Abstract
Herein, a wide-spectrum (∼678 nm) responsive Bi8(CrO4)O11 photocatalyst with a theoretical solar spectrum efficiency of 42.0% was successfully constructed. Bi8(CrO4)O11 showed highly efficient and stable photocatalytic water oxidation activity with a notable apparent quantum efficiency of 2.87% (420 nm), superior to many reported wide-spectrum driven photocatalysts. Most remarkably, its strong oxidation ability also enables the simultaneous degradation and complete mineralization for phenol, and its excellent performance is about 23.0 and 2.9 times higher than CdS and P25-TiO2, respectively. Its high activity is ascribed to the giant internal electric field induced by its large crystal dipole, which accelerates the rapid separation of photogenerated electron–hole pairs. Briefly, the discovery of wide-spectrum bismuth chromate and the mechanism of exponentially enhanced photocatalytic performance by increasing the crystal dipole throw light on improving solar energy conversion.
Keywords: bismuth chromate, dipole moment, internal electric field, water oxidation, complete mineralization
INTRODUCTION
The conversion and utilization of solar energy for chemical fuel production and environmental remediation through artificial photocatalysis have been recognized to be an ideal route to address critical energy and environmental concerns [1–3]. The full utilization of solar light is a great challenge for achieving sufficient efficiency in practical applications. In the early stages, UV light-activated materials, such as TiO2, SrTiO3, NaTaO3, etc., dominated photocatalysis study, due to the wide bandgap of conventional semiconductors and their strong redox capability of charge carriers for igniting chemical reactions [4–7]. Nonetheless, the extremely low ratio of UV photons in solar energy greatly hinders the ability to maximize the solar-to-chemical energy conversion efficiency. In recent years, a number of mixed-anion and non-oxide materials such as (oxy)nitrides and (oxy)sulfides have been developed as attractive broadband light-responsive photocatalysts [8–11]. The valence band maximums (VB) of the mixed-anion materials can be substantially regulated by hybridization of O 2p or other introduced anion orbitals, enabling both broadband light absorption and suitable band potentials for both reduction and oxidation of water [12,13]. For example, BaNbO2N, reported by Hisatomi et al., could broaden the light absorption up to 740 nm and simultaneously shows efficient water oxidation [14]. However, narrowing the bandgap of a photocatalyst weakens the driving force for redox reactions, especially water oxidation and pollutant degradation, because these reactions involve a complicated multi-electron process [15]. Therefore, the development of wide-spectrum responsive and highly efficient photocatalysts for water oxidation and pollutant degradation is a critical issue to be addressed at present.
Bi-based oxometallate materials, such as BiVO4, Bi2WO6, Bi2MoO6, etc., have been widely studied as visible-light active photocatalysts, due to their high stability, abundant resources and low toxicity [16–19]. Moreover, they also exhibit excellent photocatalytic performance in water oxidation, which is mainly benefiting from their sufficiently deep VB position as compared to the potential for water oxidation and pollutant degradation [20,21]. In particular, the BiVO4 photocatalysts present highly efficient and stable water oxidation performance, and its highest solar-to-hydrogen energy conversion efficiency of 1.2% for Z-scheme pure-water splitting by coupling with SrTiO3: La, Rh has been reported [22]. Nevertheless, their relatively wide bandgaps (about 2.5 eV) greatly limit their further application. Recently, research into Cr-based layered double hydroxide photocatalysts revealed that the hybridization of Cr 3d orbitals with O 2p orbitals in [CrO6] clusters shifts the conduction band minimum (CB) down and results in wide visible-light absorption [23,24]. Inspired by the above, the construction of bismuth chromate photocatalyst may be a desired route to achieve wide-spectrum driven, efficient, and stable photocatalytic performance.
