Abstract
Although the safety of valve-in-valve transcatheter aortic valve replacement has improved, coronary ostium obstruction remains a significant complication, with chimney stenting a possible solution to circumvent this complication. In this case, we discuss the failure of a chimney stent in a case of valve-in-valve transcatheter aortic valve replacement resulting in cardiogenic shock. (Level of Difficulty: Advanced.)
Key Words: aortic valve, percutaneous coronary intervention, tricuspid valve
Abbreviations and Acronyms: DCO, delayed coronary obstruction; ECG, electrocardiography; LMS, left main stem; RCA, right coronary artery; redo-SAVR, redo–surgical aortic valve replacement; STJ, sino-tubular junction; TAVR-ViV, transcatheter aortic valve-in-valve replacement
Graphical abstract

Although the safety of valve-in-valve transcatheter aortic valve replacement has improved, coronary ostium obstruction remains a significant complication…
The durability of bioprosthetic valves has improved drastically over the past decade despite degenerative prosthetic valve disease being inevitable. Replacement options include both redo–surgical aortic valve replacement (redo-SAVR) and transcatheter aortic valve-in-valve replacement (TAVR-ViV). Although redo-SAVR is regarded as the gold standard, patients deemed as high surgical risk according to the Society of Thoracic Surgeons score are recommended to undergo TAVR-ViV 1, 2, 3. Studies report benefits to both approaches, with a reported device success of 94.4% in ViV versus 96.6% in redo-SAVR (4). The majority of failures in the ViV cohort are due to sustained high transvalvular gradients in ViV thus deeming it unsuccessful according to the Valve Academic Research Consortium 2 endpoints. Despite the success for redo-SAVR, ViV is associated with higher EuroSCORE I scores, shorter intensive care stay, lower incidence of post-intervention stroke, and higher mean transvalvular gradients. A known complication of TAVR-ViV is coronary ostium occlusion, whether from the bioprosthetic valve leaflets obstructing flow, or by the larger TAVR prosthesis itself. In cases identified as high risk, due to anatomically determined low-lying coronary ostium and narrow aortic root, prophylactic stenting of the left main stem (LMS) may be performed, whereby the stent acts as a “chimney” shunting blood from the aorta into the coronaries 4, 5. In this case study we discuss a late-presenting failed chimney stent.
Learning Objectives
-
•
Chimney stenting can have delayed failure as a result of expanding TAVR struts.
-
•
In patients at high risk of coronary obstruction for ViV, alternative strategies to mitigate this risk should be considered (i.e., bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary obstruction technique).
-
•
In patients presenting with cardiogenic shock with LMS occlusion, there must be prompt identification, supportive therapy (i.e., Impella device [Abiomed, Danvers, Massachusetts], veno-arterial extracorporeal membrane oxygenation) with an initial attempt at percutaneous revascularization, and, if ongoing, ischemic consideration of coronary artery bypass grafting.
History of Presentation
The authors describe a case of a 74-year-old woman who presented with progressive angina and associated New York Heart Association functional class III dyspnea. This is on a background of previous SAVR 6 years earlier (21 mm Trifecta, St. Jude Medical, Inc., St. Paul, Minnesota) for severe aortic valve stenosis.
Medical History
The patient’s medical history included hypertension, hypercholesterolemia, obesity, and chronic obstructive lung disease, as well as a history of steroid-dependent polymyalgia rheumatica. Post-SAVR peak gradient was 25 mm Hg, and the mean gradient was 16 mm Hg, thus excluding patient–prosthesis mismatch.
Differential Diagnosis
Although the most likely diagnosis was severe aortic stenosis as a result of degeneration of the previously surgically implanted aortic valve, other important differentials to rule out would include an acute myocardial infarction and severe coronary artery disease.
Investigations
The 12-lead electrocardiography (ECG) at the time of presentation showed sinus rhythm with nonspecific lateral ST-segment change. Transesophageal echocardiography showed normal left ventricular size and systolic function with mild concentric hypertrophy. The aortic bioprosthesis had markedly thickened leaflets with moderate restriction of opening with a peak gradient of 78 mm Hg, a mean gradient of 40 mm Hg, and an aortic valve area of 0.4 cm2, thus confirming severe bioprosthetic aortic stenosis.
