Abstract
This case illustrates a novel percutaneous treatment of a highly vascular thoracic tumor impinging on the left atrium and right pulmonary artery by delivery of coils and alcohol ablation via a circumflex coronary artery feeder branch. (Level of Difficulty: Advanced.)
Key Words: ablation, alcohol, thoracic
Abbreviations and Acronyms: BMW, balanced middleweight; CMR, cardiac magnetic resonance imaging; CT, computed tomography; OTW, over-the-wire; SAM, systolic anterior motion
Graphical abstract
This case illustrates a novel percutaneous treatment of a highly vascular thoracic tumor impinging on the left atrium and right pulmonary artery by…
History of presentation
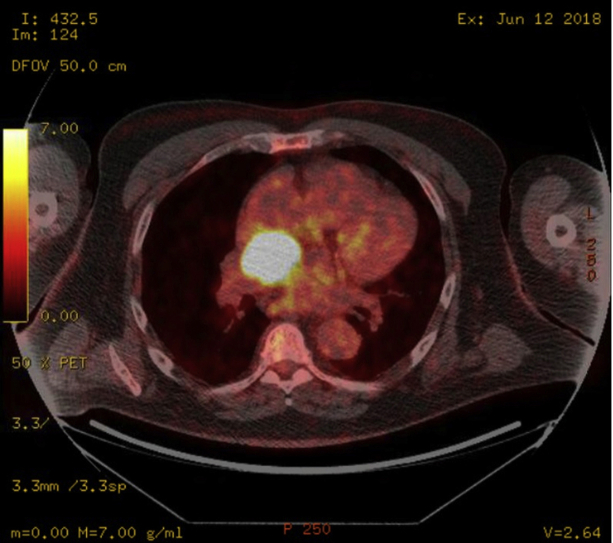
A 67-year-old male patient was referred for percutaneous management of an extracardiac mass. Past medical history was significant for stem cell transplantation for multiple myeloma, chronic kidney disease stage III, hypertension, and atrial fibrillation, whose initial surveillance positron emission tomography computed tomography (CT) showed a vascular mass measuring 3.4 × 2.3 × 2.0 cm located on the roof of the left atrium, below the right pulmonary artery and posterior to the aorta (Figures 1 and 2).
Learning Objectives
-
•
To understand feasibility of performing safe alcohol ablation and/or coil embolization of coronary feeder branch.
-
•
To explore possibilities of nonsurgical, minimally invasive management of thoracic tumor in high-risk patients.
Figure 1.
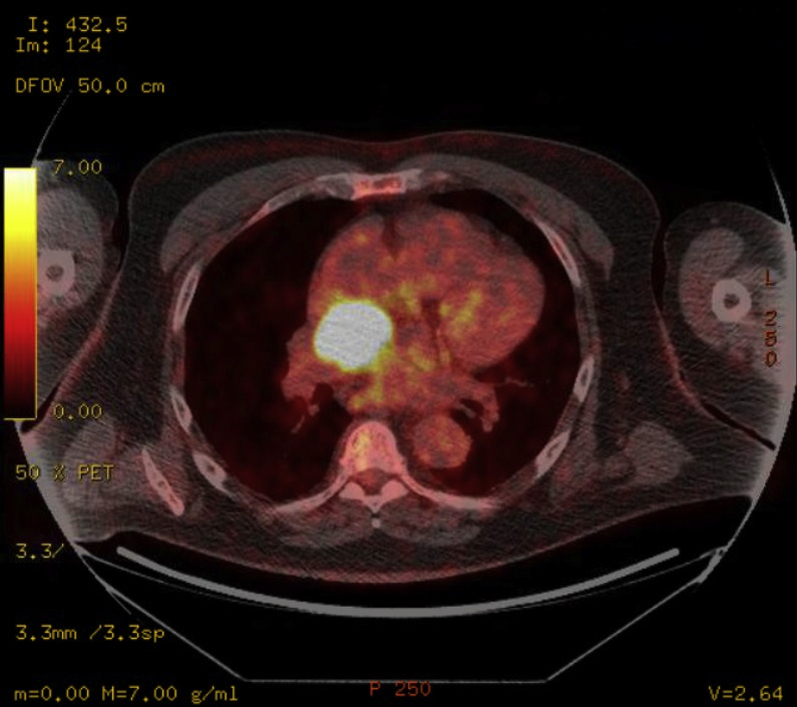
PET Scan Showing Hypermetabolic Mass 3.4 × 2.3 × 2.0 cm Based on FDG Uptake (Axial Plane)
FDG = fluorodeoxyglucose; PET = positron emission tomography.