In this work, a wide-spectrum responsive Bi8(CrO4)O11 photocatalyst was successfully constructed. Owing to the hybridization of Cr 3d with O 2p orbitals shifting the conduction band minimum down, Bi8(CrO4)O11 allows its absorption up to the entire visible region (∼678 nm) with a theoretical solar spectrum efficiency of 42.0%. Moreover, attributed to the giant internal electric field (IEF) induced by its large dipole moment, Bi8(CrO4)O11 realized evidently rapid separation of photogenerated electron–hole pairs, thus showed highly efficient photocatalytic water oxidation activity with a notable apparent quantum yield of 2.87% (420 nm), superior to many reported wide-spectrum driven photocatalysts. Most remarkably, its strong oxidation ability also enables simultaneous degradation and complete mineralization for phenol, and its excellent performance is about 23.0 and 2.9 times higher than CdS and P25–TiO2, respectively.
RESULTS AND DISCUSSION
Herein, monoclinic Bi8(CrO4)O11 nanorods (Figs S1–S3), a novel bismuth chromate photocatalyst, were successfully synthesized via a facile hydrothermal reaction. Then, density functional theory (DFT) was applied to calculate the electronic structure of this bismuth chromate. As shown in Fig. 1a, Bi8(CrO4)O11 possesses a relatively small bandgap of 1.71 eV. Moreover, the density of state of Bi8(CrO4)O11 reveals that its VB is mainly composed of O 2p and Bi 6s orbitals, in agreement with other Bi-based oxometallate photocatalysts, which could effectively avoid the self-oxidative deactivation by photogenerated holes. Also, its CB is mainly provided by the hybridization of Cr 3d orbitals with O 2p orbitals, demonstrating that the introduction of the [CrO4] cluster plays a crucial role in extending absorption into the entire visible region. Moreover, the indirect band structure of Bi8(CrO4)O11 is also confirmed by its electronic band diagram (Fig. S4a), which is in favor of confining the recombination of photogenerated electron–hole pairs. In the diffuse reflectance spectrum (DRS) (Fig. 1b), Bi8(CrO4)O11 nanorod photocatalyst displays a quite broad absorption band, practically allowing light absorption up to the entire visible region, and its highest theoretical solar utilization could reach 42.0%. Almost consistent with the above DFT result, the bandgap of Bi8(CrO4)O11 was calculated as 1.83 eV by the Kubelka–Munk function, which absolutely satisfies the thermodynamic energy criterion of water splitting [25,26]. As shown in Fig. 1c, Bi8(CrO4)O11 presents an evidently high surface photovoltage (SPV), and the response range could be extended to about 678 nm, demonstrating its wide-spectrum driven photocatalytic activity. Besides, it exhibits a positive surface photovoltage signal, meaning that the photogenerated holes are the main carriers and transfer to the surface to oxidize reactants. Therefore, the above results indicate that the Bi8(CrO4)O11 nanorod is a very promising wide-spectrum driven and stable photocatalyst.
Figure 1.
(a) The calculated density of state, (b) UV–vis–NIR DRS (the corresponding Tauc plots and a sample photograph appear in the inset), (c) the surface photovoltage spectrum, and (d) a schematic drawing of the redox potentials of Bi8(CrO4)O11.
Considering that the photocatalytic redox ability mainly depends on the energy band potential, the redox potentials of the Bi8(CrO4)O11 nanorod photocatalyst were calculated according to the DRS and Mott–Schottky plots (Fig. S5a) [27,28]. As shown in Fig. 1d, the CB of Bi8(CrO4)O11 is located at 0.12 eV vs. NHE (pH = 0), a little deeper than the reduction potential of H+/H2. Also, its VB of 1.95 eV is more positive than the oxidation potential of OH−/O2, which indicates that the photogenerated holes of Bi8(CrO4)O11 nanorod photocatalyst possess extremely strong oxidation capability, and can split water to release O2, and even completely mineralize organic pollutants under visible light.
