Abstract
The two-electron reduction of molecular oxygen represents an effective strategy to enable the green, mild and on-demand synthesis of hydrogen peroxide. Its practical viability, however, hinges on the development of advanced electrocatalysts, preferably composed of non-precious elements, to selectively expedite this reaction, particularly in acidic medium. Our study here introduces 2H-MoTe2 for the first time as the efficient non-precious-metal-based electrocatalyst for the electrochemical production of hydrogen peroxide in acids. We show that exfoliated 2H-MoTe2 nanoflakes have high activity (onset overpotential ∼140 mV and large mass activity of 27 A g−1 at 0.4 V versus reversible hydrogen electrode), great selectivity (H2O2 percentage up to 93%) and decent stability in 0.5 M H2SO4. Theoretical simulations evidence that the high activity and selectivity of 2H-MoTe2 arise from the proper binding energies of HOO* and O* at its zigzag edges that jointly favor the two-electron reduction instead of the four-electron reduction of molecular oxygen.
Keywords: hydrogen peroxide production, non-precious-metal-based electrocatalyst, molybdenum telluride, liquid phase exfoliation, zigzag edges
2H-MoTe2 nanoflake prepared from liquid phase exfoliation exhibit remarkable electrocatalystic activity for oxygen reduction to hydrogen peroxide in acids.
INTRODUCTION
Hydrogen peroxide (H2O2) is a potential energy carrier and an important commodity chemical with high industrial value [1,2]. Its low-concentration (3–9 wt%) solution is also widely used for a vast range of environmental, medical and household applications. At present, over 99% of H2O2 is produced via the energy-intensive anthraquinone oxidation process [3,4]. For economic reasons, this process only operates in centralized reactors on a large scale, and produces highly concentrated H2O2 that often has to be distributed to, and diluted at, the site of use, bringing additional complexity and challenges [5]. In addition to the anthraquinone oxidation process, H2O2 can also be directly converted from H2 and O2 in the presence of Pd-based catalysts [4,6]. The practical viability of this high-pressure conversion, however, is seriously undermined by its potential hazard of explosion. There is a pressing call to explore other effective methods for the green, mild and on-demand production of H2O2.
The electrochemical synthesis of H2O2 from oxygen reduction reaction (ORR) represents an ideal solution. ORR can take place via a four-electron pathway or a two-electron one. The former yields water as the reduction product, and is the key reaction at the cathode of fuel cells and aqueous metal-air batteries [7–9]. The latter enables the direct production of H2O2 at ambient conditions, but was substantially less investigated until very recently [5,10]. Due to the competitive nature of these two pathways, suitable electrocatalysts are required to selectively promote the two-electron ORR (2e-ORR) process. Previous theoretical study established the binding energy of HOO* (ΔGHOO*) on catalyst surface as the activity descriptor, with the highest activity achieved at the optimal ΔGHOO* ∼ 4.2 eV [11]. This selection rule was used to guide the computational screening of new alloy electrocatalysts and validated through experiments. At present, the state-of-the-art 2e-ORR electrocatalysts are Pt-Hg and Pd-Hg alloys [11,12], followed by Au alloys [13–15]. Despite their relatively high mass activity and selectivity in acids, these precious metal alloys are unlikely to be used on a large scale due to their prohibitive costs and sometimes high toxicity. On the other hand, carbon-based materials (e.g. oxidized carbon nanotubes and reduced graphene oxide) exhibit appreciable 2e-ORR activity and selectivity in alkaline solution but generally very poor performance in neutral or acidic solution [16–18]. Their potentials are also limited since H2O2 is subjected to rapid decomposition in alkaline medium. We are therefore in great need of developing non-precious-metal-based electrocatalysts with outstanding selectivity and activity for 2e-ORR in acids.
Over recent years, two-dimensional (2D) transition metal dichalcogenides (TMDs) have attracted intense research interest owing to their unique anisotropic structures and intriguing physical and chemical properties [19–21]. Many of them (as best exemplified by MoS2) are well known to be active for electrocatalytic hydrogen evolution reaction (HER) [22–24]. Computational and experimental studies suggest that their active sites are often located at the edges [25], and that the catalytic activities can be tuned via proper alloying, doping, and defect and strain engineering [26–29]. However, the potential of TMDs for other electrocatalytic reactions beyond HER remains to be explored. In this study, we demonstrate that 2H-phase molybdenum telluride (MoTe2) nanoflakes, synthesized from bulk powder via ultrasonication-assisted liquid phase exfoliation, acts as an efficient 2e-ORR electrocatalyst in acids. They are measured to catalyze the electrochemical production of H2O2 with high activity, selectivity and stability. Our theoretical calculations reveal that the high activity and selectivity can be attributed to the favorable binding of HOO* and weak binding of O* at the zigzag edges.