Figure 2.
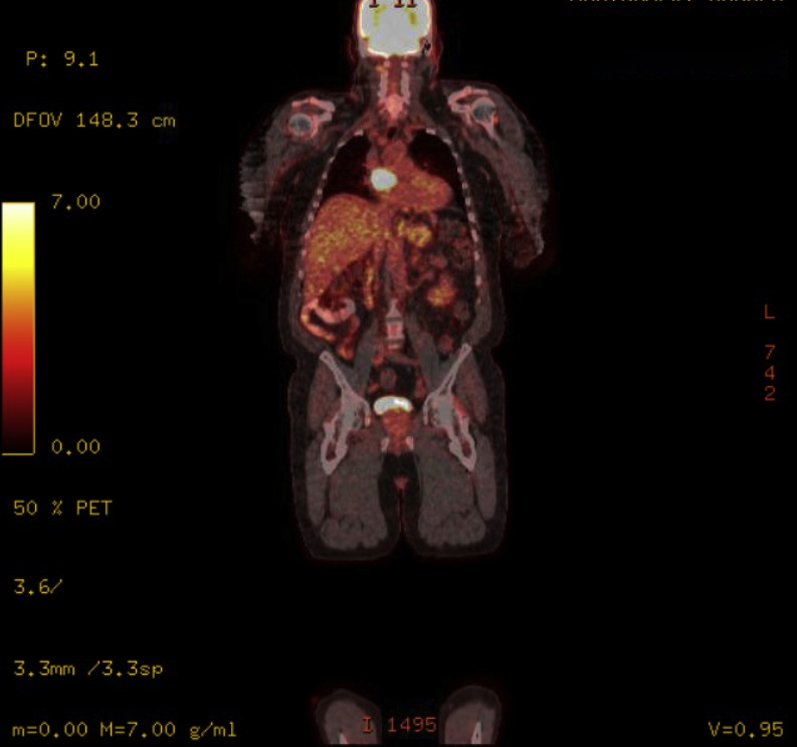
PET Scan Showing Hypermetabolic Mass 3.4 × 2.3 × 2.0 cm Based on FDG Uptake (Coronal Plane)
Abbreviations as in Figure 1.
A follow-up cardiac magnetic resonance (CMR) 6 months later revealed growth of the mass to 4.1 × 3.4 × 2.8 cm (Figures 3, 4, 5, and 6). The location of the mass was not amenable to percutaneous biopsy in an attempt to confirm diagnosis. Open surgical resection was considered and offered. Due to the patient's history of recent stem cell transplantation, subsequent immunosuppression, and baseline renal dysfunction, a heart team approach that included patient preference lead to a decision not to pursue open surgical excision. He was then referred to interventional cardiology for left heart catheterization and evaluation of potential percutaneous intervention options.
Figure 3.