We first evaluate the photocatalytic water oxidation performance over Bi8(CrO4)O11 nanorods. Figure 2a shows a comparison of the photocatalytic O2 evolution rate over different samples. It can be seen that Bi8(CrO4)O11 exhibited far superior photocatalytic water oxidation performance, and its average O2 evolution rate reached 14.94 μmol h−1, about 11.5 and 4.0 times higher than that of Bi2WO6 nanosheets [29] and commercial WO3 nanoparticles. Besides, Bi8(CrO4)O11 consequently achieved a considerable apparent quantum efficiency (AQE) 2.87% at 420 nm, even 0.65% at 650 nm (Fig. 2b), higher than many reported wide-spectrum driven photocatalysts (Table S2). In addition, the trend of AQE values for water oxidation over Bi8(CrO4)O11 is also consistent with its UV–vis DRS, further confirming that the photocatalytic water oxidation reaction is driven by its absorbed incident photons. Furthermore, after loading Co(OH)2 as co-catalyst, its photocatalytic water oxidation performance was improved by 2.1 times (Fig. S6). Just as importantly, no notable deactivation emerged over Bi8(CrO4)O11 during a continuous photocatalytic water oxidation reaction for 72 h (Fig. S7a). By comparing its XRD patterns and XPS results before and after reaction (Figs S7b and S8), it could be found that the crystal structure and composition of Bi8(CrO4)O11 after reaction show no marked change, further indicating its robust resistance to water and light corrosion.
Figure 2.
(a) A comparison of photocatalytic water oxidation activity over different photocatalysts. (b) The wavelength-dependent AQE of water oxidation over Bi8(CrO4)O11. (c) A comparison of degradation rate constants, degradation rates and TOC removal rates of phenol over different photocatalysts. (d) The wavelength-dependent degradation rate and TOC removal rate of phenol over Bi8(CrO4)O11.
Most noticeably, the excellent activity of Bi8(CrO4)O11 is also manifested in photocatalytic degradation of phenol. As shown in Fig. 2c, Bi8(CrO4)O11 showed a superior photocatalytic degradation performance for phenol under visible light, and its degradation reaction constant could reach 0.119 min−1, about 22.5 and 8.8 times higher than CdS nanowires [30] and N,N′-di(propanoic acid)-perylene-3,4,9,10-tetracarboxylic diimide (PDI) supramolecular [31,32] photocatalysts, respectively. Even its degradation activity is not inferior to P25 TiO2 under simulated sunlight, being about 2.9 times higher than the latter. Remarkably, Bi8(CrO4)O11 also presented extremely strong mineralization ability, which almost enables simultaneous degradation and complete mineralization for phenol. The total organic carbon (TOC) removal rates of phenol over Bi8(CrO4)O11 under visible light and simulated sunlight are 94.8% (degradation rate: 95.5%) and 97.3% (degradation rate: 98.1%) in 0.5 h, respectively, while that of CdS, PDI and P25 are significantly lower than their corresponding degradation rates. In particular, even under 650 nm red light irradiation, Bi8(CrO4)O11 is still able to simultaneously degrade and completely mineralize phenol (Fig. 2d), and few wide-spectrum driven photocatalysts can achieve that [33]. Besides, Bi8(CrO4)O11 also exhibited highly efficient photocatalytic formaldehyde degradation activity under visible light in a continuous-flow system, and the removal rate could be maintained at about 95% (Fig. S10c). No notable deactivation emerges during continuous measurement for 76 h.
