Abstract
Global coastal oceans as a whole represent an important carbon sink but, due to high spatial–temporal variability, a mechanistic conceptualization of the coastal carbon cycle is still under development, hindering the modelling and inclusion of coastal carbon in Earth System Models. Although temperature is considered an important control of sea surface pCO2, we show that the latitudinal distribution of global coastal surface pCO2 does not match that of temperature, and its inter-seasonal changes are substantially regulated by non-thermal factors such as water mass mixing and net primary production. These processes operate in both ocean-dominated and river-dominated margins, with carbon and nutrients sourced from the open ocean and land, respectively. These can be conceptualized by a semi-analytical framework that assesses the consumption of dissolved inorganic carbon relative to nutrients, to determine how a coastal system is a CO2 source or sink. The framework also finds utility in accounting for additional nutrients in organic forms and testing hypotheses such as using Redfield stoichiometry, and is therefore an essential step toward comprehensively understanding and modelling the role of the coastal ocean in the global carbon cycle.
Keywords: CO2 dynamics and fluxes, coastal ocean, ocean-dominated margin, river-dominated ocean margin, carbon cycle
INTRODUCTION
The coastal ocean, connecting the open ocean and the continents, provides important ecosystem services, particularly as a natural sink of atmospheric CO2 that contributes to ∼20% of the global ocean carbon uptake [1,2]. The coastal ocean is dynamic due to various physical and biogeochemical processes and its fluxes of carbon and other biogenic elements are highly variable in both time and space [3]. Quantifying the inventories and fluxes of coastal ocean carbon, essential for understanding the role of the global carbon cycle in climate variability and change, however, has been a long-standing challenge. As a result, the coastal ocean has been poorly represented in Earth System Models, in terms of both process understanding and temporal–spatial resolution [4,5]. Recent studies have further highlighted the susceptibility of coastal systems to increasingly intensified anthropogenic perturbations [6,7], adding to the challenge of understanding the already complex coastal carbon cycle and projecting its changes into the future.
Although available research has focused on carbon fluxes across several boundaries at the land–ocean interface, such as estuary–marsh interactions [3,8,9], knowledge of exchanges between ocean margins and the open ocean remains insufficient [3]. Carbon export from the coastal to open ocean was estimated as 0.75 Pg C yr−1 based on mass balance calculations, but a direct global estimate of the boundary exchanges between the coastal and open ocean is still unattainable due to data paucity [6]. The long-term wintertime data of the partial pressure of CO2 (pCO2) suggest a tendency of enhanced uptake of atmospheric CO2 in many shelf regions [10], which likely exports more dissolved inorganic carbon (DIC) to the open ocean. If this is a global trend, the uptake and export processes in coastal oceans should be included in future models of sea–air CO2 exchange [7]. In short, more efforts have focused on the river-estuarine input than the fundamental two-way or 3D exchange between ocean margins and the open ocean.
Furthermore, although the CO2 sink of coastal oceans is approaching a consensus value of 0.15–0.40 Pg C yr−1, whether a coastal ocean is a source or a sink of atmospheric CO2 varies vastly from one coastal system to another [11–19]. Based on a general latitudinal distribution featuring lower seawater pCO2 and thus strong sinks at high latitudes, and high seawater pCO2 and thus sources at low latitudes [12,17,20], temperature-induced changes in the solubility of CO2 have been considered a major control (i.e. thermal control) of coastal ocean CO2 fluxes. However, there are also other non-thermal factors at play, particularly water mass mixing (e.g. CO2 supplied by upwelling) and net primary production, resulting in deviations from this latitudinal distribution [17,19,21]. Understanding these processes in individual systems is a prerequisite for properly modelling the role of the coastal ocean in the global carbon cycle.
Thus, despite great efforts devoted to observing individual coastal systems [22–31] and synthesizing the global coastal CO2 fluxes [11–19] over the past decades, precisely modelling coastal ocean carbon either using regional models [32] or in a more comprehensive way integrating all domains of Earth’s surface [33] remains difficult, mainly due to unknown or oversimplified processes and fluxes in a highly dynamic setting [4,34]. Before an ultimate comprehensive model for predicting future coastal carbon trends at both regional and global scales can be developed, it is useful to establish a conceptual model of the coastal carbon cycle that makes full use of the limited available observations to advance our understanding of physical and biogeochemical processes.
In this study, we show the importance of non-thermal factors on coastal pCO2 and CO2 fluxes. We establish a semi-analytical framework to understand these non-thermal processes and to diagnose the CO2 source/sink nature of coastal oceans. By applying the framework to different coastal systems, we highlight the complexity of issues associated with coastal CO2 dynamics and fluxes, and demonstrate the utility of the framework, particularly in identifying pathways that advance our comprehensive understanding and modelling of the coastal carbon cycle.
RESULTS AND DISCUSSION
Non-thermal controls on coastal pCO2
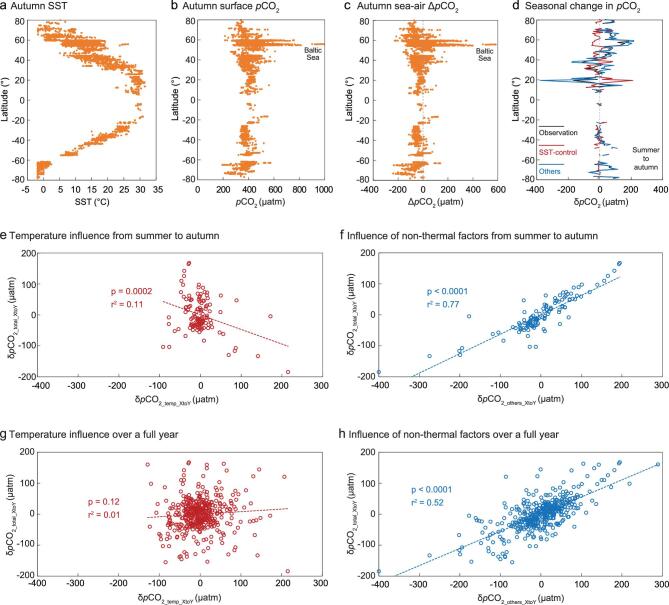
To illustrate the relative importance of temperature and non-thermal factors to coastal CO2 dynamics and fluxes, we use sea surface temperature (SST), pCO2 and sea–air ΔpCO2 (defined as the difference in pCO2 between the sea and the air, or pCO2_sea − pCO2_air) (Fig. 1a–c) data collected at distances of 50 and 100 km from the global shoreline during all four seasons (Supplementary Figs 1, 2 and 3 and Supplementary Section 1.1; see the ‘Methods’ section). Similarly to previous studies synthesizing global coastal CO2 fluxes [10,17,19], these data mainly represent observations in continental margins consisting of shelf, slope and adjacent marginal seas and excluding nearshore ecosystems in internal waters such as estuaries, lagoons or tidal marshes. While SST clearly shows high values at low latitudes and low values at high latitudes, pCO2 and sea–air ΔpCO2 have a much less defined pattern during each season (Fig. 1a–c and Supplementary Figs 1 and 2). In autumn at 50 km from the shore, for instance, there is no clear latitudinal pattern of pCO2, with comparable average values between low and high latitudes (Fig. 1b). In the Baltic Sea at ∼55°N, pCO2 even approaches 1000 μatm. This leads to extremely high sea–air ΔpCO2 of ∼600 μatm, suggesting that this high-latitude coastal system is a CO2 source (Fig. 1b and c).