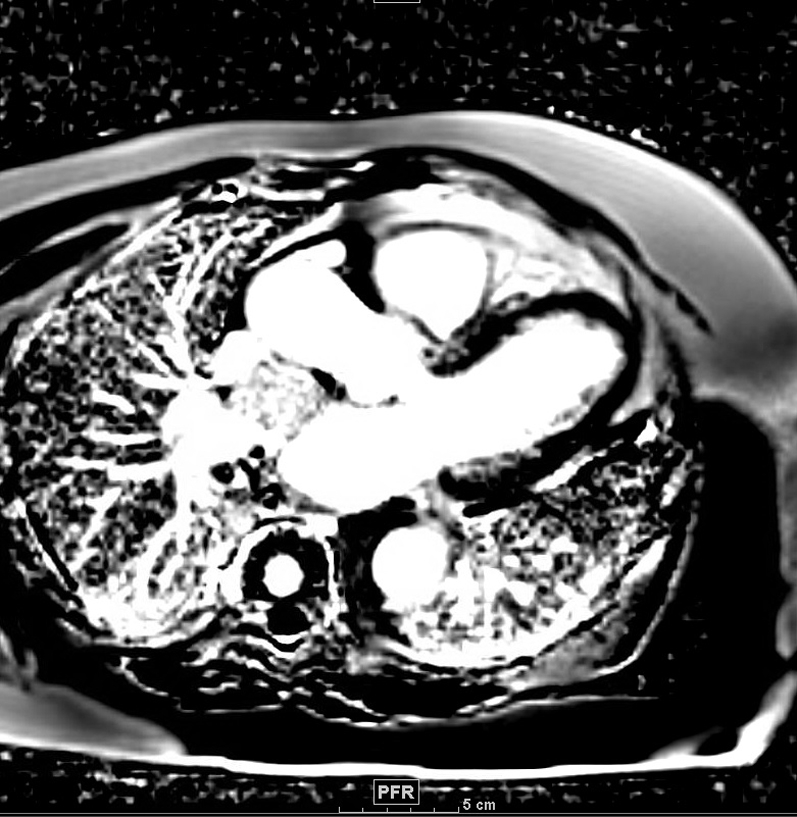
CMR, 3-Chamber View First-Pass Perfusion Image
CMR = cardiac magnetic resonance imaging.
Figure 4.
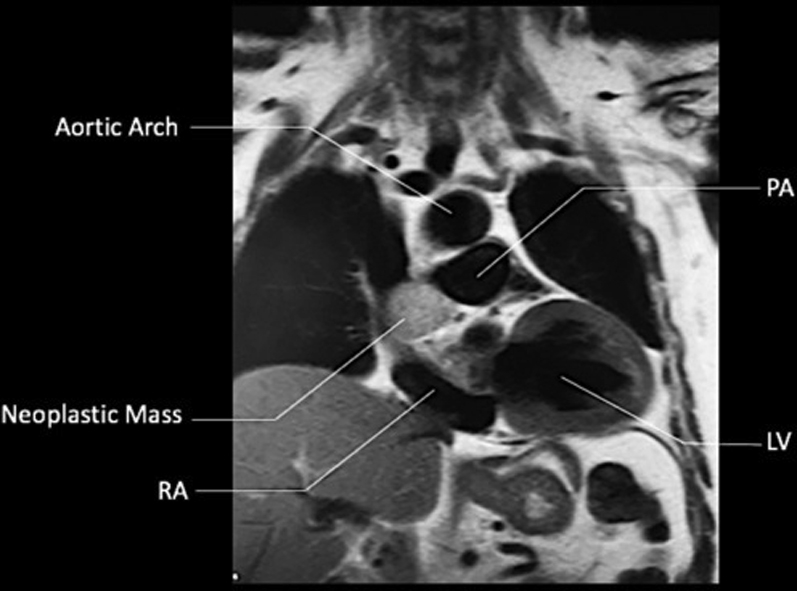
CMR, T1-Weighted SSFP (Coronal Plane)
CMR = cardiac magnetic resonance imaging; LV = left ventricle; PA = pulmonary artery; RA = right atrium; SSFP = steady-state free precession.
Figure 5.