Coronary angiography revealed mild coronary artery disease with the exception of a severely stenotic lesion (70%) in a nondominant right coronary artery (RCA), unchanged from angiography performed before the patient’s AVR.
Management
The patient was transferred to our tertiary center for heart team assessment. Given her Society of Thoracic Surgeons premature risk of mortality score of 9.1%, a TAVR was elected, and a ViV procedure was planned. A periprocedural computed tomography aortogram identified low-lying coronaries with a measured distance of 5.3 mm from the annulus to the lower border of the left main, and 7.1 mm from the annulus to the lower border of the right coronary artery. In addition, the valve-to-coronary for the left main artery was only 3.5 mm and 6.5 mm for the right coronary artery (Figure 1). In light of this, along with an identified narrow sino-tubular junction (STJ), and given the morphology of the trifecta valve, the risk of coronary occlusion was considered to be high. Subsequently, prophylactic chimney stenting of the LMS and RCA was performed (Figure 2). Biradial access was obtained by using 6-F sheaths, with right femoral artery access for the TAVR valve. Both left and right coronary systems were wired with standard coronary wires, and undeployed stents were placed within the vessel. A 23-mm trileaflet Evolut R valve (Medtronic, Minneapolis, Minnesota) was deployed under rapid pacing into the annulus. Arteriography revealed suboptimal filling of the coronaries, with close contact between the original valve leaflet and STJ necessitating stent deployment to both ostia (with intra-aortic portions). Drug-eluting stents were deployed in the LMS and RCA, extending into the aortic root, in a chimney stent fashion as previously described (6). Hemodynamic stability was maintained throughout the procedure; the invasive aortic valve gradient was ∼10 mm Hg. After stent optimization, a satisfactory angiographic result was obtained, and the patient remained stable. Post-procedural anatomical assessment was conducted via transesophageal echocardiography, which revealed appropriate deployment and good left ventricular function. Ejection fraction was estimated at 50%.
Figure 1.
Illustration of TAVI-ViV With Chimney Shunt and Subsequent Compression
