low capacity does not significantly change, suggesting a continuous inability to charge/discharge at 1C. For a 1C rate, the applied current is calculated to set the charge/discharge time to be 1 h, based on the theoretical capacity of Ge. From the published work, a value of 1384 mA h g–1 is widely acknowledged as the capacity of Ge.33,42−45 Poor capacity retention at elevated temperatures and low capacity at low temperatures reflect the respective issues of electrolyte instability and hindered Li-ion diffusion kinetics.
Figure 1.
(a) Schematic illustration of the high- and low-temperature issues of conventional graphite-based cells. (b) Discharge capacity profiles (C/2 rate) and (c) discharge capacities (at 1C) of LiCoO2||C cells from −40 to 40 °C, charged and discharged symmetrically at the same temperature. Cells were cycled over a potential window of 2.8–3.9 V. The standard 1 M LiPF6 in EC–DEC (1:1 v/v) + 3 wt % VC was chosen for testing and kept constant between tests.
Comparative testing of LiCoO2||Ge cells revealed a far superior ability of Ge NWs to adapt to temperature variations. Preliminary testing of LiCoO2||Ge with PN ratios ranging from PN1–PN2.5 revealed PN1.5 cells as the best-performing cell configuration, maximizing specific energy and power and eliminating capacity limitations of Li-deficient PN1 cells (Figure S1). As a result, PN1.5 cells were chosen as the optimal cell configuration for testing. Furthermore, performance variations from increasing the PN ratio can be attributed to two major factors: (i) excess Li to maximize anode capacity and (ii) reduced anode loading. To analyze the effect of anode loading over the PN1–PN2.5 loading range, LiCoO2||Ge cells of equivalent PN ratios (PN1.5) were compared with low (0.25 mg cm–2)- and high (0.63 mg cm–2)-loading anodes (Figure S2). Setting a constant PN1.5 isolated loading-driven electrochemical performance variations (Figure S2). Overall, both cell loadings (0.25 and 0.63 mg cm2) exhibited similar capacity trends, reaching 91.61 and 91.42% capacity retention after 100 cycles (Figure S2a). Low-loading LiCoO2||Ge delivered a slightly higher average specific capacity than high-loading LiCoO2||Ge, which is attributed to a shorter anode diffusion length. Over the entire anode loading range of 0.25–0.63 mg cm2, higher loadings did not largely affect cell performance, exhibiting similar capacity retention and an average reduction in the specific capacity of 7.1% for 1C rate. Consequently, significant performance variations of different PN ratio cells in Figure S1a (47.6% capacity loss from PN2.5 and PN1 for 1C) can therefore be largely attributed to insufficient Li of PN1 cells, leading to incomplete anode lithiation and a subsequent reduction in specific capacity, energy, and power. Insufficient Li of PN1 cells is a well understood problem and current prelithiation studies may offer a future solution to this capacity balancing issue.45−47
Employing the std. electrolyte and 1C rate, the LiCoO2||Ge cell reached 1379 mA h g–1 at 40 °C and 1351 mA h g–1 @ 20 °C, dropping to 1164 mA h g–1@0 °C (84.1% of Qth-Ge) and then reducing marginally to 1143 mA h g–1@–20 °C (Figure 2a). As the cell temperature was lowered further to −40 °C, the cell capacity fell to 362 mA h g–1 (26.2% of Qth-Ge). Despite this drop-off in capacity @–40 °C, LiCoO2||Ge reaches 97.3% of the theoretical capacity of graphite. Differential capacity plots (DCPs) of the first-cycle lithiation/delithiation of LiCoO2||Ge from −40 to 40 °C are outlined in Figure S3. “Temperature capability” testing (an analogue of commonly used rate capability) was carried out on both cell types to compare the response of both anode types to variant temperatures (Figure 2b). Cells were cycled for five cycles at a C/2 rate, beginning at −40 °C, and the temperature was increased in 20 °C increments after every fifth cycle. The results represent the response of the two material types to variations in cell temperature. For both cell types, reduced capacity at low temperatures is shown to be reversible, with both cells reaching close to their theoretical capacity when cycled at 20 °C (98.1% of Qth-Ge and 82.9% of Qth-C). The LiCoO2||Ge cell retains high capacity at temperatures as low as −20 °C (83.5% of Qth-Ge), whereas the LiCoO2||C cell returns an almost negligible capacity at this temperature. The LiCoO2||Ge cell exhibited an experimental capacity 30-fold that of LiCoO2||C at −20 °C and 40-fold at −40 °C. Between the temperature constraints of −20 to 40 °C, LiCoO2||Ge cells offer high capacity cycling, largely unaffected by variations in cell temperature. In contrast, the performance of LiCoO2||C cells was strongly dictated by cell temperature. Corresponding specific energy and power densities of these cells as a function of temperature are outlined in Figure S4.
Figure 2.
(a) Discharge capacity profiles for LiCoO2||Ge cells from −40 to 40 °C cycled at C/2. (b) “Temperature capability” testing of a LiCoO2||C (blue) and LiCoO2||Ge (red) cell from −40 to 40 °C at C/2 and (c) discharge capacities of LiCoO2||Ge cells cycled from −40 to 40 °C at a 1C rate. The standard 1 M LiPF6 in EC–DEC (1:1 v/v) + 3 wt % VC was chosen for testing and kept constant between tests.
A combination of cross-sectional scanning electron microscopy (SEM) and EIS correlated performance differences between LiCoO2||C and LiCoO2||Ge cells to RCT variations and the corresponding electrode layer thicknesses of the anodes (Figure S5). The diminished capacity of graphite at low temperatures is governed by a huge increase in RCT, suppressing interfacial Li-ion desolvation. LiCoO2||Ge exhibits a room temperature RCT of 43 Ω, rising to 1.1 × 103 Ω at −40 °C (Figure S5c,d). Comparatively, the RCT of the LiCoO2||C cell at 20 °C was found to be 682 Ω, rising to 3 × 105 Ω at −40 °C (Figure S5a,b). For Ge and graphite cells of equivalent areal capacities, LiCoO2||C cells exhibit a RCT 27× that of LiCoO2||Ge at −40 °C. The extremely high RCT of LiCoO2||C cells at −40 °C explains their inability to charge/discharge at low temperatures. Cross-sectional SEM was carried out on graphite and Ge anodes. Both anodes were capacity-matched areally with respective mass loadings of 1.74 and 0.42 mg cm–2 (Qth = 372 μA h electrode–1). SEM of the pristine anodes found that the graphite and Ge anodes have active layer thicknesses of 102 and 12 μm, respectively (Figure S5e,f). Overall, for capacity-matched anodes, the graphite layer has a thickness 8.5× that of Ge, leading to higher cell impedance associated with longer Li-ion diffusion lengths and lower surface area/volume ratios. The RT rate performance of LiCoO2||Ge and LiCoO2||C cells of equivalent areal capacities was tested and cells were compared in terms of specific capacity, energy, and power (Figure S6). Overall, LiCoO2||Ge delivered far superior energy density for every current density. Such disparity in performance is further heightened at low temperatures. The long-term stability of LiCoO2||Ge cells, employing the std. electrolyte configuration, at different temperatures was tested (Figure 2c). At 40 °C, LiCoO2||Ge exhibited rapid capacity fade, similar to LiCoO2||C, retaining 65.77% capacity after 200 cycles. Similarly, poor capacity retentions were noted at −40, −20, and 0 °C, retaining 44.09, 48.41, and 68.57%, respectively. Despite the high initial capacities at low temperatures, LiCoO2||Ge is limited by the inefficiencies of the std. electrolyte configuration.