Figure 1.
Evidence for non-thermal controls on partial pressure of CO2 (pCO2) in global coastal oceans. (a)–(c) Latitudinal distribution of sea surface temperature (SST), surface pCO2 and sea–air ΔpCO2 (defined as the difference in pCO2 between the sea and the air, or pCO2_sea − pCO2_air) in autumn. (d) Seasonal change in pCO2 (δpCO2) from summer to autumn, averaged over a 1°-latitude band; black, red and blue lines indicate δpCO2 from field observations (δpCO2_total_XtoY; XtoY means from X season to Y season; Equation (1)), solely due to temperature variations (δpCO2_temp_XtoY; Equations (2) and (3)) and controlled by other factors (δpCO2_others_XtoY; Equation (4)) such as mixing and biogeochemical processes. (e) and (f) Relationship of δpCO2_total_XtoY (black curve in (d)) to δpCO2_temp_XtoY (red curve in (d)) and δpCO2_others_XtoY (blue curve in (d)) from summer to autumn, based on departures from the average, showing that cooler SSTs in fact lead to a higher δpCO2_total_XtoY, opposite to the argument of a temperature control over this seasonal transition. Instead, non-thermal factors, such as mixing and biogeochemical processes, control δpCO2_total_XtoY. (g) and (h) Relationship of δpCO2_total_XtoY to δpCO2_temp_XtoY and δpCO2_others_XtoY between two consecutive seasons over a full inter-seasonal cycle, based on departures from their respective averages, highlighting an overall lack of control by SST changes but a strong influence of mixing and biogeochemical processes.
In addition to the latitudinal distribution of global coastal pCO2, which displays a mismatch to that of SST, we quantify the influence of temperature on pCO2 based on the experimental finding that ∂lnpCO2/∂T is 0.0423°C−1 for isochemical seawater [35,36]. Because it is nearly impossible to locate an isochemical seawater system, a reasonable approximation is to examine seasonal pCO2 changes in a system with predictable seasonal cycles. Comparing between the observed inter-seasonal pCO2 change (δpCO2_total_XtoY; XtoY means from X season to Y season) and the temperature-controlled change (δpCO2_temp_XtoY) gives the fractional amount of δpCO2 that is not controlled by temperature (δpCO2_others_XtoY; Equations (1)–(4); see the ‘Methods’ section). We infer that different latitudinal bands vary in their inter-seasonal controls (Fig. 1d and Supplementary Figs 1 and 2).
An examination of the seasonal changes from summer to autumn reveals that non-thermal factors prevail at all latitudinal bands, except from 25° to 35° in both hemispheres (Fig. 1d). This is supported by a statistically significant positive relationship between δpCO2_total_XtoY and δpCO2_others_XtoY (Fig. 1f). On the other hand, a statistically significant relationship between δpCO2_total_XtoY and δpCO2_temp_XtoY indicates that decreased SST is associated with enhanced δpCO2_total_XtoY (Fig. 1e), contrary to what is expected from the implied thermal control. This is reinforced by aggregating all δpCO2 data over two consecutive seasons in a full inter-seasonal cycle (Fig. 1g and h). Compilations of the data at 100 km from the shore give a very similar pattern of pCO2 in terms of temperature and non-thermal determinations (Supplementary Fig. 2 and Supplementary Section 1.1). Consequently, the inter-seasonal change in global coastal pCO2 is to a large extent determined by non-thermal factors including water mass mixing and net primary production [17,19,21].
This analysis assumes that coastal systems are isochemical on a global scale; however, such systems are better approximated by more fully characterizing individual ocean margin systems. We thus differentiate the influence of temperature on pCO2 from the influence of non-thermal factors in the South China Sea and the Arabian Sea (see Supplementary Section 1.2).
In the South China Sea basin, temperature predominately controls the seasonal pCO2 change from winter to spring, but both temperature and non-thermal factors play important roles in the other three inter-seasonal changes. Taking the transition from spring to summer as an example, the temperature increase augments pCO2 by 17 ± 11 μatm, but is offset by the pCO2 decrease of −23 ± 17 μatm induced by non-thermal factors, leading to a negligible inter-seasonal pCO2 change of −6 ± 13 μatm (Supplementary Table 1). The latter change mainly reflects physical and biological processes including sea–air exchange, water mass mixing and biological activity.
In the Arabian Sea, the significant seasonal pCO2 increase from spring to summer in the coastal area mainly results from the summer upwelling process delivering CO2-rich deep water to the surface; temperature plays a minor role. Moreover, the subsequent decrease in upwelling largely induces the seasonal pCO2 decrease from summer to autumn, suggesting that water mass mixing is the major control. In areas without upwelling, both temperature and non-thermal factors play an important role in the inter-seasonal pCO2 changes (Supplementary Table 2).
Thus, non-thermal factors—mainly water mass mixing such as upwelling and biological alteration such as net primary production [17,19,21]—play a substantial role in regulating coastal ocean pCO2. The latitudinal distribution of pCO2 is obscured by seasonal changes in physical and biogeochemical processes at both river–margin and margin–open ocean interfaces, which cannot be fully simulated even by latest high-resolution models [10,32]; in contrast, such boundary processes are less important for open ocean basins. An alternative way to describe coastal CO2 dynamics is therefore required.
Ocean-dominated margins versus river-dominated ocean margins
To depict the role of water mass mixing and net primary production, we conceptualize the coastal carbon cycle using a semi-analytical framework (see the ‘Methods’ section) that couples physics and biogeochemistry, and DIC and nutrients [16]. Such a semi-analytical framework highlights the boundary processes and aims to resolve the source of DIC and nutrients and their relative consumption via organic carbon production, which determines whether a coastal system is a source (i.e. an excess of DIC during consumption is removed by CO2 degassing into the atmosphere) or sink (i.e. a deficit of DIC during consumption is supplied via atmospheric CO2 input) of atmospheric CO2. In this context, the world’s coastal oceans are categorized into two distinct regimes: ocean-dominated margin (OceMar) and river-dominated ocean margin (RiOMar) [16,37], with DIC and nutrients sourced from the open ocean and land, respectively. OceMars are characterized by a concurrent non-local input of DIC and nutrients, typically from depth, and the interplay between the externally sourced DIC and nutrients through internal metabolism largely controls the CO2 source/sink nature. RiOMars are mostly shelf regions featuring major nutrient loadings from riverine inputs, which include the far-reaching area of river plumes of, for instance, the Amazon [38,39], Yangtze [27,40] and Mississippi [23,41], and exclude nearshore ecosystems such as estuaries, lagoons or tidal marshes. The large river plumes are characterized by high discharges and often show a drawdown of sea surface pCO2 resulting from increased net primary production. In these RiOMars, organic carbon production stimulated by riverine nutrients thus outweighs the remineralization of riverine organic matter, leading to sinks of atmospheric CO2.