(A) Valve-in-valve transcatheter aortic valve replacement (TAVR) with chimney shunt in place, with no kinking of the shunt. (B) Valve-in-valve TAVR with chimney shunt kinked due to radial expansion of TAVR + compression by the paravalvular seal.
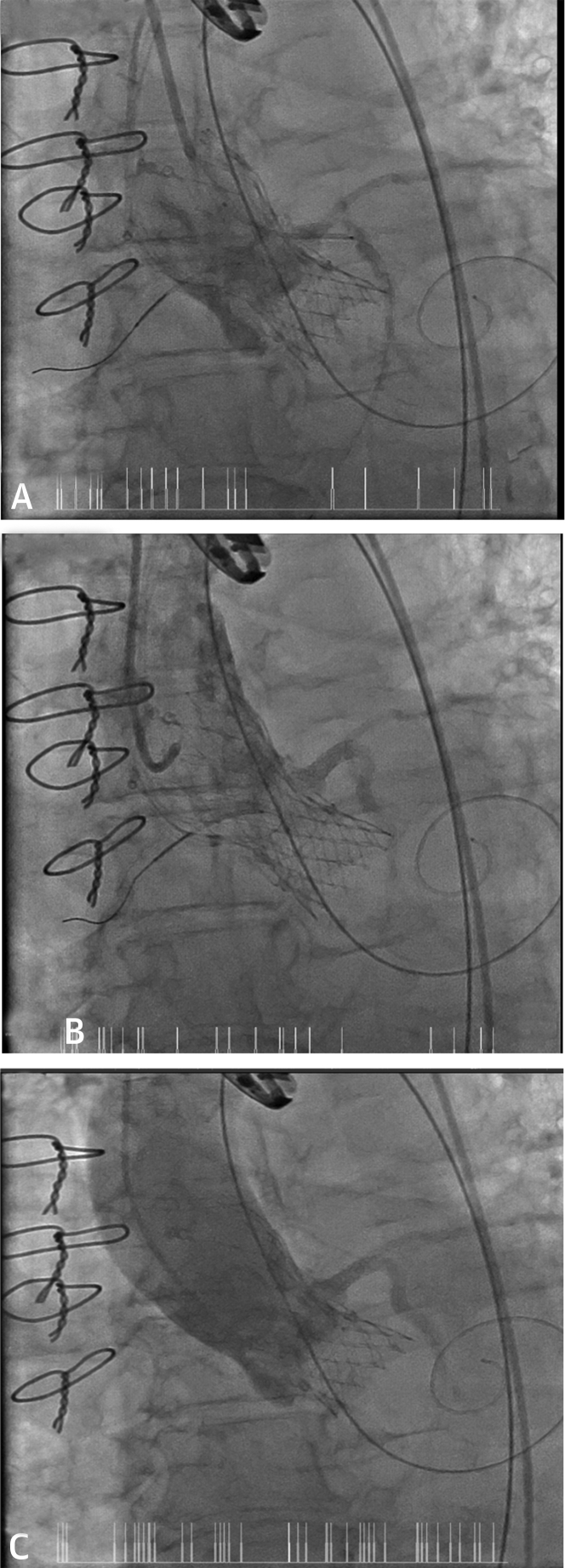
Figure 2.
Aortograms During and Post-TAVI
(A) Image showing valve deployment with the sheath in the coronary ostium for chimney stent deployment. (B and C) Aortogram after transcatheter aortic valve implantation and stent in the left main stem showing good flow in the left main stem, left anterior descending artery, and left circumflex artery.
The patient was transferred to the intensive care unit post-procedure for monitoring. Six hours after the procedure, she developed progressive cardiogenic shock, unresponsive to fluid resuscitation. An ECG showed new left bundle branch block, with a transthoracic echocardiogram identifying severe left ventricular impairment with a left ventricular ejection fraction of 35%. The patient subsequently developed chest and back pain, and respiratory compromise. She was intubated and immediately taken for repeat coronary angiography with intermittent inotropic requirement. Given the expected cardiorespiratory deterioration, the patient was commenced on veno-arterial extracorporeal membrane oxygenation.
Repeat angiography revealed a significant filling defect of the LMS, likely secondary to valve stent expansion and extrinsic compression of the chimney stent. The chimney stent was rewired, and an intravascular ultrasound catheter showed significant extraluminal asymmetrical compression (Figure 3). Serial balloon dilatations were performed, which improved flow within the vessel, and subsequent stent deployment was performed. However, given the risk of re-occlusion, the patient was transferred for emergent coronary artery bypass grafting, where she underwent a composite left internal mammary artery and saphenous vein graft to the left anterior descending coronary artery and left-posterolateral arteries. She remained stable post-operatively and was discharged to rehabilitation 6 days after her surgery. At discharge, an echocardiography revealed a peak gradient of 24 mm Hg, a mean gradient of 11 mm Hg, and an aortic valve area of 1.1 cm2. Peak troponin level was recorded at 208 ng/l, and ECG revealed sinus rhythm with left ventricular hypertrophy ascertained by voltage criteria, no Q waves, subtle inferolateral ST-segment depression, and normal PR and QTc intervals.
Figure 3.