RESULTS AND DISCUSSION
Exfoliation and characterizations of MoTe2 nanoflakes
Even though synthesis of high-quality MoTe2 nanosheets or nanoflakes by chemical vapor deposition (CVD) has been reported in literature [30,31], such a high-temperature bottom-up approach is seriously limited by its complexity and low production yield. Here, top-down liquid phase exfoliation (LPE) was employed for its notable simplicity, scalability and reproducibility [32,33]. Commercial crystalline MoTe2 powders were ultrasonicated in N-methylpyrrolidone (NMP) (see Experimental Method in the Supplementary data for details). During the ultrasonication, the weak interlayer van der Waals interactions were interrupted; the 2D bulk crystal was then exfoliated to few-layered nanoflakes stabilized by NMP (Fig. 1a). These nanoflakes were collected by centrifugation and could be readily re-dispersed in common solvents. Figure 1b showed exfoliated MoTe2 nanoflakes re-dispersed in ethanol. The dispersion was stable for weeks without obvious sediment. Powder X-ray diffraction (XRD) pattern of the product confirmed that it was composed of hexagonal 2H MoTe2, which is the most stable form of MoTe2 at low temperatures. Under scanning electron microscopy (SEM), the product was revealed to consist of nanoflakes (Fig. 1d and e). Additional SEM image and low-magnification transmission electron microscopy (TEM) images were supplemented in Fig. S1. Based on TEM statistics, the lateral size of MoTe2 nanoflakes was analyzed to be in the range of 50–350 nm with the mean length ∼143 nm (Fig. S1). Raman spectrum of the product displayed two pronounced peaks at 170 and 233 cm−1 (Fig. 1f), which are characteristic to the A1g and E2g modes of 2H MoTe2, respectively [34,35].
Figure 1.
Preparation and structural characterizations of MoTe2 nanoflakes. (a) Schematic exfoliation process of bulk MoTe2 powders to nanoflakes by LPE. (b) A photo showing the dispersion of MoTe2 nanoflakes in ethanol. (c) XRD and (d) SEM images of MoTe2 nanoflakes. (e) Histogram of lateral size distribution of MoTe2 nanoflakes estimated from multiple SEM images. (f) Raman spectrum of MoTe2 nanoflakes.
To elucidate the atomic structure of exfoliated MoTe2 nanoflakes, aberration-corrected scanning transmission electron microscopy (STEM) was utilized. Figure 2a showed a typical annular dark field (ADF) image of the product, which could be identified as 2H MoTe2 along the c-axis based on the honeycomb pattern of the atomic sites and the corresponding fast Fourier transform (FFT) pattern. The assignment was further corroborated by the excellent agreement between the experimental image and the simulated ADF image of 2H MoTe2 (0001) plane (Fig. 2b and c). Cross-sectional STEM-ADF image of MoTe2 nanoflakes was also acquired as shown in Fig. 2d. Its corresponding FFT pattern matched with the diffraction pattern of 2H MoTe2 along the [ ] zone axis. The simulated ADF image along this zone axis also agreed well with the experimental image (Fig. 2e and f). Furthermore, since the intensity of STEM-ADF image increased approximately linearly with the number of layers in thin 2D crystals, we could determine the nanoflake thickness for up to seven layers (Fig. S2) and directly examine the stacking mode between layers by quantifying the image intensity. As summarized in Fig. S3, the Mo and Te2 atoms in the monolayer honeycomb lattice of H-MoTe2 exhibited very different ADF image intensities, while the atomic sites in the bilayer honeycomb lattice showed very similar image intensity, a characteristic feature for 2H stacking with overlapped Mo+Te2 sites. This intrinsic feature of 2H-stacking MoTe2, with 180° rotation between two adjacent layers, could help us identify the edge orientations in multilayer flakes. The exposed edges, though not atomically sharp, were mostly along the zigzag directions with abundant unsaturated Mo and Te bonds (Fig. 2g), which might be responsible for the observed high electrocatalytic activity as described in the following part [36].