Modifications to the std. electrolyte with temperature-specific additives led to dramatic improvements in LiCoO2||Ge capacity retention at both high and low temperatures. First, EMC and LiBOB were chosen as high-temperature additives due to their performance-enhancing abilities in graphite-based cells at high temperatures.9,16Figure 3a illustrates the effect that varying the EMC and LiBOB content of the electrolyte has on cell stability. Initially, transitioning from a binary EC–DEC solvent to a ternary EC–DEC–EMC solvent stabilized capacity retention. In Figure 3a, the std. cell (red) retained 46.62% of its initial capacity after 250 cycles. Upon addition of EMC (blue), the capacity retention rose to 61.35%. Moreover, introducing EMC to the binary solvent mixture saw an increase in the average coulombic efficiency (CE) from 94.26 (red) to 99.68% (blue). The addition of LiBOB as an additive to the LiPF6-based electrolyte was found to notably improve capacity retention at 40 °C. LiBOB was tested as an additive at different molar concentrations (0.1, 0.25, and 0.5 M). Initially, a 0.1 M addition of LiBOB to the std. + EMC electrolyte (gray) improved capacity retention from 61.35 to 67.85%. A 0.25 M LiBOB addition (orange) proved to be the optimal composition, showing a capacity retention of 81.81% over the first 250 cycles. Increasing the LiBOB concentration further from 0.25 to 0.5 M (gold) saw a reduction in the maximum specific capacity from 1383 to 1289 mA h g–1 and a slight drop in capacity retention to 79.29%. The optimized 0.25 M LiBOB electrolyte (orange) improved the capacity fade rate threefold. The capacity of the std. cell (red) faded at a rate of 0.22% cycle–1, whereas the optimized “std. + EMC + 0.25 M LiBOB” cell (orange) exhibited a capacity fade rate of 0.07% cycle–1.
Figure 3.
(a) LiCoO2||Ge cells cycled at 40 °C varying the EMC/LiBOB content in the electrolyte. (b) LiCoO2||Ge cells cycled at −20 °C varying the PC content of the electrolyte. (c) Discharge capacity of LiCoO2||Ge cells at −40 and 0 °C with the std. electrolyte (red) and std. electrolyte + 30 wt % PC (purple). (d) Long-term cycling of LiCoO2||Ge cells at −40 °C (red), −20 °C (green), 0 °C (black), 20 °C (purple), and 40 °C (orange) with optimized electrolytes. All cells were cycled galvanostatically at a 1C rate.
To realize better low-temperature performance, PC was introduced to the standard electrolyte configuration. Figure 3b illustrates the effect that varying the PC content has on the capacity and long-term stability of LiCoO2||Ge cells cycled at −20 °C. Solutions of std. electrolyte + 10 wt % (blue), 30 wt % (green), and 50 wt % (gray) PC were tested and compared to the standard cell (red). The best-performing electrolyte compositions were found to be 10 wt % and 30 wt % PC, achieving 93.42 and 89.94% capacity retention after 200 cycles, respectively. Increasing the PC content further to 50 wt % led to a reduction in the overall cell capacity and accelerated capacity fade, retaining 69.65% capacity after 200 cycles. The poor performance of the PC-free cell can be related to the inability of the binary electrolyte to suppress electrolyte freezing at −20 °C. Images taken of the standard electrolyte and the standard electrolyte + 30 wt % PC show that at −20 °C, PC actively mitigates electrolyte freezing, which in turn promotes better cell performance (Figure S7a,b).
To analyze the versatility of this low-temperature electrolyte, its performance-enhancing ability was tested over a wider low-temperature range. In Figure 3c, the standard electrolyte cell (red) was tested versus the optimized 30 wt % PC cell (purple) at −40 and 0 °C. Similar to cells cycled at −20 °C, the introduction of PC to the electrolyte solution improves cell performance at −40 and 0 °C. At −40 °C, 30 wt % PC raised the max. capacity of the cell from 359 mA h g–1 (red) to 551 mA h g–1 (purple) and improved capacity retention from 40.11 to 54.79% after 200 cycles. At −40 °C, PC does not completely suppress electrolyte freezing (Figure S7c), leading to an increase in internal resistance and hindered Li-ion transport through the electrolyte. However, even at −40 °C, the PC-containing cell reaches 551 mA h g–1, 1.5× higher than the theoretical capacity for graphite @ RT. At 0 °C, the 30 wt % PC-containing cell increased capacity retention from 69.09 to 86.91%, over 200 cycles, slowing the capacity fade rate from 0.15 to 0.06% cycle–1.
Extended cycling of LiCoO2||Ge cells from −40 to 40 °C with the temperature-specific optimized electrolytes is shown in Figure 3d. Collectively, the discharge plots present the best-performing LiCoO2||Ge cells, employing the optimized electrolyte compositions for that temperature. At −20 °C (green), LiCoO2||Ge can function impressively at a 1C rate, reaching 1207 mA h g–1 with a capacity retention of 90.42% over 400 cycles. LiCoO2||Ge cells showed a similarly high capacity retention at 0 °C (black), retaining 87.11% after 400 cycles. LiCoO2||Ge cells with optimized electrolytes cycled at 20 °C (purple) and 40 °C (orange) retained a capacity of 82.08 and 64.51%, respectively. An interesting trend was observed as the cell temperature was reduced from 40 to −20 °C; the capacity retention improved. After 400 cycles, LiCoO2||Ge cells at 40, 20, 0, and −20 °C reached respective capacity retentions of 64.51, 82.08, 87.11, and 90.42%. Consequently, the rate of capacity fade was lowered with the lowering of cell temperature. This improved capacity retention at lower temperatures can be attributed predominantly to temperature-dependent Ge lithiation and delayed amorphization at low temperatures, which is analyzed later. Corresponding charge capacities and CEs for LiCoO2||Ge cells from −40 to 40 °C are given in Figure S8. Furthermore, voltage profiles for the 1st, 2nd, and final cycle of each cell are outlined in Figure S9.
Parallel testing of LiCoO2||C cells with these temperature-specific electrolytes yielded no notable improvements to cell performance (Figure S10). The inability of PC to improve the low-temperature performance of graphite-based cells can be attributed to the destructive behavior of PC on graphite. PC promotes solvent co-intercalation into the graphite structure, leading to poor performance and graphite decomposition.48,49 Even with PC, charging and discharging of LiCoO2||C cells are practically infeasible below −20 °C, returning 10% of Qth at −20 °C and 3% at −40 °C. The addition of EMC and LiBOB showed minimal improvement in LiCoO2||C capacity retention, increasing from 57.02 to 61.04%. The instability of the LiBOB-derived cell at 40 °C and the inability of PC to improve low-temperature performance suggest that the poor performance of graphite-based cells at temperature extremes cannot be easily rectified by modifying the electrolyte composition. Corresponding specific energies of LiCoO2||Ge and LiCoO2||C cells are depicted in Figure S11. At RT, LiCoO2||Ge delivers 174 W h kg–1 after 100 cycles, a 2× increase over that of LiCoO2||Ge (83 W h kg–1) at the same temperature. This disparity in RT specific energy of both cell types is further heightened at low temperatures, with LiCoO2||Ge returning a specific energy of 82 W h kg–1 at −40 °C, compared to 5 W h kg–1 for LiCoO2||C at −40 °C and equivalent to the specific energy of LiCoO2||C at RT (84 W h kg–1).