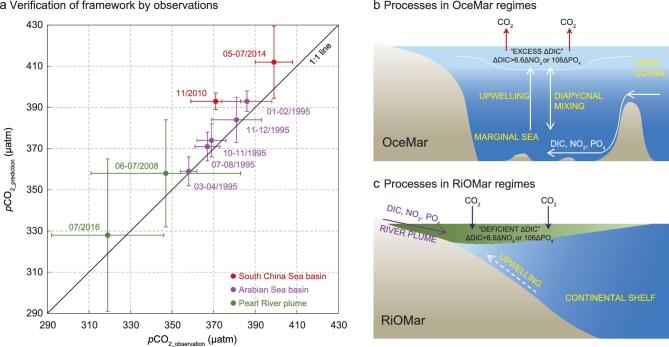
Below, we verify our semi-analytical framework using new datasets compiled from recent observations in the South China Sea, previously identified as a typical OceMar system [16]. We then apply the framework to diagnose the CO2 source/sink nature of the Arabian Sea, which is a new OceMar case, and in the Pearl River plume—a major river plume system that is a RiOMar regime.
OceMar case I: South China Sea basin
The South China Sea is the largest marginal sea of the North Pacific Ocean and it is an overall source of atmospheric CO2 [25]. Our semi-analytical framework has successfully predicted the CO2 outgassing in its basin area, consistent with field observations during summer 2009 and spring 2011 [16]. The diapycnal fluxes of materials to the euphotic zone suggest a higher DIC flux relative to phosphate (PO4) than Redfield stoichiometry [42], further implying DIC excess in the South China Sea [43]. Here, we reassess the CO2 source/sink nature using new data collected in autumn (November) 2010 and summer (May–July) 2014 in the South China Sea basin (see Supplementary Section 1.3).
Significant positive relationships between total alkalinity (TAlk) and salinity are observed in the surface mixed layer (<50 m) in both seasons, suggesting a two-endmember mixing scheme between waters immediately below the surface mixed layer and rain water, indicated by near-zero intercepts (Supplementary Fig. 7). Within this scheme, the estimated δDIC* based on PO4 (δDIC*PO4; Equation (11)) is on average 7 ± 2 μmol kg−1 in autumn 2010 and 17 ± 9 μmol kg−1 in summer 2014, which point to excess DIC removed by CO2 degassing, and are transformed to a sea–air ΔpCO2 of 13 ± 4 and 32 ± 18 μatm using Equation (14) (Supplementary Table 3). Combined with the atmospheric pCO2 field observations of ∼380 μatm in both seasons, the sea surface pCO2 (Equation (15)) in the South China Sea basin is predicted to be 393 ± 4 μatm in autumn 2010 and 412 ± 18 μatm in summer 2014, consistent with, if only slightly higher than, the field observations of 371 ± 12 and 399 ± 9 μatm (red-filled circles, Fig. 2a; Supplementary Fig. 8). Note that the estimated sea surface pCO2 based on nitrate (NO3) is essentially consistent with that based on PO4 in both seasons, which is discussed later. We thus confirm the CO2 source nature of the South China Sea.
Figure 2.
Application of our semi-analytical framework to ocean-dominated margins (OceMars) and a river-dominated ocean margin (RiOMar). (a) Verification of predicted pCO2 using our semi-analytical framework (see the ‘Methods’ section) versus the observed pCO2 in three applications: the South China Sea basin (red, two seasons), Arabian Sea basin (pink, five seasons) and Pearl River plume (green, two seasons). Horizontal and vertical bars represent one standard deviation of pCO2 values, reflecting the spatial variability of field observations and variability of our predictions, respectively. The South China Sea basin and the Arabian Sea basin, both diagnosed as a source of atmospheric CO2 by our framework, are OceMars. In these systems, DIC and nutrients are mainly from processes at the coastal and open ocean interface, whereby the open ocean-originated DIC, NO3 and PO4 are transported upward into the surface mixed layer of the marginal sea through vertical mixing and upwelling, as depicted by (b). By contrast, the Pearl River plume, diagnosed as a sink of atmospheric CO2 by our framework, is a RiOMar. In these systems, DIC, NO3 and PO4 mainly originating from rivers are transported along the plume pathway over the continental shelf, whereas contributions from the subsurface water play a minor role, as depicted by (c). The coupled DIC and nutrient consumption via organic carbon production in the surface mixed layer, or in the plume water, ultimately determine the sea–air CO2 flux of ocean margins. (b) and (c) are revised from [16].
The main processes involved in an OceMar regime are depicted in Fig. 2b. DIC and nutrients are mainly sourced from the open ocean. Through vertical mixing and upwelling, such open ocean-originated DIC, NO3 and PO4 are transported upward into the surface mixed layer, in which the consumption of DIC relative to nutrients determines whether DIC is in excess or in deficit relative to the external input. The excess DIC, as diagnosed in the South China Sea basin, is eventually released to the atmosphere, making the region a CO2 source.
OceMar case II: Arabian Sea basin
The Arabian Sea is characterized by strong seasonal cycles driven by the Asian Monsoon. There is well-defined upwelling during the southwest monsoon season due to offshore Ekman transport, which often manifests as low SST along the Arabian Peninsula [44]. The upwelling results in increased biological productivity and complicated pCO2 responses. Using the carbonate system and nutrient data collected in 1995 from five cruises in winter (January–February and November–December) during the northeast monsoon season, in spring (March–April) and autumn (October–November) during the inter-monsoon season and in summer (July–August) during the southwest monsoon season (see Supplementary Section 1.4), we estimate the CO2 source/sink nature in the Arabian Sea.
TAlk and salinity in the surface mixed layer (<75–100 m) show significant positive relationships in all seasons, suggesting an overall two-endmember mixing scheme between waters immediately below the surface mixed layer and rain water (indicated by near-zero intercepts) or a non-zero-solute freshwater (indicated by substantive intercepts) (Supplementary Fig. 10). Within this scheme, the average δDIC*PO4 values are estimated to be 23 ± 3 (January–February), 4 ± 5 (March–April), 20 ± 5 (July–August), 25 ± 6 (October–November) and 26 ± 8 (November–December) μmol kg−1, which point to excess DIC removed by CO2 degassing and are transformed to a sea–air ΔpCO2 of 37 ± 5, 6 ± 7, 30 ± 7, 37 ± 8 and 39 ± 11 μatm using Equation (14) (Supplementary Table 3). Sea surface pCO2 (Equation (15)) estimated for the five consecutive seasons are 393 ± 5, 359 ± 7, 371 ± 7, 374 ± 8 and 384 ± 11 μatm. The field-observed pCO2 values are 386 ± 12, 358 ± 4, 367 ± 6, 369 ± 7 and 381 ± 12 μatm, which agree rather well with the predicted results (pink-filled circles, Fig. 2a; Supplementary Fig. 11). Note that our analysis based on NO3 is, within error, comparable to that based on PO4 during the five seasons, which is discussed later. While the sea surface pCO2 equals the air pCO2 in the spring inter-monsoon season, in other seasons, the Arabian Sea must have acted as a weak source of CO2 to the atmosphere (Fig. 2b).
