Abstract
Cardiac rhabdomyomas in neonates may cause significant cardiac risk. Recently, sirolimus has been used to treat these lesions. The dose, duration, and monitoring for therapy are unknown. A case of sirolimus use in a premature neonate is presented. No significant adverse effects were seen. Review of published cases is included. (Level of Difficulty: Advanced.)
Key Words: cancer, congenital heart defect, echocardiography
Abbreviations and Acronyms: LVOT, left ventricular outflow tract; mTOR, mammalian target of rapamycin; TSC, tuberous sclerosis complex
Graphical abstract
Cardiac rhabdomyomas in neonates may cause significant cardiac risk. Recently, sirolimus has been used to treat these lesions. The dose, duration…
Cardiac rhabdomyomas in neonates are almost exclusively associated with tuberous sclerosis and carry significant cardiac risk, particularly when causing ventricular outflow tract obstruction due to mass effect. Recently, mammalian target of rapamycin (mTOR) inhibitors including sirolimus and everolimus have been used to treat these lesions in individual cases when surgical resection is inappropriate. The exact dose, duration, and monitoring of these medications remain unknown. This case report presents a case of sirolimus use in a premature neonate resulting in a dramatic decrease in cardiac rhabdomyoma size, although with a supratherapeutic sirolimus level. No significant adverse effects were seen. Review of other published cases is included.
Learning Objectives
-
•
With appropriate monitoring, sirolimus can be used safely in premature infants to treat cardiac rhabdomyoma.
-
•
Exact dosing, duration, and long-term safety profile of sirolimus in the neonatal and early infant setting remain unknown.
-
•
Where rapid reductions in rhabdomyoma size are needed, particularly in the newborn with hemodynamic compromise, supratherapeutic sirolimus levels may be justified.
Background
mTOR inhibitors including sirolimus and everolimus are increasingly included in the management of tuberous sclerosis complex (TSC) manifestations, including for subependymal giant-cell astrocytomas and renal angiomyolipomas. Everolimus has been approved for use in managing these conditions. Both sirolimus and everolimus have also been used “off-label” in infants with cardiac rhabdomyomas 1, 2. Published data on dosing, duration, and safety profile for sirolimus in this setting is limited to a few case reports 3, 4, 5, 6.
This case report presents a case of sirolimus use in a premature neonate with significant cardiac rhabdomyomas. Review of other published cases is included. Ethics approval and informed consent were obtained (SCHN-CCR 2018/04).
History of Presentation
A 32-year-old female gravida 4 para 4 (now) gave birth to a female infant at 33 + 4 weeks gestational age, birth weight 2.25 kg (81st centile). The infant had been diagnosed antenatally with significant cardiac rhabdomyomas with potential for left ventricular outflow tract (LVOT) obstruction and impact on both ventricular cavities (Figure 1A). The family history was significant for TSC in the mother, maternal uncle, maternal grandfather, and 2 of the infant’s siblings. Various options for postnatal management including single ventricle palliation, debulking surgery, medical therapy with an mTOR inhibitor, and comfort care were discussed antenatally.