It is well known that photocatalytic activity is closely related to the separation efficiency of photogenerated electron–hole pairs [34–36]. Previous studies have demonstrated that the IEF induced by the crystal dipole is considered to effectively boost the separation of photogenerated electron–hole pairs and enhance the photocatalytic performance exponentially, such as in Bi2MoO6, BiPO4, and BiOCl [37–39]. Therefore, to reveal the high activity mechanism of Bi8(CrO4)O11, the crystal dipoles of Bi8(CrO4)O11 and tetragonal Bi14CrO24 nanosheets (Figs S1 and S2) and their influence on the charge carrier separation and photocatalytic activity were studied. Through the Debye equation, the dipole moments of Bi8(CrO4)O11 and Bi14CrO24 were calculated to be 22.32 and 2.52 Debye (D), respectively. As shown in Fig. 3a, due to the existence of the great dipole of Bi8(CrO4)O11, the distortion of [BiOx] and [CrOy] polyhedrons induced an apparently uneven distribution of the electronic cloud between Bi–O and Cr–O, thus resulting in a giant IEF. Then, Kelvin probe force microscopy techniques were employed to reveal the IEF distribution in Bi8(CrO4)O11 and Bi14CrO24. As shown in Fig. 3b, Bi8(CrO4)O11 shows an obvious difference in the contact potential difference (CPD) between the edge and the bulk, about 202 mV (Fig. 3c), but the CPD difference over Bi14CrO24 is virtually invisible, only about 39 mV. According to the literature, the relatively large CPD difference between the two regions reflects that a relatively strong IEF is formed in the crystal [40–42], consequently demonstrating the existence of a greater IEF in Bi8(CrO4)O11.
Figure 3.
(a) A side view of the charge density difference and planar-averaged electron density difference Δρ(z) (the yellow and green areas indicate electron depletion and accumulation, respectively), (b) the surface potential image in the dark state, (c) the cross section of the surface potential distribution along the blue line ab and cd in (b) and (d) the correlation between dipole moments, internal electric field intensity, surface photovoltage and photocatalytic activity of Bi8(CrO4)O11 and Bi14CrO24.
Furthermore, the intensity of their IEF was measured via the model developed by Kanata-Kito et al. (details are given in the supplementary data online) [43,44]. It can be found that the IEF of Bi8(CrO4)O11 is 8.4 times as high as that of Bi14CrO24 (Fig. S13), well consistent with the above results. Benefiting from its greater IEF, Bi8(CrO4)O11 presented an evidently stronger surface photovoltage response and photocurrent density (Fig. S16), about 22.7 and 4.0 times higher than Bi14CrO24, respectively, revealing that a faster charge carrier transfer kinetics emerges in Bi8(CrO4)O11. As expected, Bi8(CrO4)O11 exhibited 17.2 and 153.0 times higher photocatalytic water oxidation and degradation performance than Bi14CrO24, respectively. Then, after summarizing the above results into Fig. 3d, it can be found that the IEF, charge separation efficiency and photocatalytic activity of bismuth chromate are positively correlated with their dipole moments; thus Bi8(CrO4)O11 with a greater dipole showed a significantly higher IEF, charge separation efficiency and photocatalytic performance. Therefore, as illustrated in Scheme 1, the large crystal dipole of Bi8(CrO4)O11 induces a giant IEF, which accelerates the rapid separation of photogenerated electron–hole pairs and exponentially enhances its photocatalytic performance. Most importantly, based on the above mechanism, many more efficient photocatalysts can be designed successfully by regulating the crystal dipole.
Scheme 1.

Schematic mechanism of the photocatalytic reaction over Bi8(CrO4)O11.
CONCLUSION
In conclusion, a wide-spectrum (∼678 nm) responsive Bi8(CrO4)O11 nanorod photocatalyst was constructed via the hybridization of Cr 3d with O 2p orbitals. Attributed to the giant IEF induced by its large dipole moment, Bi8(CrO4)O11 realizes evidently rapid separation of photogenerated electron–hole pairs, thus showing highly efficient photocatalytic water oxidation performance with a notable apparent quantum yield of 2.87% (420 nm), superior to many reported wide-spectrum driven photocatalysts. Most remarkably, its strong oxidation ability also enables simultaneous degradation and complete mineralization for phenol, and its excellent performance is about 23.0 and 2.9 times higher than CdS and P25-TiO2, respectively. Briefly, the discovery of wide-spectrum bismuth chromate and the mechanism of exponentially enhanced photocatalytic performance by increasing the crystal dipole throw light on designing efficient wide-spectrum photocatalysts.