RiOMar case: Pearl River plume
The Pearl River is one of the world’s major rivers, discharging 3.26 × 1011 m3 of freshwater annually and forming a river plume over the broad continental shelf of the northern South China Sea in summer. This river plume, travelling along with the coastal current, extends to hundreds of kilometres from the mouth of the Pearl River estuary and strongly modulates the shelf biogeochemistry [45–47]. Here, we use datasets collected on the northern South China Sea shelf in the summers of 2008 (June–July) and 2016 (July) to evaluate the CO2 source/sink nature of the Pearl River plume waters with salinities <33.0 (see Supplementary Section 1.5).
Due to the co-influence of both the river plume and coastal upwelling in both seasons, a three-endmember mixing scheme in the upper 100 m is identified between the plume water, offshore subsurface water and offshore surface water, as suggested by the temperature–salinity relationship (Supplementary Fig. 13). Within this scheme, the estimated δDIC*, based on NO3 (δDIC*NO3; Equation (10)), is on average −6 ± 13 μmol kg−1 in summer 2008 and −34 ± 20 μmol kg−1 in summer 2016, which indicates a DIC deficit supplied via atmospheric CO2 inputs, and these values are transformed to a sea–air ΔpCO2 of −12 ± 26 and −62 ± 37 μatm using Equation (13) (Supplementary Table 3). Combined with the field-observed atmospheric pCO2 of ∼370 and ∼390 μatm during the two seasons, the sea surface pCO2 was estimated to be 358 ± 26 and 328 ± 37 μatm (Equation (15)); both values are consistent with the calculated pCO2 of 347 ± 36 and 319 ± 27 μatm using DIC and TAlk data (green-filled circle, Fig. 2a; Supplementary Fig. 14). We thus demonstrate that the Pearl River plume is a sink of atmospheric CO2, which was stronger in summer 2016 than in summer 2008. However, our analysis based on PO4 suggests it was a CO2 source during both seasons. This inconsistency is examined later.
The main processes involved in a RiOMar regime are depicted in Fig. 2c. DIC and nutrients are mainly sourced from the river and transported along the plume pathway on the continental shelf. As in OceMars, the consumption of DIC relative to nutrients in the plume water determines whether DIC is in excess or in deficit. A DIC deficit, as identified in the Pearl River plume, is supplied by atmospheric CO2 inputs, making the region a CO2 sink.
Thus, we successfully extend the applicability of the semi-analytical framework from OceMars to RiOMars, helping to better characterize the coastal carbon cycle and resolve the CO2 dynamics and fluxes in a quantitative way. While the major source of DIC and nutrients differs between the two coastal systems due to different boundary processes, the same metabolic process coupling the externally supplied DIC and nutrients modulates the sea–air CO2 exchange in both regimes (Fig. 2b and c).
Utilities and uncertainties
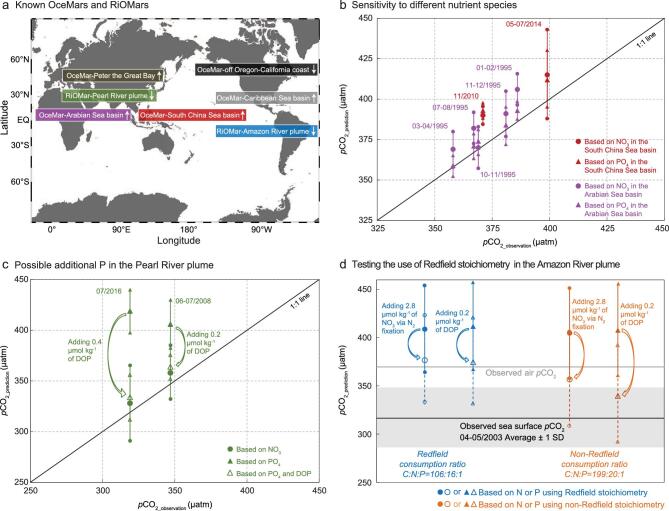
Our framework (see the ‘Methods’ section) characterizes two important carbon processes that are independent of temperature, namely physical and biogeochemical processes, in the coupled DIC–nutrient dynamics. Application of the framework strengthens the notion that, even though global coastal oceans are a sink of atmospheric CO2 as a whole, individual coastal systems are highly variable, may act as CO2 sources or sinks and need to be characterized individually. In addition to the three cases we presented here, other successful applications of our framework include the Caribbean Sea basin [16], the upwelling system of the US west coast off Oregon and California [48] and the Peter the Great Bay of the Japan/East Sea [30] (Fig. 3a). These are identified as OceMars and can be either a CO2 source or sink. However, for the Amazon River plume, a typical RiOMar system (Fig. 3a), our prediction as a source differs from observations as a sink. This is examined later.
Figure 3.
Utilities and uncertainties of our semi-analytical diagnostic framework in OceMar and RiOMar regimes. (a) Known OceMars and RiOMars, with upward and downward arrows indicating sources and sinks of atmospheric CO2, respectively. (b) Comparison between predicted pCO2 based on different nutrient species and observed pCO2 in the South China Sea basin (red, two seasons) and the Arabian Sea basin (pink, five seasons). The diagonal line indicates a perfect fit between the diagnosed and observed values, and vertical bars represent one standard deviation (1 SD) of the diagnosed pCO2 values, which are largely due to the spatial variability of the observations. The consistency between CO2 source or sink diagnoses based on NO3 or PO4 demonstrates insensitivity to the choice of nutrient species. (c) As in (b), but for the Pearl River plume (green, two seasons); in both seasons, our framework identifies an addition of P that is not accounted for by our observations but needs to be considered to bring the predicted pCO2 to that based on NO3. The added P is likely transformed from dissolved organic phosphorus (DOP). (d) As in (b), but for the Amazon River plume (blue, one season), which our framework diagnoses as a source, opposite to the observed sink. Under the Redfield stoichiometry, even with unaccounted-for N or P added, our framework still predicts a sea surface pCO2 (blue) higher than the observed air pCO2 (grey line) but, under a non-Redfield stoichiometry, the same added nutrients lead to a predicted pCO2 (orange) that is close to or within the uncertainty range due to spatial variability (grey shadow) of the observed average sea surface pCO2 (black line).