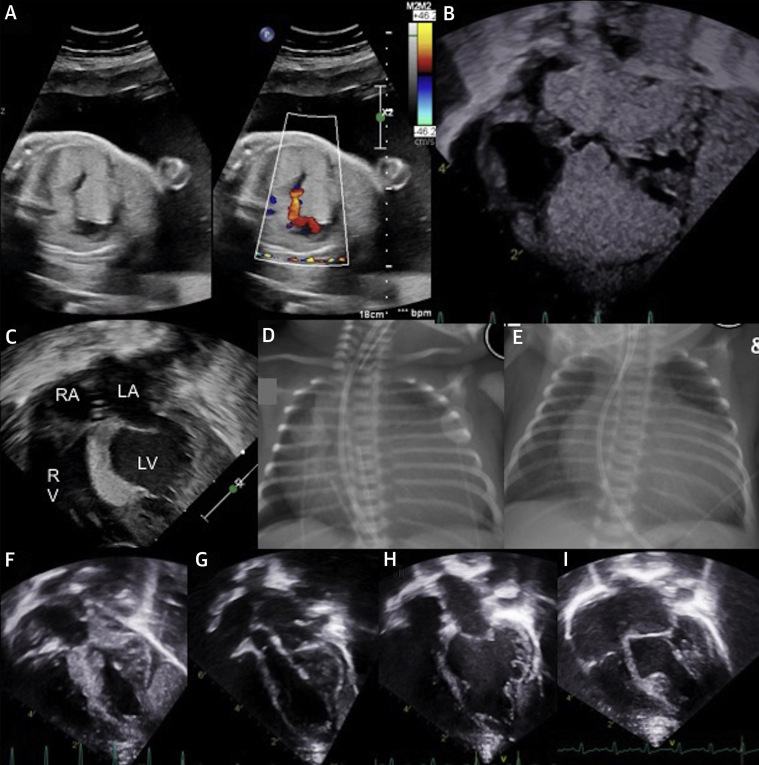
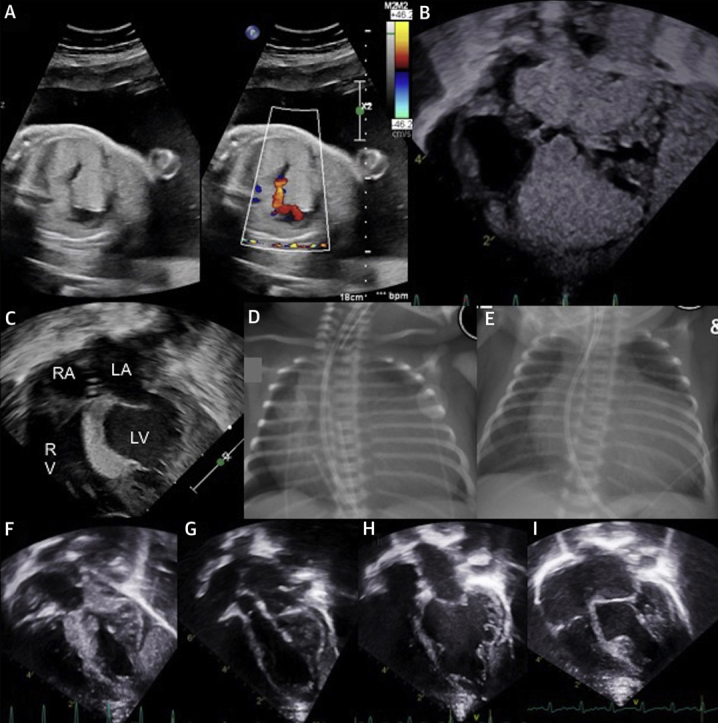
Figure 1.
Fetal and Postnatal Imaging of Cardiac Rhabdomyomas
(A) Fetal echocardiogram. (B) Postnatal echocardiogram, pre-treatment. (C) Echocardiogram after 8 days of treatment. (D) Pre-treatment chest radiography. (E) Chest radiography after 6 days of treatment. (F) Echocardiogram at 3 months of age, after 1 month of sirolimus treatment cessation. (G) Echocardiogram at 4 months of age, 5 weeks after recommencing sirolimus. (H) Echocardiogram at 8 months of age. (I) Echocardiogram at 12 months of age. LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
The infant was born with Apgar scores of 3, 5, and 6 at 1, 5, and 10 min, respectively. She required intubation and ventilation at birth in view of poor respiratory effort. Prostaglandin E1 was commenced due to concerns for potential significant LVOT obstruction.
Investigations
Postnatal echocardiography demonstrated large cardiac rhabdomyomas in the intraventricular septum encroaching on both ventricular cavities and causing LVOT obstruction (Figure 1B), although with sufficient left ventricular output for prostaglandin E1 to be ceased. Significant cardiomegaly was noted on the chest x-ray (Figure 1D).
Ultrasound of the kidneys revealed bilateral mild increased cortical echogenicity with no evidence of angiomyolipoma. Ophthalmological review identified a right supratemporal hamartoma. Cranial ultrasonography and magnetic resonance imaging of the brain were unremarkable. In the context of the clinical findings and family history, a presumptive diagnosis of TSC was made.
Management
The infant was commenced on sirolimus 0.25-mg dose once daily via a nasogastric tube on day 3 of life. The infant was having minimal trophic enteral intake at this time. Feeds were gradually graded up, and she was taking full enteral feeds by day 10. The target sirolimus trough level was 5 to 15 μg/l. The initial trough level 8 days after drug commencement (day 11 of life) was significantly higher at 69.7 μg/l. Transthoracic echocardiogram on day 11 of life demonstrated significant regression of the cardiac rhabdomyoma (Figure 1C). On the chest x-ray, the cardiac silhouette was also seen to decrease in size (Figure 1E). Following a period of dose omission until the serum sirolimus level reached 16.2 μg/l, sirolimus was recommenced at 0.125-mg dose once daily, one-half the previous dose. Sirolimus serum trough levels remained within normal limits (6.8 to 9.9 μg/l).
After the initial significant decrease in size, serial echocardiography demonstrated sustained decrease in rhabdomyoma size. The blood count, liver and renal function, and lipid profile remained normal. Prophylactic co-trimoxazole was commenced after neonatal jaundice had resolved. The infant was transferred from our institution at 4 weeks of age with outpatient follow-up arranged.