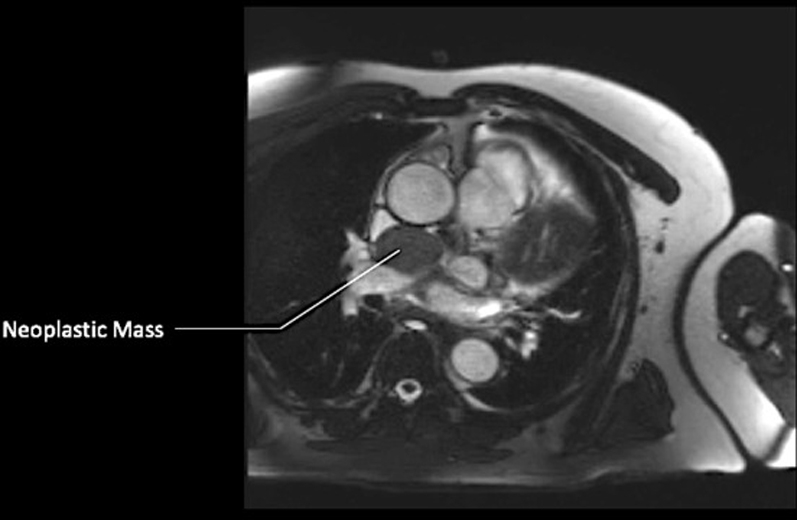
CMR, T1-Weighted SSFP Sequence With Contrast (Axial Plane)
Abbreviations as in Figure 4.
Figure 6.
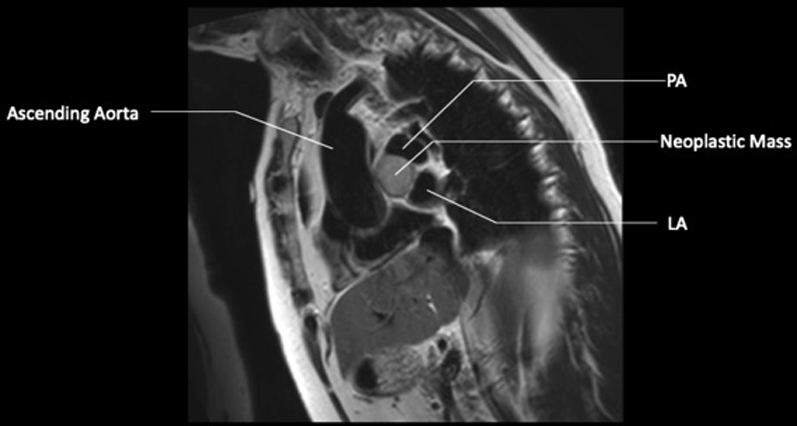
CMR, SSFP Sequence T1-Weighted Black Blood Mass 4.1 × 3.4 × 2.8 cm
LA = left atrium; other abbreviations as in Figure 4.
An extracardiac mediastinal mass can be an incidental finding in patients who undergo plain chest radiography or advanced imaging studies, such as CT or CMR, as in this patient. Symptoms, if present, may be due to direct mass effect (i.e., vascular compression–superior vena cava syndrome, hypotension due to cardiac compression, and tamponade physiology), Horner syndrome, cough, and stridor. Systemic symptoms can also be present (i.e., night sweats, weight loss, fevers), mostly due to malignant lesions (1).
Differential Diagnosis
The differential for the mass included benign and malignant etiologies such as paraganglioma, pheochromocytoma, or less likely, a plasmacytoma. The location of this mass was in the middle mediastinum; the differential of common middle mediastinal masses includes lymphadenopathy (most common causes are lymphoma, sarcoidosis, and metastatic lung cancer), benign cystic tumors (bronchogenic cysts, enteric cysts, and pericardial cysts), cardiovascular aneurysm or anomaly (thoracic aortic aneurysm, vascular ring), and esophageal tumors (2).
By imaging, this mass appeared most similar to paraganglioma, which is a tumor that arises from paraganglionic cells with a low grade of malignancy and nonepithelial origin. They are thought to arise from neural crest progenitor cells of the autonomic nervous system in extra-adrenal chromaffin tissues. These tumors mostly arise from posterior mediastinum, but occasionally may be located in middle mediastinum. Mediastinal paragangliomas are rare tumors (affecting 2 to 5 people per million per year), and they represent 0.3% of mediastinal tumors and <2% of all paragangliomas. They are usually located in the bifurcation of great vessels, similar to our patient, showing intense and homogeneous enhancement at chest CT. Paragangliomas of the middle mediastinum are generally nonfunctional and incidentally detected at chest CT, as in our case; however, sometimes symptoms related to compression of adjacent mediastinal organs (heart, great vessels, trachea, esophagus) by the tumor mass may develop, generally later in a patient’s life. Paragangliomas of the posterior mediastinum are usually functional (may secrete catecholamines, similarly to pheochromocytoma) and affect younger people (3).