The semi-analytical framework follows Redfield stoichiometry, in which the C, N and P consumption ratio is constant at 106:16:1 [42]. Treating this ratio as a global or regional constant is mostly acceptable
in the context of interpreting snapshots of the water column [49]. However, higher DIC/NO3 consumption ratios than Redfield stoichiometry have been observed, probably resulting from DIC overconsumption relative to inorganic N via the production of dissolved organic matter (DOM) [50]. This suggests that the assumption of a Redfield consumption ratio may not be always valid and must be verified. On the other hand, some of the unaccounted-for nutrients may also result in an apparently higher DIC/NO3, DIC/PO4 or NO3/PO4 consumption ratio [46].
Application of our framework to the South China Sea and the Arabian Sea illustrates that, when all nutrients are accounted for, our analysis is robust and insensitive to the choice of nutrient species. In the South China Sea basin, the estimated δDIC*NO3 agrees well with the δDIC*PO4 in both autumn 2010 and summer 2014, resulting from an average ΔNO3/ΔPO4 ratio (i.e. the apparent biological consumption of NO3 relative to PO4 obtained by Equation (8)/Equation (9)) close to the Redfield value of 16 (Supplementary Table 3). Accordingly, the estimated pCO2 values based on δDIC*NO3 and δDIC*PO4, respectively, are within error, comparable to each other during both seasons (red-filled symbols, Fig. 3b). In the Arabian Sea basin, the estimated pCO2 based on δDIC*NO3 is also within the uncertainty range of that based on δDIC*PO4, and both are consistent with field observations during the five seasons analysed (pink-filled symbols, Fig. 3b), when the ΔNO3/ΔPO4 ratios are equal to the Redfield value (Supplementary Table 3). The consistency between results from the two nutrient species suggests that consumption of C/N and C/P are well coupled in these two OceMar systems, which points to the robustness of our framework and strengthens the validity of using Redfield stoichiometry in these cases.
In the Pearl River plume, however, the estimated δDIC*PO4 values are positive in both the summers of 2008 and 2016 based on our own observations, which would imply a CO2 source (large green-filled triangles, Fig. 3c), opposite to the direction of the CO2 flux based on δDIC*NO3 (large green-filled circles, Fig. 3c). This discrepancy is supported by much higher ΔNO3/ΔPO4 ratios than the Redfield value (Supplementary Table 3), suggesting that an additional source of P not accounted for by our observations is needed to balance the NO3-based δDIC* and thus reduce the estimated pCO2 toward that based on δDIC*NO3 or field observations. This amount of P is estimated to be ∼0.2 and ∼0.4 μmol kg−1 for the summers of 2008 and 2016, respectively. Once added, the calculated pCO2 (large green-unfilled triangles, Fig. 3c) is close to the observed pCO2. The additional P can be transformed from dissolved organic phosphorus (DOP) during metabolic processes [46]. This finding of unaccounted-for DOP is confirmed by an independently observed value of approximately 0.5 μmol kg−1 in the upstream Pearl River estuary in summer 2015 [51], highlighting a utility of our framework.
Nevertheless, there is at least one case—the Amazon River plume—in which, even after accounting for additional nutrients, the predicted and observed results of sea surface pCO2 still differ. In this case, the debate regarding the validity of Redfield stoichiometry continues [50,52]. Here, using available observations of DIC, NO3 and PO4 (see Supplementary Section 1.6), our framework determines that the Amazon River plume (salinities ∼30.0–35.0) is a source of atmospheric CO2, whereas observations from April–May 2003 show it acts as a sink [38,39].
To be consistent, it would require a NO3 consumption of ∼7.4 μmol kg−1 to meet the DIC removal and drawdown of pCO2. The estimated ΔNO3 using our framework (Equation (8)) is, however, only ∼1.0 μmol kg−1 (the resulting prediction of pCO2 is denoted by a large blue-filled circle, Fig. 3d), suggesting that the majority of NO3 (∼6.4 μmol kg−1) needs to be supplied from additional sources, such as N2 fixation. Strong N2 fixation was consistently observed in the Amazon River plume [53–55], estimated to be 1404.6 μmol N m−2 d−1 in the mesohaline zone with salinities of 30.0–35.0 [56]. Combined with a mixed layer depth of ∼20 m and a water residence time of ∼40 days, the total N supplied by N2 fixation would only be 2.8 μmol kg−1, of which just a fraction could be directly transformed to NO3 and subsequently consumed during primary production. Even taking this value as a maximum of NO3 added, the resulting prediction of sea surface pCO2 is still higher than the field-observed atmospheric pCO2 (the former denoted by a large blue-unfilled circle, Fig. 3d). We therefore use another nutrient species, i.e. PO4, which leads to a finding that the estimated ΔPO4 (Equation (9)) is only ∼0.02 μmol kg−1 (the resulting prediction of pCO2 is denoted by a large blue-filled triangle, Fig. 3d) and additional PO4 of 0.53 μmol kg−1 is needed to predict the CO2 sink. DOP, with a concentration of up to 0.2 μmol kg−1, is the dominant species of P [57] (the resulting prediction of pCO2 is denoted by a large blue-unfilled triangle, Fig. 3d), which is still not sufficient to support the DIC consumption.
Although N2 fixation and DOP utilization may partially be responsible for the low NO3 and PO4 removal relative to DIC, including them in the semi-analytical framework seems incapable of reversing the prediction of their CO2 source/sink nature. We envisage that this could be a case in which Redfield stoichiometry is not fully valid. If DIC overconsumption occurred via the DOM production with higher C:N:P stoichiometry (199:20:1) [58], this would also lead to the overestimated δDIC*NO3 and δDIC*PO4 (Equations (10) and (11)). By considering both unaccounted-for nutrients and the non-Redfield stoichiometry of DOM, we can resolve the sea–air ΔpCO2 close to the CO2 sink nature of the Amazon River plume. In particular, the estimated pCO2 based on P is closer to the field-observed range of pCO2 (orange symbols, Fig. 3d). Nevertheless, this is obviously an oversimplification because DIC and inorganic nutrients are not merely transformed into DOM during biological consumption. The realistic C, N and P consumption ratio would lie in between, pointing to an uncertainty of our estimation, which warrants further study.
SUMMARY
Although global coastal oceans as a whole have been recognized as an important natural sink of atmospheric CO2, the associated physical and biogeochemical processes need to be better understood. Our finding that inter-seasonal changes of global coastal pCO2 are strongly determined by non-thermal factors has led to the establishment of our semi-analytical framework, which conceptualizes the role of water mass mixing and net primary production. The framework diagnoses CO2 dynamics and fluxes in coastal oceans in two distinctive regimes, i.e. OceMars and RiOMars. We show that coastal oceans as a CO2 sink mostly refers to RiOMars, but OceMars can be either a source or sink: e.g. the South China Sea basin is a source whereas the Oregon–California coast is a sink. Therefore, the details are critical and must be captured correctly by any global carbon cycle models, which has been a long-standing scientific challenge.