To examine the stabilizing effects of the high- and low-temperature additives, X-ray photoelectron spectroscopy (XPS) was employed to analyze the composition of the SEI. Ex situ XPS was employed to realize the exact chemical composition of the SEI layer and determine how the composition varied with the introduction of EMC/LiBOB and PC (Figure 4). XPS analysis of the effect of cycling temperature on the SEI composition using a standard electrolyte is given in Figure S12. First, XPS was carried out on Ge anodes after 25 cycles at 40 °C with and without EMC/LiBOB (Figure 4a–c). The relative concentrations of the chemical species in C1s spectra in (a) are quantified in (b). From the deconvoluted C1s spectra in (a), both SEI layers are composed predominantly of hydrocarbons (284.8 eV),50 Li ethers (286.2 eV),51 Li alkyl carbonates (287.2 eV),52 carboxylates/oxalates (288.9 eV),53 and Li2CO3/Poly(VC) (290.1 eV).54 In Figure 4a, the C1s spectra show concurrent signals for the Li2CO3/Li alkyl carbonates and poly(VC) peaks due to the carbon atoms existing in similar three-oxygen environments.52 Analysis of the deconvoluted O1s spectra for the two anode types shows the strong presence of a peak at 534 eV, indicative of poly(VC) incorporation into the SEI layer55 (Figure S13a). Poly(VC) is widely known as a vital component in the SEI layer, enhancing stability and flexibility of the passivation film.53,56−58 From the C1s spectra, the standard and the EMC/LiBOB-containing anodes do not differ drastically in their chemical makeup. The most notable difference is the emergence of a carbide B–C peak at 281 eV in the EMC/LiBOB-derived film, indicative of boron incorporation into the SEI layer.59 The analogous B–C peak can be observed at 189.1 eV in the deconvoluted B1s spectra shown in Figure S13c. The incorporation of boron into the SEI layer is demonstrated by the elemental composition data in Figure 4c. The incorporation of LiBOB as an additive to the LiPF6-based electrolyte reduced the overall fluorine content from 38.7 to 25.6% with boron accounting for 13.4% of the SEI layer upon LiBOB addition. Moreover, the F1s spectra show a higher LiF content for the EMC/LiBOB-containing cell, indicative of a more stable SEI layer60,61 (Figure S13b). A combination of partial fluorine substitution, boron incorporation, and greater LiF production collectively contributes to the enhanced capacity retention by stabilizing the SEI layer.
Figure 4.
(a–c) XPS analysis of the effect of EMC/LiBOB on the SEI layer composition at 40 °C. (a) High-resolution C1s spectra, (b) relative concentrations of chemical species from C1s spectra, and (c) elemental concentrations from the survey scan. (d–f) XPS analysis of the effect of PC on the SEI layer composition at −20 °C. (d) High-resolution C1s spectra, (e) relative concentrations of chemical species from C1s spectra, and (f) elemental concentrations from the survey scan.
Figure 4d–f quantifies the effect of introducing PC into the electrolyte mixture for cells cycled at −20 °C. The most notable difference between the two anode types in the C1s spectra is the presence of carbide species in the PC-free SEI layer (Figure 4d,e). Two peaks are present in the PC-free anode, a minor peak at 281.5 eV and a large peak at 283.8 eV, corresponding to carbide species.62 Carbide formation at low temperatures can be attributed to lithium decomposition at the anode’s edge.63 The introduction of PC was found to largely suppress carbide formation and reduce the relative concentration of the carbonate-containing species, indicative of less lithium being consumed at the anode surface. Additionally, PC was found to promote Li ether incorporation in the SEI layer. At −20 °C, Li ether is not found in the standard SEI (Figure 4d,e). In Figure 4f, the addition of PC did not lead to major variations in the elemental composition, as its addition does not introduce any additional elemental species. A notable difference between the standard and the PC-containing SEI layers is the concentration of the respective poly(VC) signals. The O1s and F1s spectra for the PC-free and PC-containing cells are shown in Figure S14. In the O1s spectra, poly(VC) accounts for 15.8% of the oxygen content in the standard SEI, which rises to 41.8% in the PC-containing SEI (Figure S14a,c). Additionally, from the F1s spectra (Figure S14b,d), the PC-containing SEI layer shows a higher LiF concentration than the standard SEI (75.4% compared to 51.9%). Enhanced low-temperature performance of PC-containing cells (as shown in Figure 3b) can therefore be collectively attributed to improving the stability of the SEI layer and suppressing electrolyte crystallization.
A combination of SEM, cyclic voltammetry (CV), and X-ray diffraction (XRD) was employed to elucidate the underlying mechanistic behavior of temperature-controlled morphology evolution of crystalline Ge NWs to a porous Ge framework (Figure 5). CV and SEM were utilized to track morphological changes in the Ge active material during cycling to determine whether the evolution of the porous network morphology is temperature-dependent. CV scans were taken from −40 to 40 °C, in 20 °C increments (Figure 5a–e). Cells were cycled for 25 cycles at a scan rate of 0.05 mV s–1. SEM images were taken of the corresponding Ge anodes after 25 cycles (Figure 5f–j). For consistency, the standard electrolyte was used throughout testing. Cells cycled at (a) −40, (b) −20, and (c) 0 °C all exhibit a gradual widening of the CV curves and an increase in peak intensity as the cell cycles. This curve widening is most prominent at −40 and −20 °C but still apparent at 0 °C. This gradual increase in peak intensity demonstrates the hindered lithiation/delithiation mechanics and slow activation of cell capacity at low temperatures. Conversely, cells cycled at (d) 20 and (e) 40 °C exhibit the characteristic electrochemical behavior for Ge-based cells. The 1st cycle involves a high degree of Li consumption and irreversible capacity loss to form the SEI layer. Successive cycles suffer reductions in peak intensity as cell capacity gradually decays. From the SEM images, a clear distinction is noted in the evolution of the Ge morphology. To better understand the transformation of individual NWs to discrete interconnected Ge islands, SEM images of pristine Ge NWs are provided in Figure S15. The characteristic porous Ge network34 is achieved after 25 cycles at (i) 20 and (j) 40 °C. As the cell temperature is lowered, the morphology of the anode approaches that of uncycled Ge NWs. At −40 °C, individual Ge NWs can still be seen after 25 cycles. SEM images are included to highlight morphological differences in post-cycled Ge NWs at temperature extremes, rather than incremental differences from temperature to temperature. Although variations in the post-cycled morphology of Ge NWs are not quantitative of phase change, they do highlight the impact of low temperatures in delaying mesh formation. The slow capacity activation of cells cycled at low temperatures combined with delayed morphology evolution highlights the critical role cell temperature plays in the performance of alloy-type anodes. Parallel testing of LiCoO2||Ge cells with optimized electrolytes revealed a similar trend in Ge morphology (Figure S16), suggesting that the evolution of the Ge morphology is driven predominantly by cell temperature rather than electrolyte composition.
Figure 5.