] zone axis. The simulated ADF image along this zone axis also agreed well with the experimental image (Fig. 2e and f). Furthermore, since the intensity of STEM-ADF image increased approximately linearly with the number of layers in thin 2D crystals, we could determine the nanoflake thickness for up to seven layers (Fig. S2) and directly examine the stacking mode between layers by quantifying the image intensity. As summarized in Fig. S3, the Mo and Te2 atoms in the monolayer honeycomb lattice of H-MoTe2 exhibited very different ADF image intensities, while the atomic sites in the bilayer honeycomb lattice showed very similar image intensity, a characteristic feature for 2H stacking with overlapped Mo+Te2 sites. This intrinsic feature of 2H-stacking MoTe2, with 180° rotation between two adjacent layers, could help us identify the edge orientations in multilayer flakes. The exposed edges, though not atomically sharp, were mostly along the zigzag directions with abundant unsaturated Mo and Te bonds (Fig. 2g), which might be responsible for the observed high electrocatalytic activity as described in the following part [36].
Figure 2.

STEM characterization of MoTe2 nanoflakes. (a) STEM ADF image of multilayer MoTe2 along the c-axis and the corresponding FFT pattern (inset). (b) Simulated ADF image of tri-layer 2H MoTe2 along the c-axis. (c) Structural model of 2H MoTe2 along the c-axis. (d) STEM ADF image of MoTe2 along the b-axis and the corresponding FFT pattern (inset). (e) Simulated ADF image of 2H MoTe2 along the b-axis. (f) Structural model of 2H MoTe2 along the b-axis. (g) Edge structures of MoTe2 nanoflakes; the dashed orange lines highlight the zigzag edges.
Electrochemical production of H2O2
To assess the electrocatalytic performance of MoTe2 nanoflakes for 2e-ORR to H2O2, they were physically mixed with graphene nanosheets exfoliated from graphite powders as the conductive additive (Fig. S4, see Experimental Method in the Supplementary data for details). Graphene nanosheets were used here instead of conventional carbon black because of their superior electrical conductivity and similar 2D geometry that could form better contacts with MoTe2 nanoflakes [37]. The electrocatalyst mixture was then loaded onto the glassy carbon disk of a rotating ring disk electrode (RRDE) with an active material loading of 10 μg cm−2. RRDE voltammograms were separately carried out in N2-saturated and O2-saturated 0.5 M H2SO4 at the electrode rotating speed of 1600 rpm (Fig. S5). Corresponding ORR polarization curves were then derived from their differences, and summarized in the lower panel of Fig. 3a. Exfoliated graphene nanosheets alone were electrochemically inert and exhibited no apparent cathodic current density till at <0 V (versus reversible hydrogen electrode or RHE, the same hereafter). Bulk MoTe2 mixed with graphene nanosheets also had a negligible activity. By stark contrast, exfoliated MoTe2 nanoflakes demonstrated a dramatically improved performance with an onset potential as positive as ∼0.56 V (corresponding to an overpotential of ∼140 mV), close to the state-of-the-art PtHg4 alloy reported in literature (∼0.6 V) [11]. Their cathodic current density continuously increased beyond the onset, and reached ∼1.9 mA cm−2 at 0 V. The broad wave centered at ∼0.3 V was likely associated with the reduction of the surface oxide. Based on the concurrently measured ring current, we further derived the H2O2 percentage in the product from MoTe2 nanoflakes (upper panel of Fig. 3a). It was shown to stay >80% at almost the entire potential region with a recorded peak value of ∼93%. Such remarkable selectivity was also comparable to Pt-Hg and Pd-Hg alloys [11,12]. In addition, the effect of catalyst loading on the geometric current density and H2O2 percentage was investigated. Increasing the loading was found to slightly enlarge the cathodic current density but adversely compromise the reaction selectivity (Fig. S6). We speculated that the higher catalyst loading helped retain produced H2O2, and caused it to be further reduced to H2O via another two-electron pathway, therefore giving rise to lower H2O2 selectivity.
Figure 3.