It is in this context that our framework has found utility while contributing to the scientific debate. On the one hand, when validated by independent observations, it helps account for additional nutrients as is the case in the Pearl River plume. On the other hand, it helps test the validity of Redfield stoichiometry as is the case in the Amazon River plume. We anticipate that the usefulness of our developed framework will inspire future studies. One natural next step would be to apply this framework to other global coastal systems. To this end, observations of DIC and nutrients from likely sources are essential, but this is not a trivial effort. International collaboration is crucial in order to gain the global picture that is composed of detailed characteristics of individual coastal ocean systems. This would also increase understanding of the associated processes, and their parameterization for inclusion in models: either mechanistic, such as our framework, or as a comprehensive global system [33], the latter being the ultimate goal of future research efforts.
METHODS
Global coastal pCO2 data sources and analysis
Global coastal SST and pCO2 data employed in Fig. 1 and Supplementary Figs 1 and 2 were extracted from the Surface Ocean CO2 Atlas (http://www.socat.info/). We selected pCO2 values at the distance of 50 and 100 km from major land masses in the SOCAT v3.0 data product [59], which cover latitudes from 82°N to 78°S and years from 1962 to 2014 (Supplementary Fig. 3).
Data analysis was based on 1°-latitude averages. To differentiate the influence of temperature on seasonal pCO2 variations from all other influences, we first calculated the total variation of field-observed pCO2 from X season to Y season (δpCO2_total_XtoY) according to Equation (1):
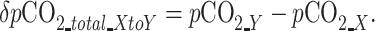 |
(1) |
pCO2_X and pCO2_Y are the 1°-latitude averaged field-observed pCO2 in X season and Y season, respectively. We then defined δpCO2_temp_XtoY as the pCO2 variation solely induced by temperature changes from X season to Y season, which was calculated as:
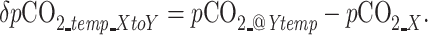 |
(2) |
pCO2_@Ytemp is the theoretical pCO2 value during Y season considering only temperature changes relative to X season, which was calculated according to Equation (3) [36]:
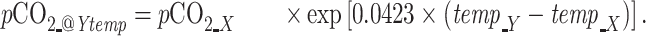 |
(3) |
temp_X and temp_Y are the 1°-latitude averaged SSTs during seasons X and Y, respectively. Finally, the difference between δpCO2_total_XtoY and δpCO2_temp_XtoY (δpCO2_others_XtoY; Equation (4)) reflects the pCO2 variability between seasons X and Y resulting from other processes (e.g. water mass mixing and net primary production) beyond the temperature effect.
 |
(4) |
The four seasonal transitions presented in Supplementary Figs 1 and 2 are changes sequentially from winter (X) to spring (Y), from spring (X) to summer (Y), from summer (X) to autumn (Y) and from autumn (X) to winter (Y). Seasons are defined as March, April and May as spring in the Northern Hemisphere and September, October and November as spring in the Southern Hemisphere.
Semi-analytical diagnostic framework of CO2 dynamics and fluxes in ocean margins
Our diagnostic framework uses the mass balance of DIC in the surface mixed layer (Equation (5)):
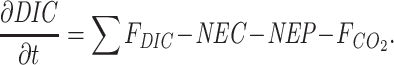 |
(5) |
Here, NEC is the net ecosystem calcification and NEP is the net ecosystem production. FDIC is the external DIC input to the surface mixed layer and FCO2 is the sea–air CO2 flux. At steady state and assuming negligible net calcification often suggested by conservative TAlk distributions, Equation (5) can be simplified to Equation (6):
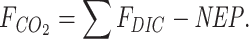 |
(6) |
The sea–air CO2 flux is thus a function of the sum of DIC inputs and net ecosystem production, or the CO2 is a net consequence of externally transported DIC and internal metabolic CO2 consumption. However, resolving Equation (6) is not a trivial task, as it requires a full solution to the 3D ocean circulation that is not always possible. Here, we couple the physical transport and biological mediation of DIC and nutrients (i.e. NO3 and PO4) within comparable timescales, by establishing a water mass mixing scheme in order to define the physical transport, or the conservative portion of DIC and nutrients from the adjacent water masses, and the constraint of the biogeochemical alteration of these non-local inputs in the upper water column of ocean margins [16]. These processes are described by
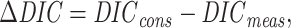 |
(7) |
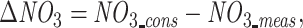 |
(8) |
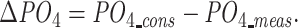 |
(9) |
The subscripts ‘cons’ and ‘meas’ in Equations (7)–(9) denote conservative-mixing-induced and field-observed values, respectively. The difference between them, represented as Δ, is the addition (negative values resulting from organic matter remineralization outweighing primary production) or removal (positive values resulting from primary production outweighing organic matter remineralization) of DIC, NO3 or PO4 beyond the mixing control. In the latter case, we subsequently use δDIC* to quantify the consumption of DIC relative to nutrients based on the classic Redfield stoichiometry of C:N:P = 106:16:1 (Equations (10) and (11)) [42], which determines whether DIC is in excess (i.e. δDIC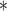 >0) or in deficit (i.e. δDIC
>0) or in deficit (i.e. δDIC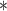 <0) relative to nutrients in the seawater body:
<0) relative to nutrients in the seawater body:
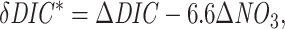 |
(10) |
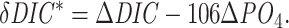 |
(11) |
Under steady-state conditions over a relatively long timescale (e.g. seasonal scale), such excesses or deficits in DIC would be degassed (i.e. CO2 source) or compensated (i.e. CO2 sink) by sea–air CO2 gas exchange. At the same time, only a fraction of δDIC* is involved in the gas exchange, depending on the Revelle factor (RF), which is referred to as the fractional change in surface seawater CO2 (∂pCO2/pCO2) over the fractional change in DIC (∂DIC/DIC) at a given temperature, salinity and TAlk (Equation (12)) [60,61]. In other words, RF quantifies the ocean’s sensitivity to an increase in atmospheric CO2, i.e.
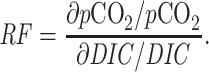 |
(12) |
In a simplified way and as an approximation, ∂DIC equals δDIC*, which is solely achieved through sea–air CO2 exchange, implying that ∂pCO2 may represent the sea–air ΔpCO2 (defined as the difference in pCO2 between the sea and the air, or pCO2_sea − pCO2_air). Given an initial balance of CO2 between the seawater and the atmosphere, the sea–air ΔpCO2 is obtained by
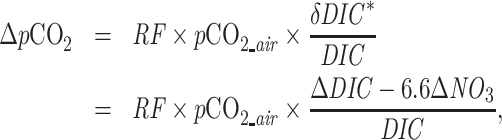 |
(13) |
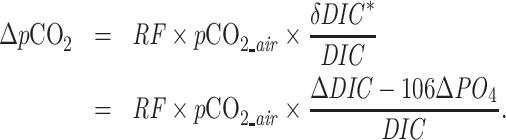 |
(14) |
The surface pCO2 in the seawater body can thus be predicted as
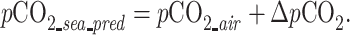 |
(15) |
Supplementary Material
Acknowledgements
We thank L. Wang, T. Huang, L. Guo, Y. Xu, Y. Li, J. Hu and D. Wang for assistance with sampling, processing and/or analysis. W. Cai and W. Zhai provided constructive comments on the manuscript. The Surface Ocean CO2 Atlas (SOCAT) is an international effort, endorsed by the International Ocean Carbon Coordination Project (IOCCP), the Surface Ocean Lower Atmosphere Study (SOLAS) and the Integrated Marine Biosphere Research (IMBeR) programme, to deliver a uniformly quality-controlled surface ocean CO2 database. The many researchers and funding agencies responsible for the collection of data and quality control are thanked for their contributions to SOCAT. Comments from three anonymous reviewers significantly improved the quality of this contribution.