CVs of LiCoO2||Ge cells at (a) −40, (b) −20, (c) 0, (d) 20, and (e) 40 °C. Cells were cycled at a scan rate of 0.05 mV s–1 between 2.8 and 3.9 V. SEM images of Ge NWs after 25 cycles at (f) −40, (g) −20, (h) 0, (i) 20, and (j) 40 °C. Stacked X-ray diffraction patterns of Ge NWs (k) after the 1st charge at −40 °C (black), −20 °C (red), and 20 °C (blue), (l) after the 1st (red), 5th (light blue), and 25th (gold) charge at −20 °C, and (m) after the 1st (black), 5th (brown), and 25th (green) charge at −40 °C. The reference patterns for Li9Ge4 (green; JCPDS card no. 73-2487), Li15Ge4 (purple; JCPDS card no. 89-3034), and Ge (orange; JCPDS card no. 89-5011) are represented by the bars at the bottom of the graphs. The std. electrolyte was used for each test.
XRD analysis of lithiated Ge anodes [charged to 3.9 V at 0.05 C for 1 cycle at −40 °C (black), −20 °C (red), and 20 °C (blue)] revealed the temperature-dependent crystalline–amorphous phase transition of Li alloying materials (Figure 5k–m). At 20 °C, the crystalline–amorphous phase transition occurs during the first cycle, as evidenced by the attenuation of the Ge (111) peak and the emergence of LixGe peaks. Peaks at 22.7 and 23.9° represent the Li9Ge4 phase, whereas the peak at 20.6° corresponds to the higher order Li15Ge4 phase. On the other hand, at low temperatures, Ge amorphization is greatly suppressed. At −40 and −20 °C, crystalline Ge persists after the 1st charge cycle. Moreover, XRD reveals no apparent LixGe phases. After five cycles at −20 °C, Ge (111) can be still observed (Figure 5l). The crystalline Ge peak disappears after 25 cycles, accompanied by the emergence of a low-order Li9Ge4 peak at 23.9°. Analogous testing was carried out for Ge cycled at −40 °C (Figure 5m). Similar to −20 °C, the crystalline Ge (111) peak is present after five cycles. However, extended cycling found that the Ge (111) peak persists after 25 cycles, with no apparent LixGe peaks visible. This agrees well with Figure 5f, which shows the presence of individual intact Ge NWs after 25 cycles at −40 °C. Low temperatures delay Ge amorphization by suppressing lithiation and this behavior is intensified as the temperature is further lowered. The results explain the trends in Figure 5 where a slow capacity activation and delayed morphology evolution at low temperatures are attributed to suppression of Ge lithiation and amorphization. This delayed lithiation behavior of alloy-type materials bodes well for long-term stable cycling at low temperatures. From Figure 5l, cells cycled at −20 °C for 25 cycles exhibit no high-order Li15Ge4 phase with only the Li9Ge4 phase present after 25 cycles. Low temperatures may behave as a natural capacity limitation, setting an upper limit on cell capacity, suppressing complete lithiation of the Ge active material, and enhancing the long-term low-temperature stability of LiCoO2||Ge cells.
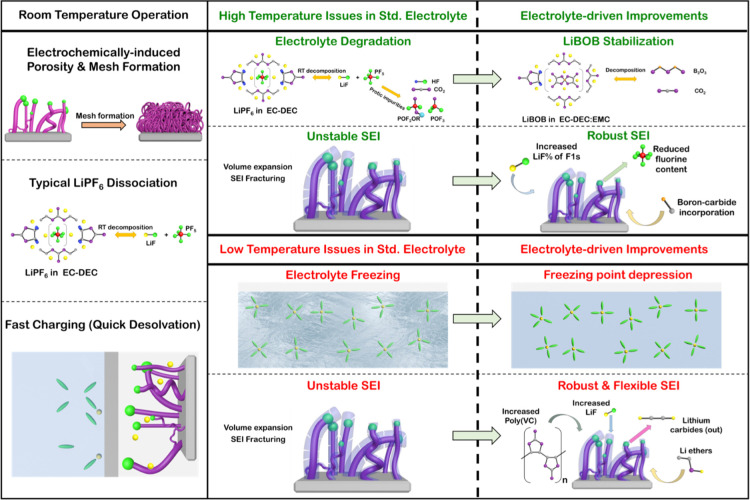
The electrolyte-driven enhancements of LiCoO2||Ge performance at both high and low temperatures are summarized in Figure 6. Rapid amorphization and mesh formation at RT improve SEI stability by generating a porous framework to facilitate large volume expansion during charge. At RT, stable dissociation of LiPF6 into LiF and PF6 aids SEI formation. A combination of short diffusion lengths and fast Li-ion desolvation mechanics promotes fast charging/discharging of Ge NWs at RT. HF/POF3 formation and gas evolution at high temperatures destabilize the cell through unstable SEI formation and eventual current collector corrosion. The dual addition of EMC and LiBOB improved high-temperature capacity retention dramatically by suppressing harmful byproduct formation through boron substitution and improved the stability of the SEI by increasing the LiF percentage of the fluorine composition and lowering the overall fluorine content. At low temperatures, the std. electrolyte freezes below −20 °C, dramatically affecting lithiation/delithiation. The introduction of PC played a dual role in suppressing electrolyte freezing and enhancing the flexibility and strength of the as-formed SEI through increases in the LiF and poly(VC) content and the incorporation of Li ethers and the suppression of lithium carbides. Modifying the electrolyte composition for temperature-specific operation can dramatically enhance the performance of Ge NW-based cells. Ensuring the stability of the electrolyte and the SEI layer facilitates stable cycling over the entire range of EV operation and beyond.
Figure 6.
Schematic summary of the temperature-dependent behavior of Ge NW-based cells. The high-temperature electrolyte instability and the low temperature freeing are overcome through modifications to the std. electrolyte, dramatically enhancing both cell capacity and capacity retention.
Conclusions
In this study, Ge NW-based Li-ion full cells were demonstrated to be extremely robust over a temperature range of −40 to 40 °C. The LiCoO2||Ge NW cells in a “standard” electrolyte adapted well to variations in cell temperature, showing high capacity and impressive cycling stability and vastly outperformed LiCoO2||graphite cells under the same conditions. Temperature-specific additives were shown to play a further role in enhancing the high- and low-temperature stability of LiCoO2||Ge cells. Propylene carbonate dramatically improved capacity retention at low temperature, by concurrently enhancing SEI flexibility and suppressing electrolyte crystallization. A binary additive mixture of EMC and lithium bis(oxalato)borate stabilized LiCoO2||Ge cells at elevated temperatures, through partial fluorine/boron substitution. This stabilizing behavior experienced by LiCoO2||Ge cells was not shown by LiCoO2||C cells, with electrolyte modifications showing no improvement in cell performance. The temperature-dependent performance of LiCoO2||Ge cells was further explained by the temperature-specific evolution of the anode morphology during cycling. We showed that delayed amorphization dramatically improved capacity retention by limiting complete lithiation during charge. The results show that alloying anodes in the form of LiCoO2||Ge cells function admirably at the extremes of temperatures experienced in transport (−40 to 40 °C), exhibiting excellent stability and high capacity and dramatically outperforming LiCoO2||C cells under the same conditions.
