Abstract
We describe a case of an adult patient with mitral valve regurgitation and the anomalous origin and course of the left circumflex coronary artery. Use of a ringless procedure or a microinvasive approach, such as transapical neochordae implantation, would have possibly avoided a life-threatening post-operative complication. (Level of Difficulty: Advanced.)
Key Words: cardiac risk, coronary vessel anomaly, computed tomography, mitral valve
Abbreviations and Acronyms: AAOCA, anomalous aortic origin of coronary artery; LCA, left coronary artery; LCX, left circumflex coronary artery; LV, left ventricular; RCS, right coronary sinus; TTE, transthoracic echocardiogram; V-A ECMO, veno-arterial extracorporeal circulatory membrane oxygenator
Central Illustration
We describe a case of an adult patient with mitral valve regurgitation and the anomalous origin and course of the left circumflex coronary artery. Use…
Physical Examination and Admission
After medical evidence of systolic murmur, a 48-year-old man was admitted at our institution for further evaluation.
Learning Objectives
-
•
If feasible, a microinvasive approach to mitral valve repair not requiring ring implantation, such as transapical neochordae implantation, may be safer than conventional surgery in the presence of an anomalous origin and course of the LCA branches.
-
•
Cardiac computed tomography scanning is essential for the proper definition of AAOCA anatomy, especially if valve surgery is planned. It may alone provide sufficient information regarding coronary anatomy and lesions, particularly in patients undergoing microinvasive procedures on cardiac valves.
Medical History
The patient’s medical history was nonsignificant, and no symptoms were reported.
Differential Diagnosis and Investigations
A transthoracic echocardiogram (TTE) (Figure 1, Video 1) revealed severe mitral valve regurgitation due to mitral posterior leaflet prolapse at the level of the median scallop (P2), left ventricular (LV) dilation (indexed LV end-diastolic volume 143 ml/m2; LV end-systolic diameter 65 cm) with preserved LV ejection fraction (65%). The TTE also prompted suspicion of an abnormal origin and course of the left circumflex coronary artery (LCX). A cardiac catheterization (including coronary angiography) revealed a right dominant coronary circulation with no obstructing lesions. It also showed a separated origin of the 2 main branches of the left coronary artery (LCA), with the LCX originating from the right coronary sinus (RCS). Furthermore, coronary angiography revealed the presence of a systolic milking at the medium portion of the LCX and prompted suspicion of its anomalous retro-aortic course (Figure 2). A cardiac computed tomography scan was then performed to clarify the coronary anatomy. It confirmed the absence of obstructive lesions of all coronary branches and the abnormal origin of the LCX from the RCS. The study also allowed precise description of the retro-aortic course of the LCX and its reduced caliber due to systolic myocardial milking (Figure 3, Video 2). Finally, no signs of myocardial regional hypoperfusion were noticed on a single-photon emission computed tomography scan. A schematic drawing of the coronary anomaly (Central Illustration) and a video showing the preoperative echocardiographic assessment of the mitral regurgitation are available online (Supplemental Figure 1, Videos 3, 4, and 5).
Figure 1.

3D Preoperative Echocardiogram
Preoperative 3-dimensional echocardiography showing prolapse of the P2 scallop of the mitral valve. AV = aortic valve; MR = mitral regurgitation; PV = pulmonary valve; TV = tricuspid valve.
Online Video 1.
Video showing the anomalous origin and course of the LCX at the preoperative 2D echocardiogram. On the left, a 4-chamber view showing the course of the anomalous coronary artery. On the right, the detail is magnified.
Figure 2.

Preoperative Angiogram
Preoperative angiography showing the anomaly origin and course of the circumflex artery. LCX = left circumflex coronary artery; RCA = right coronary artery.
Figure 3.
Preoperative Cardiac Computed Tomography Scan
Preoperative cardiac computed tomography showing the retro-aortic course of the circumflex artery. On the left, 3-dimensional reconstruction of the computed tomography images on the right. LA = left atrium; other abbreviations as in Figure 2.
Online Video 2.
Video showing the LCX milking at the angiography.