METHODS
Synthesis of samples
NaBiO3 and other chemicals were purchased from Aladdin and Sigma-Aldrich, respectively, and used without further purification. For Bi8(CrO4)O11 nanorods, 0.56 g NaBiO3 was ultrasonically dispersed in 80 mL deionized water, followed by addition of 7.35 mL 25 mmol L−1 Cr(NO)3 aqueous solution under vigorous stirring. Then the resulting solution was transferred into a 100 mL Teflon-lined stainless autoclave and maintained at 180°C for 6 h. The brown-red Bi8(CrO4)O11 was collected by centrifuge separation, rinsed thoroughly with ethanol and deionized water several times, and dried at 70°C overnight. For Bi14CrO24 nanosheets, 1 mmol Bi(NO3)3 and 0.084 mmol Cr(NO3)3 were ultrasonically dissolved in a certain concentration of mannitol aqueous solution (25 mL), followed by addition of 5 ml saturated Na2CO3 solution under vigorous stirring. Then the resulting solution was transferred into a 50 mL Teflon-lined stainless autoclave and maintained at 150°C for 12 h. The precursor was collected by centrifuge separation, rinsed thoroughly with ethanol and deionized water several times, and dried at 70°C overnight. The precursor was then calcined in a crucible at a certain temperature for 10 min under air atmosphere to yield orange-red Bi14CrO24 nanosheets.
For comparison, BiWO6 nanoplates were synthesized as in [29], and WO3 nanoparticles were purchased from Aladdin.
Characterization
XRD patterns of the samples were obtained on a Rigaku D/max-2400 X-ray diffractometer using Cu Kα1 (λ = 0.154 18 nm) at 40 kV and 200 mA, with a scan step of 0.02°. The morphologies of the samples were measured by transmission electron microscopy (TEM) on a Hitachi HT 7700 at an accelerating voltage of 100 kV and high-resolution transmission electron microscopy (HRTEM) on a JEOL JEM-2100F operated at an accelerating voltage of 200 kV. Field emission scanning electron microscopy (FESEM) on a Hitachi SU-8010 was used to further investigate the morphology. XPS measurements were performed using an ESCALAB 250Xi instrument (Thermo Scientific) with Al Kα radiation. DRS were obtained on a Cary 5000 (Varian) with BaSO4 as a reference. The surface potential images of the samples were measured by Kelvin probe force microscopy (KPFM) in ambient atmosphere on a Cypher VRS (Oxford Instruments) and a Pt/Ir-coated Si tip was used as a Kelvin tip. The surface photovoltage measurements were conducted with a home-built instrument as previously reported [45]. Photoelectrochemical measurements were performed on a CHI660E electrochemical workstation, using a standard three-electrode cell with a working electrode, a Pt-wire counter electrode and a saturated calomel reference electrode. Na2SO4 (0.1 mol L−1) was used the electrolyte solution. The working electrode was prepared by dip-coating photocatalyst slurry on ITO glass electrode (2 × 4 cm2).
Photocatalytic performance evaluation
The photocatalytic water oxidation reaction under visible-light irradiation was performed in a Pyrex top-irradiation reaction vessel with a stationary temperature at 5°C, which was connected to a glass closed gas system (Labsolar-6A, PerfectLight). 100 mg photocatalyst was suspended individually in 100 mL aqueous solution (pH = 2.5) containing 10 mmol L−1 Fe(NO3)3 as a sacrificial reagent. The suspension was then thoroughly degassed and irradiated using a 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm, light intensity 250–260 mW cm−2). The evolved gases were analyzed at given time intervals by an online gas chromatograph (GC-2002 N/TFF, TCD detector, Ar carrier, 5 Å molecular sieve column).