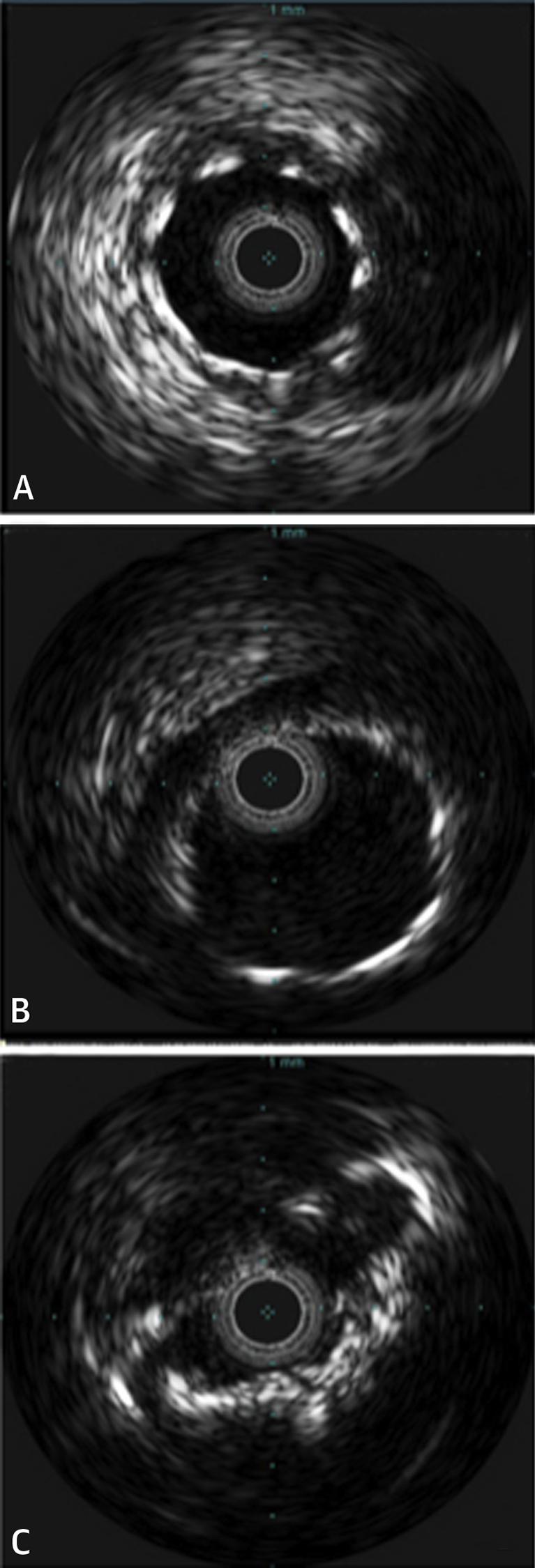
IVUS Images Immediately Post-TAVI and Following Patient Decline
(A) Well-expanded and widely patent stent in the left circumflex artery after transcatheter aortic valve implantation (TAVI). (B) Well-expanded and widely patent stent in left main (5.5 cm) after the TAVI. (C) Deformed late stent collapse in TAVI valve struts on repeat catheterization.
Discussion
This paper discusses a case of TAVR-ViV for degenerative SAVR complicated by delayed coronary obstruction and cardiogenic shock despite prophylactic chimney stenting. To the best of our knowledge, this case is the first report of a late presenting failure of the chimney stenting technique.
Although TAVR-ViV serves as a good alternative to redo-SAVR for patients deemed as high risk, the possibility of complications must be appropriately investigated. Due to the incidence of coronary obstruction with TAVR-ViV, reported as 3 to 4 times that of TAVR of a native aortic valve, and its associated high mortality rate, assessment for risk factors must be appropriately investigated (7). Risk factors associated with coronary obstruction include the intrinsic properties of the surgical bioprosthesis and anatomical factors, including narrow STJ, narrow sinuses of Valsalva, and low-lying coronary ostium.
With the risk of coronary obstruction significant in ViV, investigation using multidetector computed tomography imaging can be used, which has been shown to reduce the incidence of ViV-induced coronary obstruction (8). Due to the anatomically determined low-lying coronary ostia, it was considered appropriate to prophylactically protect the coronary ostium with a chimney stent. As described, this approach failed, with delayed coronary occlusion resulting in cardiogenic shock experienced 6 h post-intervention. The failure of the chimney stent is hypothesized to be due to the progressive expansion of the nitinol-based self-expanding scaffold, which initially did not compromise stent integrity but over time, with progressive scaffold expansion, resulted in stent compression. An alternative and previously reported cause for coronary occlusion includes repositioning of the surgically implanted aortic valve upon valve expansion, which inevitably obstructs the coronaries despite coronary protection; however, this would have presented acutely at the time of TAVR-ViV (9).