The infant’s postnatal course was complicated by respiratory distress requiring surfactant, mechanical ventilation for 12 days, and noninvasive respiratory support until day 35. There were concerns about possible seizures on day 3 of life that were not evident on the electroencephalogram. Given the presumptive diagnosis of TSC, levitiracetam was prophylactically commenced.
The infant continued on sirolimus therapy until 2 months of age at which time, in the context of persistent neutropenia 0.3 to 0.6 × 109/l and with sustained decrease in rhabdomyoma size, a decision was made to cease sirolimus therapy. Levitiracetam therapy was also weaned and ceased as an additional possible cause for neutropenia. No further seizure activity was seen.
Subsequent transthoracic echocardiography at 3 months of age (Figure 1F) demonstrated an increase in rhabdomyoma size with no change to the neutrophil count. The sirolimus was recommenced at 0.125 mg daily, resulting once again in reduction of the rhabdomyoma (Figure 1G).
Discussion
In this case, treatment with sirolimus was associated with a substantial decrease in the size of a massive cardiac rhabdomyoma in a premature neonate with TSC. Of note, with cessation of treatment for 1 month, the rhabdomyomas demonstrated a substantial increase in size with regression again upon recommencement of sirolimus, suggesting an effect of treatment rather than just natural history as the cause for regression.
Initial treatment with sirolimus resulted in trough levels well above the recommended maximum adult range (5 to 15 μg/l). Although speculative, it is possible that the rapid reduction in rhabdomyoma size in this case is related to the high blood levels of sirolimus. These unanticipated high levels were not associated with significant adverse effects. Neonatal dosing of mTOR inhibitors is not well established. Sirolimus’ liquid formulation provides a distinct advantage for use in neonates, although everolimus is now available in a dispersible tablet form. Everolimus is known to have greater oral bioavailability and, due to differences in systemic clearance time, reaches steady-state levels sooner after initiation. Limited pharmacokinetic data from pediatric dialysis patients suggests that the time to maximum concentration is slightly longer in younger children and clearance adjusted for weight is higher (2). The systemic absorption of both sirolimus and everolimus is significantly decreased when taken with a meal high in fat (2). The initial fasting status of the neonate in the current case may have contributed to the supratherapeutic sirolimus.
Safety data for sirolimus use in pediatric patients, particularly in neonates, is not well established. Safety data in pediatric renal transplant patients showed an increased risk of renal impairment, elevated lipid profile, and infection in children when receiving sirolimus in combination with calcineurin inhibitors and steroids (2).
A total of 5 cases of sirolimus use in neonates with cardiac rhabdomyomas were found in published reports and are summarized in Table 1. Variation in dose, duration, and magnitude of effect were noted. A similar supratherapeutic level (42.1 μg/l) was reported in a premature neonate weighing 2.2 kg at time of drug initiation. The dose of 0.25 mg once daily was also used in this infant, and similarly no adverse effects were seen (3). A report of use in a term neonate who received 0.5 mg oral daily resulted in a slightly elevated trough level 26 μg/l and a “dramatic reduction” in LVOT tumor was once again seen by day 5 of treatment (4).
Table 1.
Use of Sirolimus in Management of Neonatal Cardiac Rhabdomyoma
| First Author (Year), Country (Ref. #) | Case(s) | Treatment (Age, Dose, Trough Levels, Duration) | Monitoring | Prophylactic Co-Trimoxazole? | Results, Follow-Up |
|---|---|---|---|---|---|
| Breathnach et al. (2014), Ireland (4) | Term neonate (born 38 of 40 weeks), LVOT obstruction due to cardiac rhabdomyoma. | Treatment started day 10 of life, 0.5 mg oral daily (7 days), reduced to 0.4 mg oral daily due elevated trough level (26 ng/ml). Treated until 34 days of age (total 24 days). | Trough level, full blood count, electrolytes, renal and liver function, triglycerides. | Yes | Decrease in size of LVOT tumor by day 5 of treatment; “dramatic reduction” by day 24 of treatment. Treatment ceased at 34 days of age (day 24). Follow-up to age 8 months, stable LVOT gradient. |