Electrochemical performance of MoTe2 nanoflakes. (a) (Lower panel) polarization curves of MoTe2 nanoflakes, bulk MoTe2 powders and graphene nanosheets alone, respectively and (upper panel) the corresponding ring currents (dashed line) and H2O2 percentage (solid line). (b) Derived mass activity of MoTe2 nanoflakes in comparison with those of Pt/Pd-Hg alloys [11,12] and Au-based catalysts [14,15] estimated from literature. (c) Polarization curves of MoTe2, MoS2 and MoSe2 nanoflakes, respectively. (d) Polarization curves, ring currents and H2O2 percentage of MoTe2 nanoflakes at the initial state and after certain numbers of cycles during the accelerated durability test. The electrolyte in use was O2-saturated 0.5 M H2SO4; the electrode rotating speed was 1600 rpm.
In order to allow direct activity comparison with other reported 2e-ORR electrocatalysts in acids, we normalized the H2O2 partial current of MoTe2 nanoflakes over the mass of active material as presented in Fig. 3b. The mass activity was calculated to be in the range of ∼10–102 A g−1 between 0.3–0.45 V, which, although still not as magnificent as the state-of-the-art Pt-Hg and Pd-Hg alloys [11,12], was superior to Au alloys [14,15] and carbon-based materials [38,39]. For example, the mass activity of MoTe2 nanosheets at 0.4 V was 27 A g−1—which is ∼7–10 times greater than those of Au-Pd alloys [14,15] and N-doped carbon [38,39]. Interestingly, MoTe2 seemed to be quite unique among TMD materials for the electrochemical production of H2O2. Its sulfide and selenide analogues—MoS2 and MoSe2 nanoflakes likewise exfoliated from corresponding bulk powders and hybridized with graphene nanosheets—exhibited significantly worse activities (Fig. 3c).
Stability is another key parameter in the electrocatalyst assessment. Despite the concern over the susceptibility of MoTe2 to oxidation, we found that exfoliated MoTe2 nanoflakes had very decent electrochemical stability under the 2e-ORR working condition, at least for several days. To demonstrate this, our electrocatalyst was subjected to an accelerated durability test by rapidly cycling between 0 and 0.3 V at 100 mV s−1 in O2-saturated electrolyte for a predetermined number of cycles, and then measuring its polarization curves. Figure 3d compared the polarization curves at the initial state, after 2500 cycles and after 5000 cycles. The measured disk polarization curves remained largely similar except for the reduction wave at ∼0.3 V presumably due to the partial surface oxidation during the durability test. There was only a slight decrease in the potential-dependent H2O2 percentage likely as a result of the catalyst oxidation. Moreover, even after MoTe2 nanoflakes were aged overnight (>12 h) in the O2-saturated electrolyte at the open-circuit potential, only slight current decay occurred and no apparent selectivity decay were observed as compared to the fresh electrode (Fig. S7).
DFT calculations
In order to understand the origin of the high activity and selectivity of MoTe2 nanoflakes, spin-unrestricted density functional theory (DFT) calculations were carried out to simulate the 2e-ORR process on both the basal plane and edge site of 2H MoTe2 as well as several other materials. The basal plane slab was modeled using a 4 × 4 × 1 supercell (Fig. S8). Previous experimental studies on MoS2 demonstrated that its most stable edges were zigzag-type with the Mo atoms covered by 50% S coverage [40,41]. As a result, the Mo-edge slab in our study was constructed with 50% Te coverage and a periodicity of 3 Mo atoms, which was determined to be the most stable configuration (Fig. S9). 2e-ORR to H2O2 generally involves two elemental steps: molecular O2 is first transformed to HOO* via a proton-coupled electron transfer, followed by the protonation and reduction of HOO* to yield H2O2. Previous study established that the binding energy of HOO* (ΔGHOO*) was an effective activity descriptor, and its optimal value was found to be ∼4.2 eV [42]. Following this guiding principle, ΔGHOO* on different surface sites was computed and compared. Figure 4a showed the optimized structures of HOO* adsorbed on the basal plane or edge of 2H MoTe2. ΔGHOO* calculations suggested that the H2O2 formation was overwhelmingly challenging on the basal plane (ΔGHOO* = 5.34 eV). Introduction of Te vacancies to the basal plane was also not helpful. For example, HOO* could not even be produced over the single Te vacancy on the basal plane as it would be spontaneously dissociated to O* adsorbed on the Te vacancy site and HO* adsorbed on the nearby Te site (Fig. S10). By contrast, the reaction could readily proceed at the zigzag edge with ΔGHOO* = 4.32 eV. Such an observation was interestingly reminiscent of the structure-dependent HER activities of 2H-MoS2 and many other TMD materials. Furthermore, the theoretical overpotential (ηt) of 2e-ORR at the edge of MoTe2 was estimated by |ΔGHOO*/e − 4.22 V| and calculated to be 100 mV, which agreed reasonably well with the experimental value (∼140 mV), and was sufficiently close to that of PtHg4(110) surface (ΔGHOO* = 4.28 eV and ηt = 60 mV) [11]. It unambiguously corroborated the high 2e-ORR activity of 2H MoTe2. Worth noting is that the armchair edge of MoTe2 (Fig. S11) was not catalytically active for 2e-ORR because it bound HOO* too weakly (ΔGHOO* = 4.60 eV). For the purpose of comparison, the HOO* adsorption on Pt(111) was calculated to be considerably stronger than the ideal (ΔGHOO* = 3.98 eV), and the HOO* adsorption on MoS2 and MoSe2 edges significantly weaker than the ideal (ΔGHOO* = 4.60 eV and 4.59 eV on MoS2 and MoSe2, respectively, Fig. 4b). The observed inefficiency of MoS2 and MoSe2 for 2e-ORR was therefore rationalized in spite of their structural similarity to MoTe2.