Experimental Section
Sample Preparation
Graphite slurries were prepared via a typical slurry processing technique. Graphite anodes consisted of 90 wt % graphite, 5 wt % carbon black, and 5 wt % carboxymethyl cellulose (CMC). Carbon black was homogeneously dispersed in a 1.5 wt % solution of CMC in H2O. The mixture was left to stir for 6 h. Graphite was added slowly and incrementally, with constant stirring to ensure homogeneity of the slurry mixture. The slurry was left to stir overnight prior to casting. Cu foil (purchased from Pi-Kem), with a thickness 9 μm, was used as the current collector. The slurry mixture was cast on the Cu foil using a heated vacuum doctor blade. A blade height of 25 μm achieved the desired 1.74 ± 0.13 mg cm–2 anode mass loading. The anode tape was dried under vacuum for 12 h@120 °C. Prior to cell assembly, anodes with an area of 0.64 cm2 were punched out and stored in an Ar-filled glovebox.
A previously published, rapid solvent-free protocol was employed for high-density growth of germanium NWs via a vapor–solid–solid (VSS) mechanism (Figure S17).64 Briefly, the reaction proceeds through thermal decomposition of the organometallic germanium precursor, diphenylgermane (DPG). Ge NWs were grown on stainless steel (SS), allowing them to be used directly as battery anodes. SS foil (purchased from Pi-Kem Ltd.) with a thickness of 0.1 mm was sanded prior to evaporation to increase the surface area and improve adhesion of the thermally evaporated catalytic film. The P600 grit sandpaper was used in the sanding process. Afterward, the SS substrates were washed in acetone to remove residual particles. Substrates with an area of 0.64 cm2 were punched out and a 2 nm coating of Cu (Kurt J. Lesker 99.999%) was thermally evaporated onto the SS substrate. The substrate was placed on a Stuart CD162 hotplate/digital stirrer at 420 °C in an argon-filled glovebox. To contain the decomposing vapor, a SS confiner was placed over the substrate. The Ge precursor was dropcast onto the substrate, by injecting it through the top of the confiner. High-density growth of Ge NWs was achieved after 30 s. The mass loading of the Ge NW substrates was found to correlate directly to the volume of the precursor used. An appropriate amount of DPG (2–10 μL) was dropcast onto the SS substrate, depending on the desired anode mass loading (0.25–0.62 mg cm–2). Anodes were stored under argon prior to cell assembly to minimize native oxide growth.
Cell Assembly and Electrochemical Analysis
Two-electrode Swagelok-type cells were assembled in an Ar-filled glovebox. LCO cathode tapes were purchased from NEI Corporation with an active mass loading of 6.2 mg cm–2. Cathodes with an area of 0.64 cm2 were punched out. No further treatment of the Ge or graphite anodes was required prior to cell assembly. Both anode types were matched in terms of areal capacity with a constant PN ratio for both cell types (LiCoO2||C and LiCoO2||Ge). A PN ratio of 1.5 was kept constant throughout testing. The PN ratio denotes the capacity ratio between the positive (P) and negative (N) electrodes. Previous work by our group highlighted the importance of capacity balancing for full-cell configurations.65 For PN1.5 cells, the active mass loadings of Ge and graphite were set at 0.42 and 1.57 mg cm–2, respectively. The electrodes were separated from each other using a porous polypropylene Celgard separator paper. A total of 1 M LiPF6 in EC–DEC (1:1 v/v) + VC (3 wt %) was chosen as the standard (std.) electrolyte throughout testing with modifications made to the solution for high- and low-temperature cycling. The electrochemical performances of these cells were analyzed through galvanostatic cycling within a 2.8–3.9 V potential window, using a BioLogic MPG-2 multichannel potentiostat. For low-temperature cycling (−40 to 0 °C), the PC content of the electrolyte was varied. Electrolyte solutions (1 M LiPF6 in EC–DEC (1:1) + 3 wt % VC) with 0, 10, 30, and 50 wt % PC were made up and tested as potential electrolyte compositions for low-temperature cycling. EMC and LiBOB were tested as high-temperature additives. Initially, 1 M LiPF6 in EC–DEC–EMC (1:1:1) + 3 wt % VC was formulated and denoted as “std. + EMC”. LiBOB was tested in different molar concentrations (0.1, 0.25, and 0.5 M) by dissolving it in a ternary EC–DEC–EMC (1:1:1) mixture and incorporating it into the std. + EMC solution.
Analogous to rate capability testing, temperature capability testing increased cell temperature stepwise, analyzing the electrochemical response of the cell to variations in cell temperature. Using a combination of freezers and incubators, cells were first cycled for five cycles at −40 °C. After five cycles, temperature was raised to −20 °C and the cell was left idle on discharge overnight to allow for temperature equalization. This was repeated up as far as 40 °C, with the temperature increased in 20 °C increments after every five cycles. Cell response was analyzed by plotting discharge capacity as a function of cell temperature from −40 to 40 °C. EIS was carried out using Biologic BT-Lab V1.65 software. Cells were subjected to low current slow partial charge to 3.7 V to stabilize the open-circuit potential. The cells were subsequently allowed to relax to the open circuit, until the potential drift became low enough to remove DC bias. The EIS spectra were then recorded with a peak amplitude of 10 mV, between 20 mHz and 100 kHz. Equivalent circuit models were then fitted to the EIS data using the EIS fitting function of Biologic BT-Lab V1.65 software.
Ex Situ Characterization
SEM was carried out using a Hitachi SU-70 system with an accelerating voltage of 8 kV. The cells were disassembled in an Ar-filed glovebox. The electrode was soaked in acetonitrile overnight before being successively washed in 0.1 mM acetic acid, deionized water, and ethanol. This process removes the SEI layer on the anode surface along with any lingering electrolyte material and residual lithium content.66 XRD was achieved using a PANalytical X’Pert PRO MRD with a radiation source consisting of Cu Kα (λ = 1.5418 Å) and an X’celerator detector. To analyze lithiated Ge anodes under XRD, atmospheric oxidation had to be kept at a minimum. This was achieved by taping the Ge anodes to glass slides using Scotch tape. The tape ensured that the Ge anodes remained in the charged state and removed contact with the atmosphere. Background scans of the blank glass slide with Scotch tape allowed for the identification of crystalline peaks associated with the samples. XPS was carried out using the Kratos AXIS ULTRA spectrometer with Al Kα 1486.58 eV. Prior to XPS, substrate pre-treatment involved washing off the residual electrolyte by immersing the anode in dimethyl carbonate (DMC) for 1 h and allowing it to dry in an Ar environment. The core level binding energies were determined by taking the charge reference of C1s at 284.8 eV. The narrow regions were analyzed using 20 eV with the survey spectra analyzed with a pass energy of 160 eV. The broad peaks were fitted using a Gaussian–Lorenzian mixed-type fit with the narrow regions constructed using a Shirley-type background.
Acknowledgments
This work was made possible by the joint financial support of the Irish Research Council (IRC) and Intel Ireland, under the IRC Enterprise Award Scheme (contract no. EPSPG/2017/233). H.G. acknowledges SIRG under grant 18/SIRG/5484. This work was further supported by the Science Foundation Ireland (SFI) under the Principal Investigator Program to K.M.R. under contract no. 16/IA/4629 and under grant no. SFI 16/M-ERA/3419. K.M.R. further acknowledges IRCLA/2017/285 and SFI Research Centres MaREI, AMBER and CONFIRM 12/RC/2278_P2, 12/RC/2302_P2, and 16/RC/3918.