The AQE for water oxidation was measured using a 300 W Xe lamp (FX300, PerfectLight) with different band-pass filters of 420, 450, 500, 550, 600, and 650 nm (FWHM = 15 nm). The irradiation area was controlled as 1.2 × 1.2 cm2. The average intensity was determined by an optical power meter (S310C connected to a PM100D console, Thorlabs). The AQE was calculated as follows:
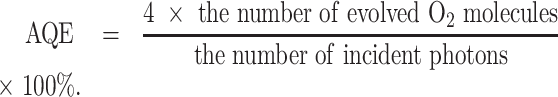 |
The photodegradation reactions were carried in quartz tube reactors with a 50 mL 10 ppm phenol pollutant solution and 25 mg photocatalyst powders. The reaction solution was kept at 35°C by a recirculating cooling water system. The visible-light source was obtained from a 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm). Before light irradiation, the suspension solutions were first ultrasonically dispersed for 5 min and then magnetically stirred for 1 h in the dark to reach adsorption–desorption equilibrium. At certain time intervals, a suspension (2 mL) was extracted and centrifuged to remove the photocatalysts. The concentration of phenol pollutants was determined by a high-performance liquid chromatography (HPLC) system (Shimadzu LC-20AT) with a C18 reversed-phase column, and the total organic carbon (TOC) in the aqueous solution was analyzed using a TOC analyzer (Multi N/C 2100, Analytik Jena AG).
Supplementary Material
FUNDING
This work was supported by the National Natural Science Foundation of China (21437003, 21673126, 21761142017, 21621003 and 21872077) and the Collaborative Innovation Center for Regional Environmental Quality.
Conflict of interest statement. None declared.
REFERENCES
- 1. Tong H, Ouyang S, Bi Yet al. Nano-photocatalytic materials: possibilities and challenges. Adv Mater 2012; 24: 229–51. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmann MR, Martin ST, Choi Wet al. Environmental applications of semiconductor photocatalysis. Chem Rev 1995; 95: 69–96. [Google Scholar]
- 3. Hisatomi T, Domen K.. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat Catal 2019; 2: 387–99. [Google Scholar]
- 4. Ma Y, Wang X, Jia Yet al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem Rev 2014; 114: 9987–10043. [DOI] [PubMed] [Google Scholar]
- 5. Garcia-Esparza AT, Shinagawa T, Ould-Chikh Set al. An oxygen-insensitive hydrogen evolution catalyst coated by a molybdenum-based layer for overall water splitting. Angew Chem Int Ed 2017; 56: 5780–4. [DOI] [PubMed] [Google Scholar]
- 6. Kato H, Asakura K, Kudo A. Highly efficient water splitting into H2 and O2 over lanthanum-doped NaTaO3 photocatalysts with high crystallinity and surface nanostructure. J Am Chem Soc 2003; 125: 3082–9. [DOI] [PubMed] [Google Scholar]
- 7. Zhang P, Ochi T, Fujitsuka Met al. Topotactic epitaxy of SrTiO3 mesocrystal superstructures with anisotropic construction for efficient overall water splitting. Angew Chem Int Ed 2017; 56: 5299–303. [DOI] [PubMed] [Google Scholar]
- 8. Maeda K, Teramura K, Lu Det al. Noble-metal/Cr2O3 core/shell nanoparticles as a cocatalyst for photocatalytic overall water splitting. Angew Chem Int Ed 2006; 45: 7806–9. [DOI] [PubMed] [Google Scholar]
- 9. Shi R, Ye HF, Liang Fet al. Interstitial P-doped CdS with long-lived photogenerated electrons for photocatalytic water splitting without sacrificial agents. Adv Mater 2018; 30: 1705941. [DOI] [PubMed] [Google Scholar]