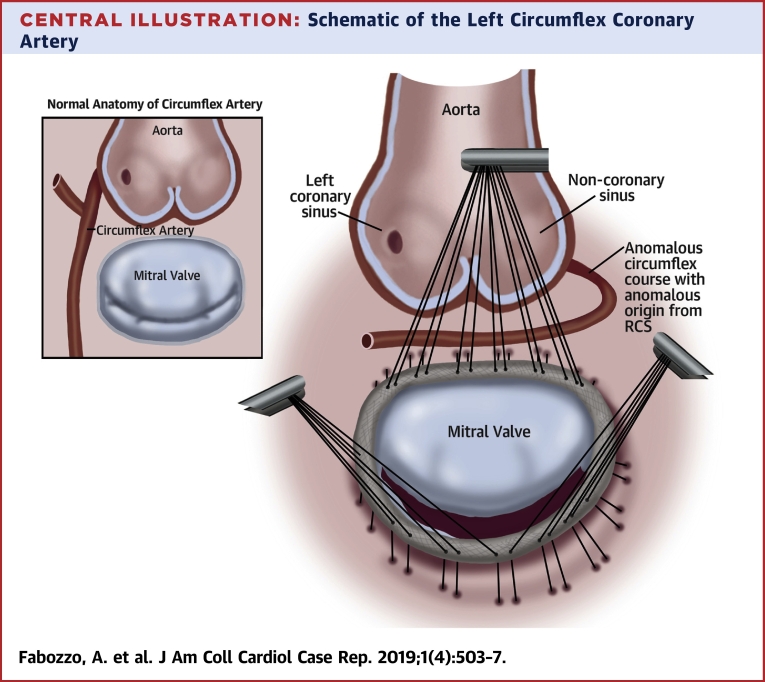
Central Illustration.
Schematic of the Left Circumflex Coronary Artery
Schematic of the left circumflex coronary artery anomalous aortic origin and course during mitral valve repair with a complete prosthetic ring compared to normal course (upper left box). RCS = right coronary sinus.
Online Video 3.
Video showing mitral valve regurgitation in long axis view at the preoperative TTE.
Online Video 4.
Video showing mitral valve regurgitation in 4-chamber view at the preoperative TTE.
Online Video 5.
Video showing a 3D imaging of the mitral valve at the preoperative
Management
The case was discussed in the heart team, and the need for a concomitant myocardial revascularization procedure was excluded. A conventional (surgical) mitral valve repair was chosen. Surgery was performed, as usual, under general anesthesia and mechanical ventilation. A median sternotomy permitted access to the heart. Once exposed, the superior and inferior venae cavae were both cannulated, as well as the aorta. After full heparinization, cardiopulmonary bypass was instituted. After administration of cold cardioplegia and aortic cross-clamping, the left atrium was opened. An accurate inspection of the mitral valve confirmed the prolapse of the posterior leaflet at the level of P2. Mitral valve was thus repaired by P2 resection and sliding of P1 and P3 adjacent scallops. Finally, annular stabilization was obtained with the implantation of a prosthetic ring (saddle 34 mm). Results of an intraoperative “pressure test” showed good valve competency. The heart cavities were properly de-aired before aortic cross-clamping removal. The patient did not experience any complications during cardiopulmonary bypass discontinuation. Hemodynamic variables seemed stable, and no major bleeding was observed.
After chest closure, the patient experienced ventricular tachycardia. A DC shock at 260 J restored a regular rhythm, but hemodynamic instability and the high risk of other arrhythmic events required implantation of peripheral veno-arterial extracorporeal circulatory membrane oxygenator (VA-ECMO) support. Subsequently, the patient underwent an emergent coronary angiogram (Figure 4), which revealed a mechanical occlusion of the LCX due to annular ring implantation. An immediate percutaneous coronary intervention attempt was unsuccessful. Coronary bypass grafting was judged not feasible in that setting because of the short extent and small caliber of the LCX peripheral branches and due to the patient’s critical condition. The patient was therefore transferred to our post-operative intensive care unit. Hemodynamic parameters were stable under inotropes infusion and ECMO support (∼3.5 l/min). The peak value of post-operative troponin I was 142.800 ng/l. The patient was gradually weaned from the ECMO support, which was finally removed on post-operative day 4. Oro-tracheal extubation was rapidly achieved, and the inotropic support was gradually discontinued. A pre-discharge echocardiogram showed mild to moderate mitral regurgitation and a moderate impairment of LV contractility (ejection fraction 47%).
Figure 4.

Postoperative Angiogram
Postoperative angiography showing a mitral prosthetic ring in place occluding the mid-portion of the circumflex artery (small black arrow). ECMO = extracorporeal membrane oxygenator; IVC = inferior vena cava; LCX = left circumflex artery; MV = mitral valve.
Follow-Up
No other significant complications occurred, and the patient was discharged to a rehabilitation unit on post-operative day 12 in good clinical condition.
Discussion
Transapical minimally invasive procedures to repair the mitral valve are increasingly becoming an alternative option to conventional mitral surgery (1). Nevertheless, the absence of ring implantation has always been considered a major drawback of the procedure (2). The current report describes the case of an adult patient diagnosed with severe mitral regurgitation and anomalous aortic origin of the LCX coronary artery, for which a microinvasive procedure would have possibly avoided a life-threatening post-operative complication.