Figure 4.
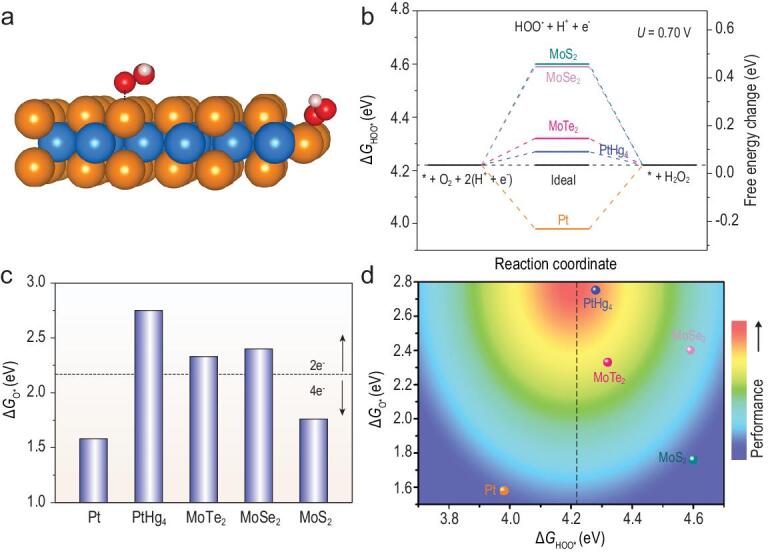
DFT simulations of the 2e-ORR pathway on 2H MoTe2. (a) Optimized structure of HOO* adsorbed on the basal plane or edge site; blue, orange, red and white spheres represent Mo, Te, O and H atoms, respectively. (b) ΔGHOO* for 2e-ORR to H2O2 on Pt [11], PtHg4 [11], MoTe2, MoSe2 and MoS2 at the equilibrium potential of UO2/H2O2 = 0.70 V and the corresponding free-energy profiles. (c) ΔGO* of Pt [11], PtHg4 [11], MoTe2, MoSe2 and MoS2; the position of the dashed line (2.17 eV) denotes the average value of ΔGO* on Pt (typical 4e-ORR electrocatalyst) and PtHg4 (typical 2e-ORR electrocatalyst), which was proposed to be the boundary between 4e and 2e selectivity. (d) 2D ‘heat map’ for 2e-ORR performance (both activity and selectivity); the dashed line represents the optimal ΔGHOO* for H2O2 production.