FUNDING
This work was supported by the National Key Scientific Research Project (2015CB954000 and 2015CB954001) sponsored by the Ministry of Science and Technology of China, and by the National Natural Science Foundation of China (91328202).
Conflict of interest statement. None declared.
REFERENCES
- 1. Sabine CL, Feely RA, Gruber Net al. . The oceanic sink for anthropogenic CO2. Science 2004; 305: 367–71. [DOI] [PubMed] [Google Scholar]
- 2. Khatiwala S, Primeau F, Hall T. Reconstruction of the history of anthropogenic CO2 concentrations in the ocean. Nature 2009; 462: 346–9. [DOI] [PubMed] [Google Scholar]
- 3. Cai W-J. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annu Rev Mar Sci 2011; 3: 123–45. [DOI] [PubMed] [Google Scholar]
- 4. Holt JT, Harle J, Proctor Ret al. . Modelling the global coastal ocean. Philos Trans R Soc A 2009; 367: 939–51. [DOI] [PubMed] [Google Scholar]
- 5. Bonan GB, Doney SC. Climate, ecosystems, and planetary futures: the challenge to predict life in earth system models. Science 2018; 359: eaam8328. [DOI] [PubMed] [Google Scholar]
- 6. Regnier P, Friedlingstein P, Ciais Pet al. . Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 2013; 6: 597–607. [Google Scholar]
- 7. Bauer JE, Cai W-J, Raymond PAet al. . The changing carbon cycle of the coastal ocean. Nature 2013; 504: 61–70. [DOI] [PubMed] [Google Scholar]
- 8. Andersson AJ, Mackenzie FT. Shallow-water oceans: a source or sink of atmospheric CO2? Front Ecol Environ 2004; 2: 348–53. [Google Scholar]
- 9. Duarte CM, Middelburg JJ, Caraco N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005; 2: 1–8. [Google Scholar]
- 10. Laruelle GG, Cai W-J, Hu Xet al. . Continental shelves as a variable but increasing global sink for atmospheric carbon dioxide. Nat Commun 2018; 9: 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borges A, Delille B, Frankignoulle M. Budgeting sinks and sources of CO2 in the coastal ocean: diversity of ecosystems counts. Geophys Res Lett 2005; 32: L14601. [Google Scholar]
- 12. Cai W-J, Dai M, Wang Y. Air-sea exchange of carbon dioxide in ocean margins: a province-based synthesis. Geophys Res Lett 2006; 33: L12603. [Google Scholar]
- 13. Chen C-TA, Borges AV. Reconciling opposing views on carbon cycling in the coastal ocean: continental shelves as sinks and near-shore ecosystems as sources of atmospheric CO2. Deep-Sea Res II 2009; 56: 578–90. [Google Scholar]
- 14. Laruelle GG, Dürr HH, Slomp CPet al. . Evaluation of sinks and sources of CO2 in the global coastal ocean using a spatially-explicit typology of estuaries and continental shelves. Geophys Res Lett 2010; 37: L15607. [Google Scholar]
- 15. Chen C-TA, Huang T-H, Chen Y-Cet al. . Air-sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 2013; 10: 6509–44. [Google Scholar]
- 16. Dai M, Cao Z, Guo Xet al. . Why are some marginal seas sources of atmospheric CO2? Geophys Res Lett 2013; 40: 2154–8. [Google Scholar]
- 17. Laruelle GG, Lauerwald R, Pfeil Bet al. . Regionalized global budget of the CO2 exchange at the air-water interface in continental shelf seas. Glob Biogeochem Cycle 2014; 28: 1199–214. [Google Scholar]
- 18. Bourgeois T, Orr JC, Resplandy Let al. . Coastal ocean uptake of anthropogenic carbon. Biogeosciences 2016; 13: 4167–85. [Google Scholar]
- 19. Laruelle GG, Landschützer P, Gruber Net al. . Global high-resolution monthly pCO2 climatology for the coastal ocean derived from neural network interpolation. Biogeosciences 2017; 14: 4545–61. [Google Scholar]
- 20. Cai W-J, Dai M. Comment on ‘enhanced open ocean storage of CO2 from shelf sea pumping'. Science 2004; 306: 1477c. [DOI] [PubMed] [Google Scholar]
- 21. Gruber N. Carbon at the coastal interface. Nature 2015; 517: 148–9. [DOI] [PubMed] [Google Scholar]
- 22. Cai W-J, Chen L, Chen Bet al. . Decrease in the CO2 uptake capacity in an ice-free Arctic Ocean basin. Science 2010; 329: 556–9. [DOI] [PubMed] [Google Scholar]
- 23. Lohrenz SE, Cai W-J, Chen Fet al. . Seasonal variability in air-sea fluxes of CO2 in a river-influenced coastal margin. J Geophys Res 2010; 115: C10034. [Google Scholar]
- 24. Omar AM, Olsen A, Johannessen Tet al. . Spatiotemporal variations of f CO2 in the North Sea. Ocean Sci 2010; 6: 77–89. [Google Scholar]
- 25. Zhai W, Dai M, Chen Bet al. . Seasonal variations of air-sea CO2 fluxes in the largest tropical marginal sea (South China Sea) based on multiple-year underway measurements. Biogeosciences 2013; 10: 7775–91. [Google Scholar]
- 26. Evans W, Mathis JT, Cross JNet al. . Sea-air CO2 exchange in the western Arctic coastal ocean. Glob Biogeochem Cycle 2015; 29: 1190–209. [Google Scholar]
- 27. Guo X, Zhai W, Dai Met al. . Air-sea CO2 fluxes in the East China Sea based on multiple-year underway observations. Biogeosciences 2015; 12: 5495–514. [Google Scholar]
- 28. Laruelle GG, Lauerwald R, Rotschi Jet al. . Seasonal response of air-water CO2 exchange along the land-ocean aquatic continuum of the northeast north American coast. Biogeosciences 2015; 12: 1447–58. [Google Scholar]
- 29. Volta C, Laruelle GG, Regnier P. Regional carbon and CO2 budgets of North Sea tidal estuaries. Estuar Coast Shelf Sci 2016; 176: 76–90. [Google Scholar]
- 30. Chou W-C, Tishchenko PY, Chuang K-Yet al. . The contrasting behaviors of CO2 systems in river-dominated and ocean-dominated continental shelves: a case study in the East China Sea and the Peter the Great Bay of the Japan/East Sea in summer 2014. Mar Chem 2017; 195: 50–60. [Google Scholar]
- 31. Geilfus N-X, Pind M-L, Else BGTet al. . Spatial and temporal variability of seawater pCO2 within the Canadian Arctic archipelago and Baffin Bay during the summer and autumn 2011. Cont Shelf Res 2018; 156: 1–10. [Google Scholar]