Investigations
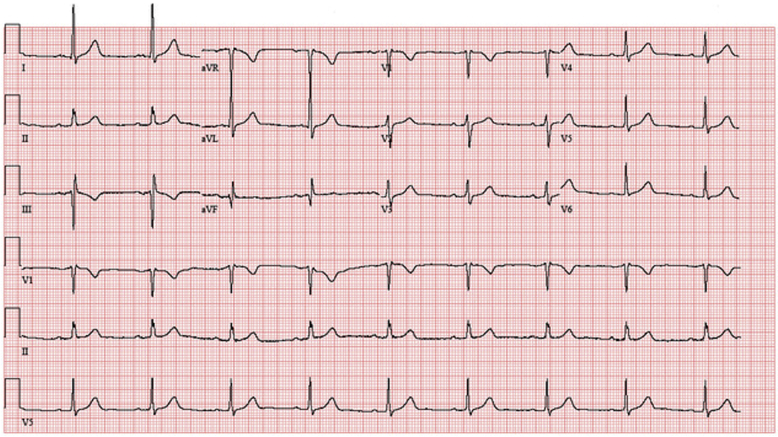
Coronary angiography identified a large feeder branch from the proximal left circumflex coronary artery and a small feeder branch from the right coronary artery to the extracardiac tumor (Figures 7, 8, and 9, Videos 1, 2, 3, 4, 5, 6, and 7). The authors identified this as the source circulation to the mass as the vascular supply was discretely extracardiac on angiogram, and it was encasing the shape of the mass. Remaining coronary anatomy was free of atherosclerotic disease. The levels of 24-h urine fractionated metanephrines and catecholamines were normal as shown in an initial testing for possible secreting paraganglioma or thoracic pheochromocytoma. Electrocardiogram showed normal sinus rhythm with voltage criteria for left ventricular hypertrophy and nonspecific T-wave inversions in lead V1 and lead III (Figure 10).
Figure 7.
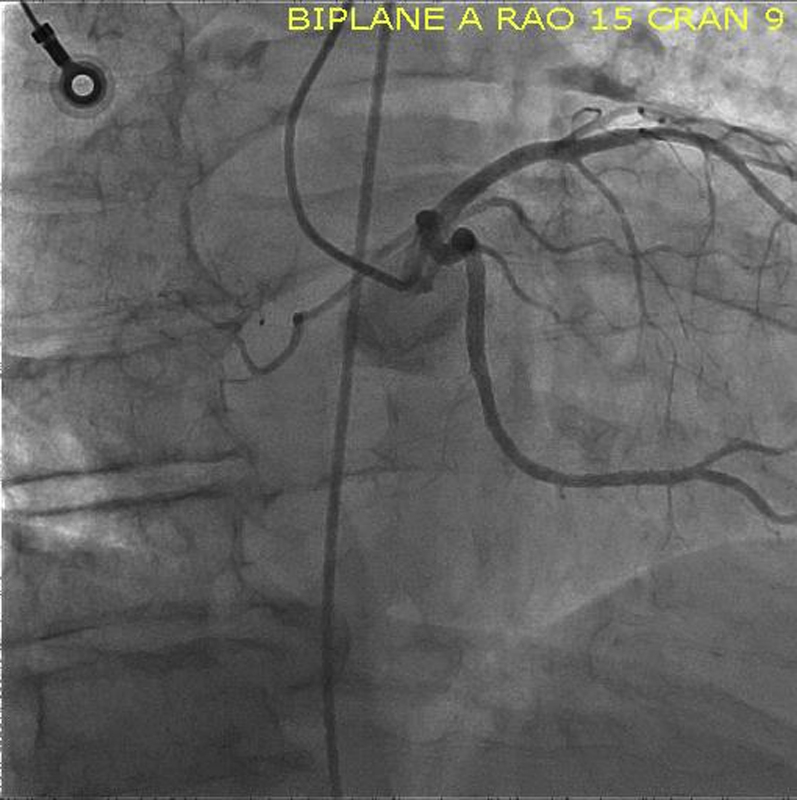
Angiogram Image, RAO Caudal View of the Left Coronary Artery, Focused on Tumor
RAO = right anterior oblique.