Of note, a favorable ΔGHOO* value does not necessarily guarantee high H2O2 selectivity since 2e-ORR and 4e-ORR share the same initial step. The further transformation of HOO* to O* (4e-ORR pathway) would have to be effectively suppressed in order to achieve high H2O2 selectivity. As a result, weak O* binding energy (ΔGO*) on the catalyst surface becomes a prerequisite. Based on this rationale, we could understand the predominant 4e-ORR selectivity on Pt owing to its very strong O* affinity (ΔGO* = 1.58 eV on Pt(111)), as well as the predominant 2e-ORR selectivity on PtHg4 owing to its very weak O* affinity (ΔGO* = 2.75 eV on PtHg4(110)) (Fig. 4c) [11]. Our calculations showed that the edge of 2H MoTe2 had ΔGO* = 2.33 eV, which, albeit still not as positive as PtHg4, was sufficiently large to render MoTe2 H2O2-selective. It is also worth mentioning that MoS2 relatively favored 4e-ORR, and MoSe2 relatively favored 2e-ORR even though both of them had negligible activities. At last, based on the previous volcano for 2e-ORR built upon the sole activity descriptor ΔGHOO* [11], we further compiled our computation results on a 2D heat map as shown in Fig. 4d. We implicitly assumed that the hottest spot was located at ΔGHOO* = 4.22 eV and ΔGO* = 2.75 eV. The ΔGO* of PtHg4 (2.75 eV) was chosen as a reference given the fact that, in contrast to HOO*, there is not explicit criteria for denoting the optimal O* affinity. According to this definition, the closer to the hottest spot, the better 2e-ORR performance, and vice versa. Among different materials under investigation, PtHg4 was in the region of the greatest activity and followed by MoTe2, while MoSe2, MoS2 and Pt were quite far away from the hot area.
CONCLUSION
In summary, we for the first time introduced 2H-MoTe2 nanoflakes as a high-performance catalyst for electrochemical H2O2 production in acids. MoTe2 nanoflakes were exfoliated from the bulk powder via LPE in NMP. They were determined to have small thickness of a few nm, lateral sizes of 100∼500 nm and with preferentially exposed zigzag edges. When physically mixed with graphene nanosheets as the conductive additive, the MoTe2 nanoflakes demonstrated excellent activity and selectivity for 2e-ORR in 0.5 M H2SO4, with an onset potential at ∼0.56 V (η ∼140 mV), a large mass activity of 27 A g−1 at 0.4 V and high H2O2 selectivity up to ∼93%. Such a performance was far superior to those of Au alloys and N-doped carbon reported in literature, and approaching that of the state-of-the-art PtHg4 alloy. MoTe2 nanoflakes also exhibited impressive chemical and electrochemical stability in an accelerated durability test and overnight aging experiment. Finally, detailed DFT calculations showed that the high activity and selectivity of 2H MoTe2 originated from the favorable binding of HOO* and weak binding of O* at the zigzag edges, and thereby directly correlated the electrocatalytic performance with the unique anisotropic structure of MoTe2. Our study here unveiled the unexpected potential of MoTe2 nanoflakes as a non-precious-metal-based electrocatalyst for H2O2 production in acids, and might open a new pathway toward the catalyst design for this challenging electrochemical reaction.
Supplementary Material
Contributor Information
Xuan Zhao, Institute of Functional Nano and Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou 215123, China.
Yu Wang, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China.
Yunli Da, College of Material Science and Opto-Electronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China.
Xinxia Wang, Institute of Functional Nano and Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou 215123, China.
Tingting Wang, Institute of Functional Nano and Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou 215123, China.
Mingquan Xu, School of Physical Sciences and CAS Key Laboratory of Vacuum Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Xiaoyun He, School of Physics, CRANN and AMBER Centers, Trinity College Dublin, Dublin 2, Ireland.
Wu Zhou, School of Physical Sciences and CAS Key Laboratory of Vacuum Sciences, University of Chinese Academy of Sciences, Beijing 100049, China.
Yafei Li, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, China.
Jonathan N Coleman, School of Physics, CRANN and AMBER Centers, Trinity College Dublin, Dublin 2, Ireland.
Yanguang Li, Institute of Functional Nano and Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials and Devices, Soochow University, Suzhou 215123, China.
FUNDING
Y.G.L. acknowledges the support of the Ministry of Science and Technology of China (2017YFA0204800), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Collaborative Innovation Center of Suzhou Nano Science and Technology. Y.F.L. acknowledges the support of the National Natural Science Foundation of China (21873050). W.Z. is grateful for the financial support from the National Natural Science Foundation of China (51622211).
AUTHOR CONTRIBUTIONS
Y.G.L. conceived the project and designed the experiments. X.Z. and X.W. prepared the material and conducted electrochemical measurements. X.H. and J.N.C. contributed to the material preparation method. T.W. assisted in electrochemical measurements. Y.D., M.X. and W.Z. performed the STEM analysis. Y.W. and Y.F.L. conducted the theoretical calculations. X.Z., Y.W., Y.D. and Y.G.L. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Conflict of interest statement . None declared.