Glossary
Abbreviations
- CE
coulombic efficiency
- CMC
carboxymethyl cellulose
- CV
cyclic voltammetry
- DCP
differential capacity plot
- DEC
diethyl carbonate
- DMC
dimeythl carbonate
- DPG
diphenylgermane
- EC
ethyl carbonate
- EIS
electrochemical impedance spectroscopy
- EMC
ethyl methyl carbonate
- EV
electric vehicle
- ICE
internal combustion engine
- LiBOB
lithium bis(oxalato)borate
- NW
nanowire
- PC
propylene carbonate
- Qth
theoretical capacity
- RT
room temperature
- RCT
rate capability testing
- RCT
charge transfer resistance
- SEI
solid electrolyte interphase
- SS
stainless steel
- TEM
transmission electron microscopy
- VSS
vapor–solid–solid
- XPS
X-ray photoelectron spectroscopy
- XRD
X-ray diffraction
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsaem.0c02928.
Performance comparison of LiCoO2||Ge NWs to published work of similar materials, first-cycle DCPs of LiCoO2||Ge cells, temperature capability testing of LiCoO2||Ge (red) and LiCoO2||C (blue) cells, and Nyquist plots of LiCoO2||C cells and LiCoO2||Ge cells (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Ramadass P.; Haran B.; White R.; Popov B. N. Capacity fade of Sony 18650 cells cycled at elevated temperatures: Part I. Cycling performance. J. Power Sources 2002, 112, 606–613. 10.1016/s0378-7753(02)00474-3. [DOI] [Google Scholar]
- Kawamura T.; Okada S.; Yamaki J.-i. Decomposition reaction of LiPF6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. 10.1016/j.jpowsour.2005.05.084. [DOI] [Google Scholar]
- Shojaei S.; McGordon A.; Robinson S.; Marco J. Improving the Performance Attributes of Plug-in Hybrid Electric Vehicles in Hot Climates through Key-Off Battery Cooling. Energies 2017, 10, 2058. 10.3390/en10122058. [DOI] [Google Scholar]
- Jeffs J.; McGordon A.; Widanage W. D.; Robinson S.; Picarelli A. In Use of a thermal battery with a heat pump for low temperature electric vehicle operation. 2017 IEEE Vehicle Power and Propulsion Conference (VPPC); IEEE, 2017; pp 1–5.
- Roberts A.; Brooks R.; Shipway P. Internal combustion engine cold-start efficiency: A review of the problem, causes and potential solutions. Energy Convers. Manage. 2014, 82, 327–350. 10.1016/j.enconman.2014.03.002. [DOI] [Google Scholar]
- Gumus M. Reducing cold-start emission from internal combustion engines by means of thermal energy storage system. Appl. Therm. Eng. 2009, 29, 652–660. 10.1016/j.applthermaleng.2008.03.044. [DOI] [Google Scholar]
- Li Q.; Jiao S.; Luo L.; Ding M. S.; Zheng J.; Cartmell S. S.; Wang C.-M.; Xu K.; Zhang J.-G.; Xu W. Wide-temperature electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. 10.1021/acsami.7b04099. [DOI] [PubMed] [Google Scholar]
- Li Q.; Lu D.; Zheng J.; Jiao S.; Luo L.; Wang C.-M.; Xu K.; Zhang J.-G.; Xu W. Li+-desolvation dictating lithium-ion battery’s low-temperature performances. ACS Appl. Mater. Interfaces 2017, 9, 42761–42768. 10.1021/acsami.7b13887. [DOI] [PubMed] [Google Scholar]
- Xu K.; Zhang S.; Jow T. R.; Xu W.; Angell C. A. LiBOB as salt for lithium-ion batteries: a possible solution for high temperature operation. Electrochem. Solid-State Lett. 2002, 5, A26–A29. 10.1149/1.1426042. [DOI] [Google Scholar]
- Yang H.; Zhuang G. V.; Ross P. N. Jr Thermal stability of LiPF6 salt and Li-ion battery electrolytes containing LiPF6. J. Power Sources 2006, 161, 573–579. 10.1016/j.jpowsour.2006.03.058. [DOI] [Google Scholar]
- Sayed F. N.; Rodrigues M.-T. F.; Kalaga K.; Gullapalli H.; Ajayan P. M. Curious case of positive current collectors: Corrosion and passivation at high temperature. ACS Appl. Mater. Interfaces 2017, 9, 43623–43631. 10.1021/acsami.7b12675. [DOI] [PubMed] [Google Scholar]
- Gnanaraj J. S.; Zinigrad E.; Asraf L.; Gottlieb H. E.; Sprecher M.; Schmidt M.; Geissler W.; Aurbach D. A detailed investigation of the thermal reactions of LiPF6 solution in organic carbonates using ARC and DSC. J. Electrochem. Soc. 2003, 150, A1533–A1537. 10.1149/1.1617301. [DOI] [Google Scholar]
- Krause L. J.; Lamanna W.; Summerfield J.; Engle M.; Korba G.; Loch R.; Atanasoski R. Corrosion of aluminum at high voltages in non-aqueous electrolytes containing perfluoroalkylsulfonyl imides; new lithium salts for lithium-ion cells. J. Power Sources 1997, 68, 320–325. 10.1016/s0378-7753(97)02517-2. [DOI] [Google Scholar]
- Zhang S. S.; Xu K.; Jow T. R. LiBOB-based gel electrolyte Li-ion battery for high temperature operation. J. Power Sources 2006, 154, 276–280. 10.1016/j.jpowsour.2005.03.196. [DOI] [Google Scholar]
- Zinigrad E.; Larush-Asraf L.; Salitra G.; Sprecher M.; Aurbach D. On the thermal behavior of Li bis (oxalato) borate LiBOB. Thermochim. Acta 2007, 457, 64–69. 10.1016/j.tca.2007.03.001. [DOI] [Google Scholar]
- Kim Y. Investigation of the gas evolution in lithium ion batteries: effect of free lithium compounds in cathode materials. J. Solid State Electrochem. 2013, 17, 1961–1965. 10.1007/s10008-013-2050-2. [DOI] [Google Scholar]
- Fan J. On the discharge capability and its limiting factors of commercial 18650 Li-ion cell at low temperatures. J. Power Sources 2003, 117, 170–178. 10.1016/s0378-7753(03)00354-9. [DOI] [Google Scholar]
- Senyshyn A.; Mühlbauer M. J.; Dolotko O.; Ehrenberg H. Low-temperature performance of Li-ion batteries: The behavior of lithiated graphite. J. Power Sources 2015, 282, 235–240. 10.1016/j.jpowsour.2015.02.008. [DOI] [Google Scholar]
- Nobili F.; Mancini M.; Dsoke S.; Tossici R.; Marassi R. Low-temperature behavior of graphite–tin composite anodes for Li-ion batteries. J. Power Sources 2010, 195, 7090–7097. 10.1016/j.jpowsour.2010.05.001. [DOI] [Google Scholar]
- Zhang S. S.; Xu K.; Jow T. R. Low temperature performance of graphite electrode in Li-ion cells. Electrochim. Acta 2002, 48, 241–246. 10.1016/s0013-4686(02)00620-5. [DOI] [Google Scholar]
- Zhang S. S.; Xu K.; Jow T. R. Charge and discharge characteristics of a commercial LiCoO2-based 18650 Li-ion battery. J. Power Sources 2006, 160, 1403–1409. 10.1016/j.jpowsour.2006.03.037. [DOI] [Google Scholar]
- Zhang S. S.; Xu K.; Jow T. R. Electrochemical impedance study on the low temperature of Li-ion batteries. Electrochim. Acta 2004, 49, 1057–1061. 10.1016/j.electacta.2003.10.016. [DOI] [Google Scholar]
- Wang B.; Li X.; Qiu T.; Luo B.; Ning J.; Li J.; Zhang X.; Liang M.; Zhi L. High volumetric capacity silicon-based lithium battery anodes by nanoscale system engineering. Nano Lett. 2013, 13, 5578–5584. 10.1021/nl403231v. [DOI] [PubMed] [Google Scholar]
- Tarascon J.-M.; Armand M.. Issues and challenges facing rechargeable lithium batteries. In Materials for Sustainable Energy: A Collection of Peer-Reviewed Research and Review Articles from Nature Publishing Group; World Scientific, 2011; pp 171–179. [Google Scholar]