Figure 8.
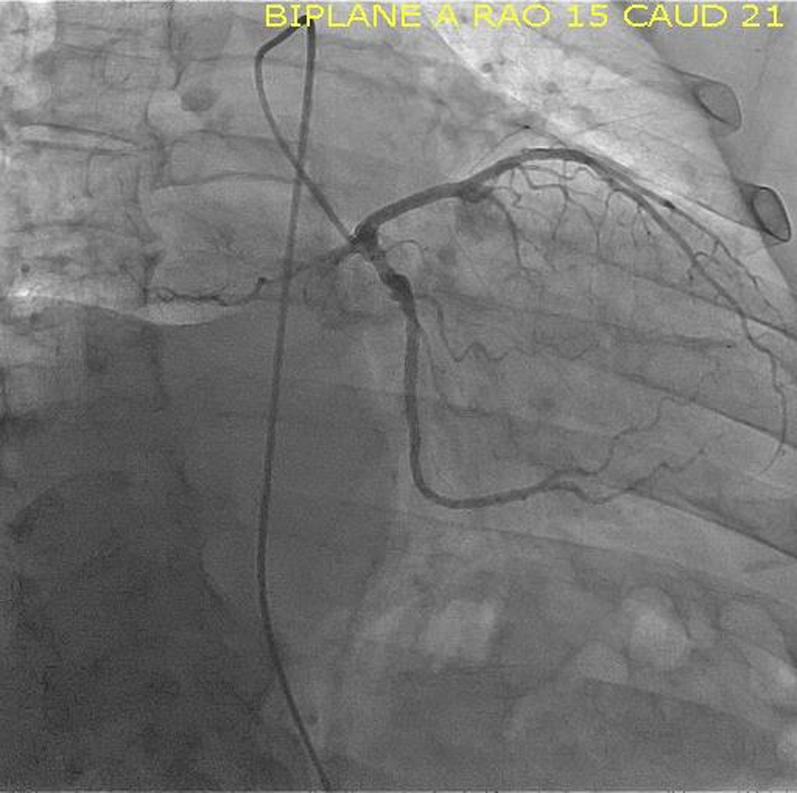
Angiogram Image, RAO Caudal View of Left Coronary Artery
RAO = right anterior oblique.
Figure 9.
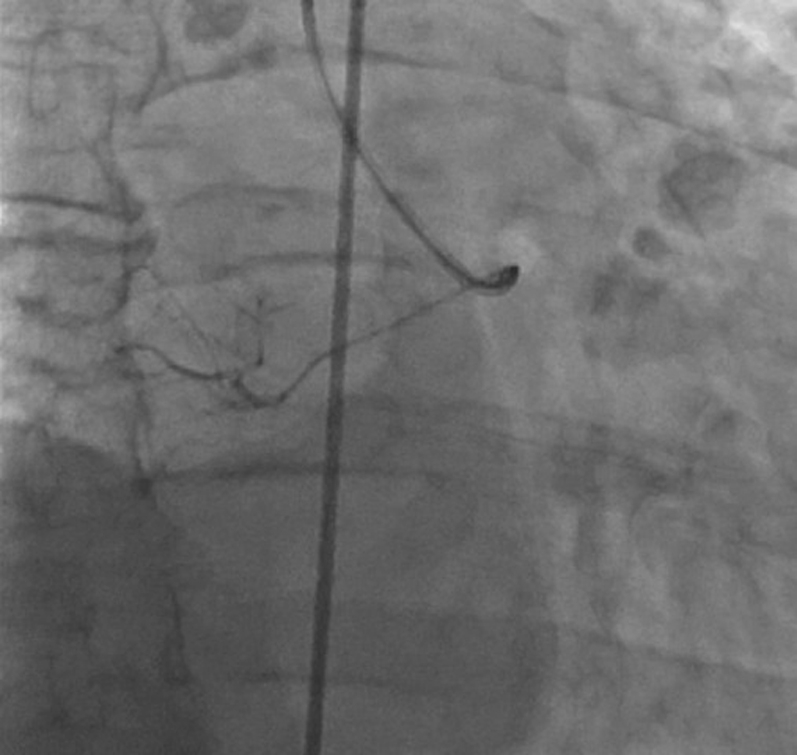
Injection of Contrast Through Microcatheter Showing Vascular Supply to the Tumor
Online Video 1.