REFERENCES
- 1. Fukuzumi S, Yamada Y, Karlin KD. Hydrogen peroxide as a sustainable energy carrier: electrocatalytic production of hydrogen peroxide and the fuel cell. Electrochim Acta 2012; 82: 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ciriminna R, Albanese L, Meneguzzo Fet al. Hydrogen peroxide: a key chemical for today's sustainable development. ChemSusChem 2016; 9: 3374–81. [DOI] [PubMed] [Google Scholar]
- 3. Samanta C. Direct synthesis of hydrogen peroxide from hydrogen and oxygen: an overview of recent developments in the process. Appl Catal A-Gen 2008; 350: 133–49. [Google Scholar]
- 4. Campos-Martin JM, Blanco-Brieva G, Fierro JLG. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process. Angew Chem Int Ed 2006; 45: 6962–84. [DOI] [PubMed] [Google Scholar]
- 5. Yang S, Verdaguer-Casadevall A, Arnarson Let al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal 2018; 8: 4064–81. [Google Scholar]
- 6. Edwards JK, Freakley SJ, Lewis RJet al. Advances in the direct synthesis of hydrogen peroxide from hydrogen and oxygen. Catal Today 2015; 248: 3–9. [Google Scholar]
- 7. Shao MH, Chang QW, Dodelet JPet al. Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev 2016; 116: 3594–657. [DOI] [PubMed] [Google Scholar]
- 8. Kulkarni A, Siahrostami S, Patel Aet al. Understanding catalytic activity trends in the oxygen reduction reaction. Chem Rev 2018; 118: 2302–12. [DOI] [PubMed] [Google Scholar]
- 9. Li YG, Dai HJ. Recent advances in zinc-air batteries. Chem Soc Rev 2014; 43: 5257–75. [DOI] [PubMed] [Google Scholar]
- 10. Jiang YY, Ni PJ, Chen CXet al. Selective electrochemical H2O2 production through two-electron oxygen electrochemistry. Adv Energy Mater 2018; 8: 1801909. [Google Scholar]
- 11. Siahrostami S, Verdaguer-Casadevall A, Karamad Met al. Enabling direct H2O2 production through rational electrocatalyst design. Nat Mater 2013; 12: 1137–43. [DOI] [PubMed] [Google Scholar]
- 12. Verdaguer-Casadevall A, Deiana D, Karamad Met al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett 2014; 14: 1603–8. [DOI] [PubMed] [Google Scholar]
- 13. Zheng Z, Ng YH, Wang DWet al. Epitaxial growth of Au-Pt-Ni nanorods for direct high selectivity H2O2 production. Adv Mater 2016; 28: 9949–55. [DOI] [PubMed] [Google Scholar]
- 14. Jirkovský JS, Panas I, Ahlberg Eet al. Single atom hot-spots at Au–Pd nanoalloys for electrocatalytic H2O2 production. J Am Chem Soc 2011; 133: 19432–41. [DOI] [PubMed] [Google Scholar]
- 15. Pizzutilo E, Freakley SJ, Cherevko Set al. Gold-palladium bimetallic catalyst stability: consequences for hydrogen peroxide selectivity. ACS Catal 2017; 7: 5699–705. [Google Scholar]
- 16. Lu ZY, Chen GX, Siahrostami Set al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat Catal 2018; 1: 156–62. [Google Scholar]
- 17. Kim HW, Ross MB, Kornienko Net al. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat Catal 2018; 1: 282–90. [Google Scholar]
- 18. Liu YM, Quan X, Fan XFet al. High-yield electrosynthesis of hydrogen peroxide from oxygen reduction by hierarchically porous carbon. Angew Chem Int Ed 2015; 54: 6837–41. [DOI] [PubMed] [Google Scholar]
- 19. Chhowalla M, Shin HS, Eda Get al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat Chem 2013; 5: 263–75. [DOI] [PubMed] [Google Scholar]
- 20. Butler SZ, Hollen SM, Cao LYet al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013; 7: 2898–926. [DOI] [PubMed] [Google Scholar]
- 21. Lv R, Robinson JA, Schaak REet al. Transition metal dichalcogenides and beyond: synthesis, properties, and applications of single- and few-layer nanosheets. Acc Chem Res 2015; 48: 56–64. [DOI] [PubMed] [Google Scholar]