| Lee et al. (2017), South Korea (3) | Premature neonate (born 28 of 40 weeks, birth weight 1.170 kg), LVOT obstruction due to cardiac rhabdomyoma. | Treatment started day 18 of life (CGA 30 + 4, weight 2.2 kg), 0.25 mg oral daily (14 days), reduced to 0.12 mg oral daily due to elevated trough level (42.1 ng/ml). Treated until 75 days of age (total 57 days). | Trough level, full blood count, electrolytes, renal and liver function, lipid profiling. | Yes | Decrease in size of LVOT tumor at day 15 of treatment, further reduction noted at days 22 and 43 of treatment. Treatment ceased at 75 days of age (day 57). Symptom-free at follow-up to 7 months of age. |
| Pendse and Deepika (2017), Australia (5) | Neonate with cardiac rhabdomyoma in intraventricular septum. | Not mentioned. | Not mentioned. | Not mentioned. | Cardiac arrest at day 29 of life, deceased. |
| Weiland et al. (2017), United States (6) | Two cases of neonatal cardiac rhabdomyoma. Case 1: Cardiac rhabdomyoma in apex of heart encroaching on left and right ventricle cavities. Case 2: LVOT obstruction due to cardiac rhabdomyoma. |
Case 1: Treatment started with 0.3 mg (0.1 mg/kg) daily. Trough level at 4 weeks post-commencement elevated 22.5 ng/ml. Case 2: Treatment started with 0.1 mg/kg twice daily, reduced to 0.05 mg/kg/dose at day 12 of treatment as trough level elevated (24.3 ng/dl), further reduced to 0.03 mg/kg/dose after 1 month of treatment as trough level elevated (12.1 ng/dl), resulting level 9.1 ng/dl. |
Trough level, other monitoring not specified. | Not mentioned. | Case 1: 74% reduction in tumor volume by day 11 of treatment, further reduction by 12% at 4 weeks. Interval size increase at follow-up 9 months after sirolimus cessation. Case 2: Decrease in size of tumor by day 12 of treatment (to approximately one-fourth of original size), LV free wall tumor decreased by >50%. |
CGA = corrected gestational age; LN = left ventricular; LVOT = left ventricular outflow tract.
There remains a paucity of data regarding optimal duration of therapy once the desired regression of cardiac rhabdomyoma is achieved. Our case demonstrates that regrowth of rhabdomyomas may occur with sirolimus cessation and that drug recommencement may result in a further response.
Inadequate information exists on the safety of sirolimus and everolimus in pregnancy, although both are capable of crossing the placenta and in rat models have been shown toxic to the fetus (1). However, there is a single published case report of successful antenatal use of sirolimus for the treatment of fetal rhabdomyoma (7).
Follow-Up
The infant continues on sirolimus at 0.125 mg daily at 12 months of age. Repeat imaging at 8 (Figure 1H) and 12 months of age (Figure 1I) demonstrated a sustained reduction in rhabdomyoma size. No further adverse side effects have been noted.
Conclusions
This case highlights the efficacy of sirolimus in the treatment of massive cardiac rhabdomyoma in TSC. The authors speculate that rapid reductions in the size of the rhabdomyoma were the result of unanticipated supratherapeutic sirolimus blood levels. Nonetheless these levels were well tolerated and not associated with side effects. Exact dosing, duration, and long-term safety profile of sirolimus in the neonatal and early infant setting remain unknown. However, where rapid reductions in rhabdomyoma size are needed, particularly in the newborn with hemodynamic compromise, supratherapeutic levels may be justified. This case report adds to the evidence that, with careful monitoring, sirolimus should be considered when managing infants with significant cardiac rhabdomyomas.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Therapeutic Goods Administration. Therapeutic Goods Administration Product and Consumer Medicine Information 2018. January 15, 2018. ebs.tga.gov.au Available at: Accessed March 27, 2019.
- 2.MacKeigan J.P., Krueger D.A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro Oncol. 2015;17:1550–1559. doi: 10.1093/neuonc/nov152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.J., Song E.S., Cho H.J., Choi Y.Y., Ma J.S., Cho Y.K. Rapid regression of obstructive cardiac rhabdomyoma in a preterm neonate after sirolimus therapy. Biomed Hub. 2017;2 doi: 10.1159/000460813. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breathnach C., Pears J., Franklin O., Webb D., McMahon C.J. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199–e1202. doi: 10.1542/peds.2013-3293. [DOI] [PubMed] [Google Scholar]
- 5.Pendse A., Wagh D. A case report of giant cardiac rhabdomyoma. J Paediatr Child Health. 2017;53 Suppl 2:77–78. [Google Scholar]
- 6.Weiland M.D., Bonello K., Hill K.D. Rapid regression of large cardiac rhabdomyomas in neonates after sirolimus therapy. Cardiol Young. 2018;28:485–489. doi: 10.1017/S104795111700244X. [DOI] [PubMed] [Google Scholar]
- 7.Barnes B.T., Procaccini D., Crino J. Maternal sirolimus therapy for fetal cardiac rhabdomyomas. N Engl J Med. 2018;378:1844–1845. doi: 10.1056/NEJMc1800352. [DOI] [PMC free article] [PubMed] [Google Scholar]