This phenomenon of delayed coronary obstruction post-TAVR has been studied by Jabbour et al. (10), who was able to correlate an association between delayed coronary obstruction and ViV. Furthermore, the use of self-expanding valves was associated with a higher rate of coronary obstruction compared with balloon-expanding valves (0.36% vs. 0.11%; p < 0.001), which may be attributable to ongoing expansion after acute deployment of the valve.
Follow-Up
The patient remained in a stable condition at her local hospital for >6 months. Unfortunately, she died due to cardiac arrest months later.
Conclusions
In this case, we discuss a complication of TAVR-ViV whereby a patient develops delayed and progressively worsening cardiogenic shock despite coronary protection with prophylactic chimney shunting. This delayed presentation may be due to the progressive and continual expansion of self-expanding valves. In such situations, alternative protection strategies should also be considered.
Footnotes
Dr. Bhindi is a proctor for Medtronic and Edwards Lifesciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Holmes D.R., Brennan J.M., Rumsfeld J.S. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA Cardiol. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 2.Webb J.G., Mack M.J., White J.M. Transcatheter aortic valve implantation within degenerated aortic surgical bioprostheses: PARTNER 2 Valve-in-Valve Registry. J Am Coll Cardiol. 2017;69:2253–2262. doi: 10.1016/j.jacc.2017.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Duncan A., Davies S., Di Mario C., Moat N. Valve-in-valve transcatheter aortic valve implantation for failing surgical aortic stentless bioprosthetic valves: a single-center experience. J Thorac Cardiovasc Surg. 2015;150:91–98. doi: 10.1016/j.jtcvs.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Silaschi M., Wendler O., Seiffert M. Transcatheter valve-in-valve implantation versus redo surgical aortic valve replacement in patients with failed aortic bioprostheses. Interact Cardiovasc Thorac Surg. 2016;24:63–70. doi: 10.1093/icvts/ivw300. [DOI] [PubMed] [Google Scholar]
- 5.Romano V., Buzzatti N., Latib A., Colombo A., Montorfano M. Chimney technique for coronary obstruction after aortic valve in valve: pros and cons. Eur Heart J Cardiovasc Imaging. 2018;19:1194. doi: 10.1093/ehjci/jey092. [DOI] [PubMed] [Google Scholar]
- 6.Ohrlander T., Sonesson B., Ivancev K., Resch T., Dias N., Malina M. The chimney graft: a technique for preserving or rescuing aortic branch vessels in stent-graft sealing zones. J Endovasc Ther. 2008;15:427–432. doi: 10.1583/07-2315.1. [DOI] [PubMed] [Google Scholar]
- 7.Dvir D., Leipsic J., Blanke P. Coronary obstruction in transcatheter aortic valve-in-valve implantation: preprocedural evaluation, device selection, protection, and treatment. Circ Cardiovascular Interv. 2015;8 doi: 10.1161/CIRCINTERVENTIONS.114.002079. [DOI] [PubMed] [Google Scholar]
- 8.Suri R.M., Webb J., Mack M. TCT-688 one year results of transcatheter aortic valve therapy for failed surgical bioprostheses—PARTNER II Valve-in-Valve Registry. J Am Coll Cardiol. 2014;64:B201. [Google Scholar]
- 9.Ribeiro H.B., Nombela-Franco L., Urena M. Coronary obstruction following transcatheter aortic valve implantation: a systematic review. J Am Coll Cardiol Intv. 2013;6:452–461. doi: 10.1016/j.jcin.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Jabbour R.J., Tanaka A., Finkelstein A. Delayed coronary obstruction after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71:1513–1524. doi: 10.1016/j.jacc.2018.01.066. [DOI] [PubMed] [Google Scholar]