- 10. Chowdhury FA, Trudeau ML, Guo Het al. A photochemical diode artificial photosynthesis system for unassisted high efficiency overall pure water splitting. Nat Commun 2018; 9: 1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiao X, Chen Z, Li Xet al. Defect-mediated electron-hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. J Am Chem Soc 2017; 139: 7586–94. [DOI] [PubMed] [Google Scholar]
- 12. Cui J, Liu T, Qi Yet al. A wide visible light driven complex perovskite Ba (Mg1/3Ta2/3)O3-xNy photocatalyst for water oxidation and reduction. J Mater Chem A 2017; 5: 18870–7. [Google Scholar]
- 13. Abeysinghe D, Skrabalak SE.. Toward shape-controlled metal oxynitride and nitride particles for solar energy applications. ACS Energy Lett 2018; 3: 1331–44. [Google Scholar]
- 14. Hisatomi T, Katayama C, Moriya Yet al. Photocatalytic oxygen evolution using BaNbO2N modified with cobalt oxide under photoexcitation up to 740 nm. Energ Environ Sci 2013; 6: 3595–9. [Google Scholar]
- 15. Maeda K, Lu D, Domen K. Oxidation of water under visible-light irradiation over modified BaTaO2N photocatalysts promoted by tungsten species. Angew Chem Int Ed 2013; 52: 6488–91. [DOI] [PubMed] [Google Scholar]
- 16. Wang L, Xu K, Cui Wet al. Monolayer epitaxial heterostructures for selective visible-light-driven photocatalytic NO oxidation. Adv Funct Mater 2019; 29: 1808084. [Google Scholar]
- 17. Li P, Chen X, He Het al. Polyhedral 30-faceted BiVO4 microcrystals predominantly enclosed by high-index planes promoting photocatalytic water-splitting activity. Adv Mater 2018; 30: 1703119. [DOI] [PubMed] [Google Scholar]
- 18. Cao S, Shen B, Tong Tet al. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv Funct Mater 2018; 28: 1800136. [Google Scholar]
- 19. Zhou Y, Zhang Y, Lin Met al. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat Commun 2015; 6: 8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li D, Liu Y, Shi Wet al. Crystallographic-orientation-dependent charge separation of BiVO4 for solar water oxidation. ACS Energy Lett 2019; 4: 825–31. [Google Scholar]
- 21. Kong HJ, Won DH, Kim Jet al. Sulfur-doped g-C3N4/BiVO4 composite photocatalyst for water oxidation under visible light. Chem Mater 2016; 28: 1318–24. [Google Scholar]
- 22. Wang Q, Hisatomi T, Suzuki Yet al. Particulate photocatalyst sheets based on carbon conductor layer for efficient Z-scheme pure-water splitting at ambient pressure. J Am Chem Soc 2017; 139: 1675–83. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y, Zhang S, Li Bet al. A family of visible-light responsive photocatalysts obtained by dispersing CrO6 octahedra into a hydrotalcite matrix. Chem Eur J 2011; 17: 13175–81. [DOI] [PubMed] [Google Scholar]
- 24. Silva CG, Bouizi Y, Fornés Vet al. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water. J Am Chem Soc 2009; 131: 13833–9. [DOI] [PubMed] [Google Scholar]
- 25. Chen S, Takata T, Domen K. Particulate photocatalysts for overall water splitting. Nat Rev Mater 2017; 2: 17050. [Google Scholar]
- 26. Kong D, Zheng Y, Kobielusz Met al. Recent advances in visible light-driven water oxidation and reduction in suspension systems. Mater Today 2018; 21: 897–924. [Google Scholar]
- 27. Gelderman K, Lee L, Donne S. Flat-band potential of a semiconductor: using the Mott–Schottky equation. J Chem Educ 2007; 84: 685–8. [Google Scholar]
- 28. Tao X, Zhao Y, Mu Let al. Bismuth tantalum oxyhalogen: a promising candidate photocatalyst for solar water splitting. Adv Energy Mater 2018; 8: 1701392. [Google Scholar]