- Geaney H.; Bree G.; Stokes K.; Collins G. A.; Aminu I. S.; Kennedy T.; Ryan K. M. Enhancing the performance of germanium nanowire anodes for Li-ion batteries by direct growth on textured copper. Chem. Commun. 2019, 55, 7780–7783. 10.1039/c9cc03579f. [DOI] [PubMed] [Google Scholar]
- Stokes K.; Geaney H.; Sheehan M.; Borsa D.; Ryan K. M. Copper Silicide Nanowires as Hosts for Amorphous Si Deposition as a Route to Produce High Capacity Lithium-Ion Battery Anodes. Nano Lett. 2019, 19, 8829–8835. 10.1021/acs.nanolett.9b03664. [DOI] [PubMed] [Google Scholar]
- Son I. H.; Park J. H.; Kwon S.; Park S.; Rümmeli M. H.; Bachmatiuk A.; Song H. J.; Ku J.; Choi J. W.; Choi J.-m. Silicon carbide-free graphene growth on silicon for lithium-ion battery with high volumetric energy density. Nat. Commun. 2015, 6, 7393. 10.1038/ncomms8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane E.; Kennedy T.; Geaney H.; Dickinson C.; Ryan K. M. Synthesis of tin catalyzed silicon and germanium nanowires in a solvent–vapor system and optimization of the seed/nanowire interface for dual lithium cycling. Chem. Mater. 2013, 25, 1816–1822. 10.1021/cm400367v. [DOI] [Google Scholar]
- Doherty J.; McNulty D.; Biswas S.; Moore K.; Conroy M.; Bangert U.; O’Dwyer C.; Holmes J. D. Germanium tin alloy nanowires as anode materials for high performance Li-ion batteries. Nanotechnology 2020, 31, 165402. 10.1088/1361-6528/ab6678. [DOI] [PubMed] [Google Scholar]
- Profatilova I. A.; Stock C.; Schmitz A.; Passerini S.; Winter M. Enhanced thermal stability of a lithiated nano-silicon electrode by fluoroethylene carbonate and vinylene carbonate. J. Power Sources 2013, 222, 140–149. 10.1016/j.jpowsour.2012.08.066. [DOI] [Google Scholar]
- Chan C. K.; Peng H.; Liu G.; McIlwrath K.; Zhang X. F.; Huggins R. A.; Cui Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31. 10.1038/nnano.2007.411. [DOI] [PubMed] [Google Scholar]
- Peng K.; Jie J.; Zhang W.; Lee S.-T. Silicon nanowires for rechargeable lithium-ion battery anodes. Appl. Phys. Lett. 2008, 93, 033105. 10.1063/1.2929373. [DOI] [Google Scholar]
- Chockla A. M.; Klavetter K. C.; Mullins C. B.; Korgel B. A. Solution-grown germanium nanowire anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 4658–4664. 10.1021/am3010253. [DOI] [PubMed] [Google Scholar]
- Kennedy T.; Mullane E.; Geaney H.; Osiak M.; O’Dwyer C.; Ryan K. M. High-performance germanium nanowire-based lithium-ion battery anodes extending over 1000 cycles through in situ formation of a continuous porous network. Nano Lett. 2014, 14, 716–723. 10.1021/nl403979s. [DOI] [PubMed] [Google Scholar]
- Kim H.; Son Y.; Park C.; Cho J.; Choi H. C. Catalyst-free direct growth of a single to a few layers of graphene on a germanium nanowire for the anode material of a lithium battery. Angew. Chem., Int. Ed. 2013, 52, 5997–6001. 10.1002/anie.201300896. [DOI] [PubMed] [Google Scholar]
- Lin H.-P.; Chua D.; Salomon M.; Shiao H.-C.; Hendrickson M.; Plichta E.; Slane S. Low-temperature behavior of Li-ion cells. Electrochem. Solid-State Lett. 2001, 4, A71. 10.1149/1.1368736. [DOI] [Google Scholar]
- Friesen A.; Horsthemke F.; Mönnighoff X.; Brunklaus G.; Krafft R.; Börner M.; Risthaus T.; Winter M.; Schappacher F. M. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J. Power Sources 2016, 334, 1–11. 10.1016/j.jpowsour.2016.09.120. [DOI] [Google Scholar]
- Cho H.-M.; Choi W.-S.; Go J.-Y.; Bae S.-E.; Shin H.-C. A study on time-dependent low temperature power performance of a lithium-ion battery. J. Power Sources 2012, 198, 273–280. 10.1016/j.jpowsour.2011.09.111. [DOI] [Google Scholar]
- Yamaki J.; Takatsuji H.; Kawamura T.; Egashira M. Thermal stability of graphite anode with electrolyte in lithium-ion cells. Solid State Ionics 2002, 148, 241–245. 10.1016/s0167-2738(02)00060-7. [DOI] [Google Scholar]
- Lux S. F.; Lucas I. T.; Chevalier J. S.; Richardson T. J.; Kostecki R. M. Time-dependent determination of hf formation in lipf6-containing electrolytes in different cell types by spectroscopic ellipsometry. ECS Trans. 2013, 50, 27. 10.1149/05001.0027ecst. [DOI] [Google Scholar]
- Myung S.-T.; Hitoshi Y.; Sun Y.-K. Electrochemical behavior and passivation of current collectors in lithium-ion batteries. J. Mater. Chem. 2011, 21, 9891–9911. 10.1039/c0jm04353b. [DOI] [Google Scholar]
- Xiao X.; Li X.; Zheng S.; Shao J.; Xue H.; Pang H. Nanostructured germanium anode materials for advanced rechargeable batteries. Adv. Mater. Interfaces 2017, 4, 1600798. 10.1002/admi.201600798. [DOI] [Google Scholar]
- Vanhellemont J.; Śpiewak P.; Sueoka K.. On the Solubility and Diffusivity of the Intrinsic Point Defects in Germanium; American Institute of Physics, 2007. [Google Scholar]
- Yuan F.-W.; Tuan H.-Y. Scalable solution-grown high-germanium-nanoparticle-loading graphene nanocomposites as high-performance lithium-ion battery electrodes: an example of a graphene-based platform toward practical full-cell applications. Chem. Mater. 2014, 26, 2172–2179. 10.1021/cm5002016. [DOI] [Google Scholar]
- Baasner A.; Reuter F.; Seidel M.; Krause A.; Pflug E.; Härtel P.; Dörfler S.; Abendroth T.; Althues H.; Kaskel S. The Role of Balancing Nanostructured Silicon Anodes and NMC Cathodes in Lithium-Ion Full-Cells with High Volumetric Energy Density. J. Electrochem. Soc. 2020, 167, 020516. 10.1149/1945-7111/ab68d7. [DOI] [Google Scholar]
- Forney M. W.; Ganter M. J.; Staub J. W.; Ridgley R. D.; Landi B. J. Prelithiation of silicon–carbon nanotube anodes for lithium ion batteries by stabilized lithium metal powder (SLMP). Nano Lett. 2013, 13, 4158–4163. 10.1021/nl401776d. [DOI] [PubMed] [Google Scholar]
- Klett M.; Gilbert J. A.; Trask S. E.; Polzin B. J.; Jansen A. N.; Dees D. W.; Abraham D. P. Electrode behavior RE-visited: monitoring potential windows, capacity loss, and impedance changes in Li1. 03 (Ni0. 5Co0. 2Mn0. 3) 0.97 O2/silicon-graphite full cells. J. Electrochem. Soc. 2016, 163, A875. 10.1149/2.0271606jes. [DOI] [Google Scholar]
- Chung G.-C.; Kim H.-J.; Yu S.-I.; Jun S.-H.; Choi J.-w.; Kim M.-H. Origin of graphite exfoliation an investigation of the important role of solvent cointercalation. J. Electrochem. Soc. 2000, 147, 4391. 10.1149/1.1394076. [DOI] [Google Scholar]
- Abe T.; Kawabata N.; Mizutani Y.; Inaba M.; Ogumi Z. Correlation between cointercalation of solvents and electrochemical intercalation of lithium into graphite in propylene carbonate solution. J. Electrochem. Soc. 2003, 150, A257. 10.1149/1.1541004. [DOI] [Google Scholar]
- Beamson G.High resolution XPS of organic polymers. The Scienta ESCA 300 Database, 1992.