- 32. Lachkar Z, Gruber N. Response of biological production and air-sea CO2 fluxes to upwelling intensification in the California and canary current systems. J Mar Syst 2013; 109: 149–60. [Google Scholar]
- 33. Mackenzie FT, Lerman A, Ver LMB. Role of the continental margin in the global carbon balance during the past three centuries. Geology 1998; 26: 423–6. [Google Scholar]
- 34. Hofmann EE, Cahill B, Fennel Ket al. . Modeling the dynamics of continental shelf carbon. Annu Rev Mar Sci 2011; 3: 93–122. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi T, Olafsson J, Goddard JGet al. . Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: a comparative study. Glob Biogeochem Cycle 1993; 7: 843–78. [Google Scholar]
- 36. Takahashi T, Sutherland SC, Sweeney Cet al. . Global Sea-air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep-Sea Res II 2002; 9–10: 1601–22. [Google Scholar]
- 37. McKee BA, Aller RC, Allison MAet al. . Transport and transformation of dissolved and particulate materials on continental margins influenced by major rivers: benthic boundary layer and seabed processes. Cont Shelf Res 2004; 24: 899–926. [Google Scholar]
- 38. Körtzinger AA. Significant CO2 sink in the tropical Atlantic Ocean associated with the Amazon River plume. Geophys Res Lett 2003; 30: 2287. [Google Scholar]
- 39. Cooley SR, Coles VJ, Subramaniam Aet al. . Seasonal variations in the Amazon plume-related atmospheric carbon sink. Glob Biogeochem Cycle 2007; 21: GB3014. [Google Scholar]
- 40. Tseng C-M, Liu K-K, Gong G-Cet al. . CO2 uptake in the East China Sea relying on Changjiang runoff is prone to change. Geophys Res Lett 2011; 38: L24609. [Google Scholar]
- 41. Guo X, Cai W-J, Huang W-Jet al. . Carbon dynamics and community production in the Mississippi River plume. Limnol Oceanogr 2012; 57: 1–17. [Google Scholar]
- 42. Redfield AC, Ketchum BH, Richards FA. The influence of organisms on the composition of seawater. In: Hill MN (ed.). The Sea. New York: Wiley, 1963, 26–77. [Google Scholar]
- 43. Du C, Liu Z, Kao S-Jet al. . Diapycnal fluxes of nutrients in an oligotrophic oceanic regime: the South China Sea. Geophys Res Lett 2017; 44: 11510–8. [Google Scholar]
- 44. Rixen T, Ittekkot V. Nitrogen deficits in the Arabian Sea, implications from a three component mixing analysis. Deep-Sea Res II 2005; 52: 1879–91. [Google Scholar]
- 45. Cao Z, Dai M, Zheng Net al. . Dynamics of the carbonate system in a large continental shelf system under the influence of both a river plume and coastal upwelling. J Geophys Res 2011; 116: G02010. [Google Scholar]
- 46. Han A, Dai M, Kao S-Jet al. . Nutrient dynamics and biological consumption in a large continental shelf system under the influence of both a river plume and coastal upwelling. Limnol Oceanogr 2012; 57: 486–502. [Google Scholar]
- 47. Wu K, Dai M, Li Xet al. . Dynamics and production of dissolved organic carbon in a large continental shelf system under the influence of both river plume and coastal upwelling. Limnol Oceanogr 2017; 62: 973–88. [Google Scholar]
- 48. Cao Z, Dai M, Evans Wet al. . Diagnosing CO2 fluxes in the upwelling system off the Oregon-California coast. Biogeosciences 2014; 11: 6341–54. [Google Scholar]
- 49. Martz T, Send U, Ohman MDet al. . Dynamic variability of biogeochemical ratios in the Southern California current system. Geophys Res Lett 2014; 41: 2496–501. [Google Scholar]
- 50. Sambrotto RN, Savidge G, Robinson Cet al. . Elevated consumption of carbon relative to nitrogen in the surface ocean. Nature 1993; 363: 248–50. [Google Scholar]
- 51. Li R, Xu J, Li Xet al. . Spatiotemporal variability in phosphorus species in the Pearl River estuary: influence of the river discharge. Sci Rep 2017; 7: 13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Banse K. Uptake of inorganic carbon and nitrate by marine plankton and the Redfield ratio. Glob Biogeochem Cycle 1994; 8: 81–4. [Google Scholar]
- 53. Voss M, Croot P, Lochte Ket al. . Patterns of nitrogen fixation along 10°N in the tropical Atlantic. Geophys Res Lett 2004; 31: L23S09. [Google Scholar]
- 54. Foster RA, Subramaniam A, Mahaffey Cet al. . Influence of the Amazon River plume on distributions of free-living and symbiotic cyanobacteria in the western tropical North Atlantic Ocean. Limnol Oceanogr 2007; 52: 517–32. [Google Scholar]
- 55. Olson EM, McGillicuddy DJ, Dyhrman STet al. . The depth-distribution of nitrogen fixation by Trichodesmium spp. colonies in the tropical–subtropical North Atlantic. Deep-Sea Res I 2015; 104: 72–91. [Google Scholar]
- 56. Subramaniam A, Yager PL, Carpenter EJet al. . Amazon River enhances diazotrophy and carbon sequestration in the tropical North Atlantic Ocean. Proc Natl Acad Sci USA 2008; 105: 10460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sohm JA, Capone DG. Zonal differences in phosphorus pools, turnover and deficiency across the tropical North Atlantic Ocean. Glob Biogeochem Cycle 2010; 24: GB2008. [Google Scholar]
- 58. Hopkinson CS, Vallino JJ. Efficient export of carbon to the deep ocean through dissolved organic matter. Nature 2005; 433: 142–5. [DOI] [PubMed] [Google Scholar]
- 59. Bakker DCE, Pfeil B, Landa CSet al. . A multi-decade record of high-quality fCO2 data in version 3 of the surface ocean CO2 atlas (SOCAT). Earth Sys Sci Data 2016; 8: 383–413. [Google Scholar]
- 60. Revelle R, Suess HE. Carbon dioxide exchange between atmosphere and ocean and the question of an increase of atmospheric CO2 during the past decades. Tellus 1957; 9: 18–27. [Google Scholar]
- 61. Sundquist ET, Plummer LN, Wigley TML. Carbon dioxide in the ocean surface: the homogenous buffer factor. Science 1979; 204: 1203–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.