Mitral valve annuloplasty can result in an iatrogenic lesion of the LCX because, after its origin, it lies within the atrioventricular groove. Although predictable, this complication is poorly described in the literature, and its incidence is unknown (3, 4, 5). The risk of mechanical coronary occlusion seems to be higher in patients with an anomalous course of the LCX (5). An anomalous aortic origin of a coronary artery (AAOCA) is, per se, a rare condition with an estimated incidence of 1%, with the anomalous LCA originating from the RCS in 0.15% (6). It is a common cause of sudden cardiac death and often represents an accidental finding during autopsy (7). If an AAOCA is diagnosed, surgery is indicated to avoid predictable ischemic events, for which the evidence of a slit-like orifice and interarterial and/or intramural course of the anomalous coronary are risk factors. Alternatively, myocardial revascularization is rarely recommended, even if another cardiac-associated procedure is planned (8). Therefore, “no-risk” variants of AAOCA, such as a retro-aortic course, do not require intervention as they are not related to ischemic events or sudden death (9,10).
In the current case, the retro-aortic course of the LCX was known because it was pre-operatively described with a multimodality imaging approach, and the absence of signs of ischemia contraindicated myocardial revascularization of any sort. The primary surgical indication was severe mitral regurgitation, mainly due to prolapse of the central portion (P2) of the posterior leaflet. Although anatomically feasible (type A/B and no significant tricuspid regurgitation and/or tricuspid annular dilation [1]), the heart team judged a minimal invasive transapical approach to be improper. The choice of a conventional surgical approach was, indeed, considered most appropriate in the presence of an AAOCA due to the increased likelihood of unforeseen anatomy and for the predictably increased possibility of a perioperative complication.
Prolapse of the scallop P2 of the posterior leaflet was recognized as the main mechanism of mitral regurgitation and, as usual, surgery entailed maneuvers at the level of the leaflets’ body and annular stabilization (to avoid its further dilation). Annular stabilization with complete annular band, over a ringless repair or an incomplete mitral band, was performed as standard, clearly underestimating the concomitant ischemic risk. The most adequate theoretical choice, however, led to a life-threatening surgical complication.
Conclusions
In patients undergoing mitral valve repair and presenting associated “no-risk” subtypes of AAOCA involving the LCX, a partial annuloplasty ring or a band should be considered, as well as nonresection techniques, if repair durability is not compromised. Minimal invasive strategies that avoid annular manipulation, such as transapical artificial chordae implantation, may offer a reasonable alternative for select eligible patients.
Acknowledgment
The authors are grateful to Dr. Chiara Tessari for capturing the figures and their elaborations.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Informed consent was obtained for this case.
Appendix
For a supplemental figure and videos, please see the online version of this paper.
Appendix
References
- 1.Colli A., Manzan E., Aidietis A. An early European experience with transapical off-pump mitral valve repair with NeoChord implantation. Eur J Cardiothorac Surg. 2018;54:460–466. doi: 10.1093/ejcts/ezy064. [DOI] [PubMed] [Google Scholar]
- 2.Gerosa G. Invited commentary. Ann Thorac Surg. 2018;106:445–446. doi: 10.1016/j.athoracsur.2018.02.090. [DOI] [PubMed] [Google Scholar]
- 3.Mantilla R., Legarra J.J., Pradas G. Percutaneous coronary intervention for iatrogenic occlusion of the circumflex artery after mitral annuloplasty. Rev Esp Cardiol. 2004;57:702–704. In Spanish. [PubMed] [Google Scholar]
- 4.Veinot J.P., Acharya V.C., Bedard P. Compression of anomalous circumflex coronary artery by a prosthetic valve ring. Ann Thorac Surg. 1998;66:2093–2094. doi: 10.1016/s0003-4975(98)01082-0. [DOI] [PubMed] [Google Scholar]
- 5.Vaishnava P., Pyo R., Filsoufi F., Sharma S. Compression of an anomalous left circumflex artery after aortic and mitral valve replacement. Ann Thorac Surg. 2011;92:1887–1889. doi: 10.1016/j.athoracsur.2011.04.095. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka O., Hobbs R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn. 1990;21:28–40. doi: 10.1002/ccd.1810210110. [DOI] [PubMed] [Google Scholar]
- 7.Liberthson R.R. Sudden death from cardiac causes in children and young adults. N Engl J Med. 1996;334:1039–1044. doi: 10.1056/NEJM199604183341607. [DOI] [PubMed] [Google Scholar]
- 8.Feins E.N., De Faria Yeh D., Bhatt A.B. Anomalous aortic origin of a coronary artery: surgical repair with anatomic- and function-based follow-up. Ann Thorac Surg. 2016;101:169–175. doi: 10.1016/j.athoracsur.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Roberts W.C., Shirani J. The four subtypes of anomalous origin of the left main coronary artery from the right aortic sinus (or from the right coronary artery) Am J Cardiol. 1992;70:119–121. doi: 10.1016/0002-9149(92)91406-t. [DOI] [PubMed] [Google Scholar]
- 10.Fabozzo A., DiOrio M., Newburger J.W. Anomalous aortic origin of coronary arteries: a single-center experience. Semin Thoracic Surg. 2016;28:791–800. doi: 10.1053/j.semtcvs.2016.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.