- Andersson A. M.; Herstedt M.; Bishop A. G.; Edström K. The influence of lithium salt on the interfacial reactions controlling the thermal stability of graphite anodes. Electrochim. Acta 2002, 47, 1885–1898. 10.1016/s0013-4686(02)00044-0. [DOI] [Google Scholar]
- Dedryvère R.; Gireaud L.; Grugeon S.; Laruelle S.; Tarascon J.-M.; Gonbeau D. Characterization of lithium alkyl carbonates by X-ray photoelectron spectroscopy: experimental and theoretical study. J. Phys. Chem. B 2005, 109, 15868–15875. 10.1021/jp051626k. [DOI] [PubMed] [Google Scholar]
- Dalavi S.; Guduru P.; Lucht B. L. Performance enhancing electrolyte additives for lithium ion batteries with silicon anodes. J. Electrochem. Soc. 2012, 159, A642. 10.1149/2.076205jes. [DOI] [Google Scholar]
- Etacheri V.; Haik O.; Goffer Y.; Roberts G. A.; Stefan I. C.; Fasching R.; Aurbach D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes. Langmuir 2012, 28, 965–976. 10.1021/la203712s. [DOI] [PubMed] [Google Scholar]
- Kennedy T.; Brandon M.; Laffir F.; Ryan K. M. Understanding the influence of electrolyte additives on the electrochemical performance and morphology evolution of silicon nanowire based lithium-ion battery anodes. J. Power Sources 2017, 359, 601–610. 10.1016/j.jpowsour.2017.05.093. [DOI] [Google Scholar]
- El Ouatani L.; Dedryèvre R.; Siret C.; Biensan P.; Reynaud S.; Iratçabal P.; Gonbeau D. The effect of vinylene carbonate additive on surface film formation on both electrodes in Li-ion batteries. J. Electrochem. Soc. 2009, 156, A103–A113. 10.1149/1.3029674. [DOI] [Google Scholar]
- Michan A. L.; Parimalam B. S.; Leskes M.; Kerber R. N.; Yoon T.; Grey C. P.; Lucht B. L. Fluoroethylene carbonate and vinylene carbonate reduction: Understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chem. Mater. 2016, 28, 8149–8159. 10.1021/acs.chemmater.6b02282. [DOI] [Google Scholar]
- Brown Z. L.; Jurng S.; Lucht B. L. Investigation of the Lithium Solid Electrolyte Interphase in Vinylene Carbonate Electrolytes Using Cu|| LiFePO4 Cells. J. Electrochem. Soc. 2017, 164, A2186. 10.1149/2.0021712jes. [DOI] [Google Scholar]
- Jacobsohn L. G.; Schulze R. K.; Maia da Costa M. E. H.; Nastasi M. X-ray photoelectron spectroscopy investigation of boron carbide films deposited by sputtering. Surf. Sci. 2004, 572, 418–424. 10.1016/j.susc.2004.09.020. [DOI] [Google Scholar]
- Wang M.; Peng Z.; Luo W.; Ren F.; Li Z.; Zhang Q.; He H.; Ouyang C.; Wang D. Tailoring lithium deposition via an SEI-functionalized membrane derived from LiF decorated layered carbon structure. Adv. Energy Mater. 2019, 9, 1802912. 10.1002/aenm.201802912. [DOI] [Google Scholar]
- Zhang L.; Zhang K.; Shi Z.; Zhang S. LiF as an artificial SEI layer to enhance the high-temperature cycle performance of Li4Ti5O12. Langmuir 2017, 33, 11164–11169. 10.1021/acs.langmuir.7b02031. [DOI] [PubMed] [Google Scholar]
- Ismail I.; Noda A.; Nishimoto A.; Watanabe M. XPS study of lithium surface after contact with lithium-salt doped polymer electrolytes. Electrochim. Acta 2001, 46, 1595–1603. 10.1016/s0013-4686(00)00758-1. [DOI] [Google Scholar]
- Ng B.; Coman P. T.; Faegh E.; Peng X.; Karakalos S. G.; Jin X.; Mustain W. E.; White R. E. Low-Temperature Lithium Plating/Corrosion Hazard in Lithium-Ion Batteries: Electrode Rippling, Variable States of Charge, and Thermal and Nonthermal Runaway. ACS Appl. Energy Mater. 2020, 3, 3653–3664. 10.1021/acsaem.0c00130. [DOI] [Google Scholar]
- Mullane E.; Kennedy T.; Geaney H.; Ryan K. M. A rapid, solvent-free protocol for the synthesis of germanium nanowire lithium-ion anodes with a long cycle life and high rate capability. ACS Appl. Mater. Interfaces 2014, 6, 18800–18807. 10.1021/am5045168. [DOI] [PubMed] [Google Scholar]
- Geaney H.; Bree G.; Stokes K.; McCarthy K.; Kennedy T.; Ryan K. M. Highlighting the importance of full-cell testing for high performance anode materials comprising Li alloying nanowires. J. Electrochem. Soc. 2019, 166, A2784–A2790. 10.1149/2.0291913jes. [DOI] [Google Scholar]
- Kim G.-T.; Kennedy T.; Brandon M.; Geaney H.; Ryan K. M.; Passerini S.; Appetecchi G. B. Behavior of germanium and silicon nanowire anodes with ionic liquid electrolytes. ACS Nano 2017, 11, 5933–5943. 10.1021/acsnano.7b01705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.