- 29. Zhang C, Zhu Y.. Synthesis of square Bi2WO6 nanoplates as high-activity visible-light-driven photocatalysts. Chem Mater 2005; 17: 3537–45. [Google Scholar]
- 30. Jiang W, Liu Y, Zong Ret al. Photocatalytic hydrogen generation on bifunctional ternary heterostructured In2S3/MoS2/CdS composites with high activity and stability under visible light irradiation. J Mater Chem A 2015; 3: 18406–12. [Google Scholar]
- 31. Liu D, Wang J, Bai Xet al. Self-assembled PDINH supramolecular system for photocatalysis under visible light. Adv Mater 2016; 28: 7284–90. [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Shi W, Liu Det al. Supramolecular organic nanofibers with highly efficient and stable visible light photooxidation performance. Appl Catal B Environ 2017; 202: 289–97. [Google Scholar]
- 33. Yang MQ, Gao M, Hong Met al. Visible-to-NIR photon harvesting:progressive engineering of catalysts for solar-powered environmental purification and fuel production. Adv Mater 2018; 30: 1802894. [DOI] [PubMed] [Google Scholar]
- 34. Chen X, Shi R, Chen Qet al. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019; 59: 644–50. [Google Scholar]
- 35. Che W, Cheng W, Yao Tet al. Fast photoelectron transfer in (Cring)-C3N4 plane heterostructural nanosheets for overall water splitting. J Am Chem Soc 2017; 139: 3021–6. [DOI] [PubMed] [Google Scholar]
- 36. Chen R, Pang S, An Het al. Charge separation via asymmetric illumination in photocatalytic Cu2O particles. Nat Energy 2018; 3: 655–63. [Google Scholar]
- 37. Morris MR, Pendlebury SR, Hong Jet al. Effect of internal electric fields on charge carrier dynamics in a ferroelectric material for solar energy conversion. Adv Mater 2016; 28: 7123–8. [DOI] [PubMed] [Google Scholar]
- 38. Li J, Cai L, Shang Jet al. Giant enhancement of internal electric field boosting bulk charge separation for photocatalysis. Adv Mater 2016; 28: 4059–64. [DOI] [PubMed] [Google Scholar]
- 39. Jiang J, Zhao K, Xiao Xet al. Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets. J Am Chem Soc 2012; 134: 4473–6. [DOI] [PubMed] [Google Scholar]
- 40. Zhu J, Pang S, Dittrich Tet al. Visualizing the nano cocatalyst aligned electric fields on single photocatalyst particles. Nano Lett 2017; 17: 6735–41. [DOI] [PubMed] [Google Scholar]
- 41. Zhu J, Fan F, Chen Ret al. Direct imaging of highly anisotropic photogenerated charge separations on different facets of a single BiVO4 photocatalyst. Angew Chem Int Ed 2015; 54: 9111–4. [DOI] [PubMed] [Google Scholar]
- 42. Chen R, Fan F, Dittrich Tet al. Imaging photogenerated charge carriers on surfaces and interfaces of photocatalysts with surface photovoltage microscopy. Chem Soc Rev 2018; 47: 8238–62. [DOI] [PubMed] [Google Scholar]
- 43. Kanata-Kito T, Matsunaga M, Takakura Het al. Photoreflectance characterization of built-in potential in MBE-produced as-grown GaAs surface. Proc SPIE 1990; 1286: 56–66. [Google Scholar]
- 44. Lefebvre P, Allègre J, Gil Bet al. Time-resolved photoluminescence as a probe of internal electric fields in GaN-(GaAl)N quantum wells. Phys Rev B 1999; 59: 15363–7. [Google Scholar]
- 45. Zhang Z, Zhu Y, Chen Xet al. A full-spectrum metal-free porphyrin supramolecular photocatalyst for dual functions of highly efficient hydrogen and oxygen evolution. Adv Mater 2019; 31: 1806626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