- 22. Xu J, Zhang JJ, Zhang WJet al. Interlayer nanoarchitectonics of two-dimensional transition-metal dichalcogenides nanosheets for energy storage and conversion applications. Adv Energy Mater 2017; 7: 1700571. [Google Scholar]
- 23. Ding Q, Song B, Xu Pet al. Efficient electrocatalytic and photoelectrochemical hydrogen generation using MoS2 and related compounds. Chem 2016; 1: 699–726. [Google Scholar]
- 24. Yan Y, Xia BY, Xu ZCet al. Recent development of molybdenum sulfides as advanced electrocatalysts for hydrogen evolution reaction. ACS Catal 2014; 4: 1693–705. [Google Scholar]
- 25. Gholamvand Z, McAteer D, Harvey Aet al. Electrochemical applications of two-dimensional nanosheets: the effect of nanosheet length and thickness. Chem Mater 2016; 28: 2641–51. [Google Scholar]
- 26. Jayabal S, Saranya G, Wu Jet al. Understanding the high-electrocatalytic performance of two-dimensional MoS2 nanosheets and their composite materials. J Mater Chem A 2017; 5: 24540–63. [Google Scholar]
- 27. Zhu CR, Gao DQ, Ding Jet al. TMD-based highly efficient electrocatalysts developed by combined computational and experimental approaches. Chem Soc Rev 2018; 47: 4332–56. [DOI] [PubMed] [Google Scholar]
- 28. Tan CL, Lai ZC, Zhang H. Ultrathin two-dimensional multinary layered metal chalcogenide nanomaterials. Adv Mater 2017; 29: 1701392. [DOI] [PubMed] [Google Scholar]
- 29. Zeng M, Li YG. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J Mater Chem A 2015; 3: 14942–62. [Google Scholar]
- 30. Empante TA, Zhou Y, Klee Vet al. Chemical vapor deposition growth of few layer MoTe2 in the 2H, 1T', and 1T phases: tunable properties of MoTe2 films. ACS Nano 2017; 11: 900–5. [DOI] [PubMed] [Google Scholar]
- 31. Zhou L, Xu K, Zubair Aet al. Large-area synthesis of high-quality uniform few-layer MoTe2. J Am Chem Soc 2015; 137: 11892–5. [DOI] [PubMed] [Google Scholar]
- 32. Coleman JN, Lotya M, O’Neill Aet al. Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science 2011; 331: 568–71. [DOI] [PubMed] [Google Scholar]
- 33. Nicolosi V, Chhowalla M, Kanatzidis MGet al. Liquid exfoliation of layered materials. Science 2013; 340: 1226419. [Google Scholar]
- 34. Ruppert C, Aslan OB, Heinz TF. Optical properties and band gap of single- and few-layer MoTe2 crystals. Nano Lett 2014; 14: 6231–6. [DOI] [PubMed] [Google Scholar]
- 35. Guo HH, Yang T, Yamamoto Met al. Double resonance Raman modes in monolayer and few-layer MoTe2. Phys Rev B 2015; 91: 205415. [Google Scholar]
- 36. Zhou W, Zou XL, Najmaei Set al. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett 2013; 13: 2615–22. [DOI] [PubMed] [Google Scholar]
- 37. Hernandez Y, Nicolosi V, Lotya Met al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 2008; 3: 563–8. [DOI] [PubMed] [Google Scholar]
- 38. Fellinger TP, Hasche F, Strasser Pet al. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J Am Chem Soc 2012; 134: 4072–5. [DOI] [PubMed] [Google Scholar]
- 39. Park J, Nabae Y, Hayakawa Tet al. Highly selective two-electron oxygen reduction catalyzed by mesoporous nitrogen-doped carbon. ACS Catal 2014; 4: 3749–54. [Google Scholar]
- 40. Lauritsen JV, Kibsgaard J, Helveg Set al. Size-dependent structure of MoS2 nanocrystals. Nat Nanotechnol 2007; 2: 53–8. [DOI] [PubMed] [Google Scholar]
- 41. Wang ZY, Li H, Liu Zet al. Mixed low-dimensional nanomaterial: 2D ultranarrow MoS2 inorganic nanoribbons encapsulated in quasi-1D carbon nanotubes. J Am Chem Soc 2010; 132: 13840–7. [DOI] [PubMed] [Google Scholar]
- 42. Viswanathan V, Hansen HA, Rossmeisl Jet al. Unifying the 2e− and 4e− reduction of oxygen on metal surfaces. J Phys Chem Lett 2012; 3: 2948–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