LAO Caudal Angiogram of Left Coronary Artery
Online Video 2.
LAO Angiogram of Right Coronary Artery
Online Video 3.
RAO Caudal Angiogram of Left Coronary Artery (Zoomed-Out View)
Online Video 4.
RAO Caudal Angiogram of Left Coronary Artery
Online Video 5.
RAO Caudal Angiogram of Left Coronary Artery
Online Video 6.
RAO Cranial Angiogram of Right Coronary Artery
Online Video 7.
0.014-Inch BMW Guidewire Inserted Into the Circumflex Coronary Artery and Into the Feeder Branch
Figure 10.
Electrocardiogram, Normal Sinus Rhythm With Voltage Criteria for Left Ventricular Hypertrophy and Nonspecific T-Wave Inversions in Lead V1 and Lead III
Management
After identification of the source circulation to the mass, the authors proceeded with the intervention. The authors ascertained that the intervention would not jeopardize important normal structures because the vascular supply to the mass was extracardiac, and there was no contrast extravasation into surrounding tissues on angiography. Using a Judkins left guide catheter, the left coronary artery was engaged. A 0.014-inch balanced middleweight (BMW) guidewire was inserted into the circumflex coronary artery and into the feeder branch that supplied the tumor (Video 8). After removing the guidewire, 2 Trufill coils (3 × 20 mm) (Codman Neuro [Johnson & Johnson], Raynham, Massachusetts) were deployed through a Transit microcatheter (Codman Neuro [Johnson & Johnson]) into the distal segment of the feeder coronary branch. There was significant, albeit not complete, reduction in flow (Videos 9, 10, and 11). Thus, the BMW guidewire was reinserted, and the microcatheter was exchanged for a 2.0 × 6-mm Sprinter over-the-wire (OTW) balloon (Medtronic, Dublin, Ireland) that was used to occlude the feeder branch. After the guidewire was removed, angiography through the balloon confirmed complete occlusion by the balloon without reflux into the circumflex coronary artery (Video 12). A total of 1.5 ml of ethanol was then administered through the OTW balloon (Video 13). There was a transient asymptomatic change in rhythm from sinus bradycardia to sinus arrest and junctional escape rhythm of 45 to 60 beats/min. Finally, the balloon was removed over a guidewire, and on angiography, the authors noted patent left circumflex and left anterior descending coronary arteries with occlusion of the feeder coronary branch without further visualization of extracardiac tumor (Figures 11 and 12, Videos 14, 15, and 16). After monitoring on telemetry overnight, the patient was discharged home the following morning.
Online Video 8.
Contrast Injection Through Transit Microcatheter
Online Video 9.
Delivery of First Trufill Coil Through Microcatheter (3 × 20 mm)
Online Video 10.
Delivery of Second Trufill Coil Through Microcatheter (3 × 20 mm)
Online Video 11.
Contrast Injection of Left Coronary Artery Post-Coil Delivery, RAO Caudal View
Online Video 12.
Contrast Injection of Left Coronary Artery After Inflation of the OTW Balloon
Online Video 13.
Injection of 1.5 ml of Ethanol Through OTW Balloon
Figure 11.
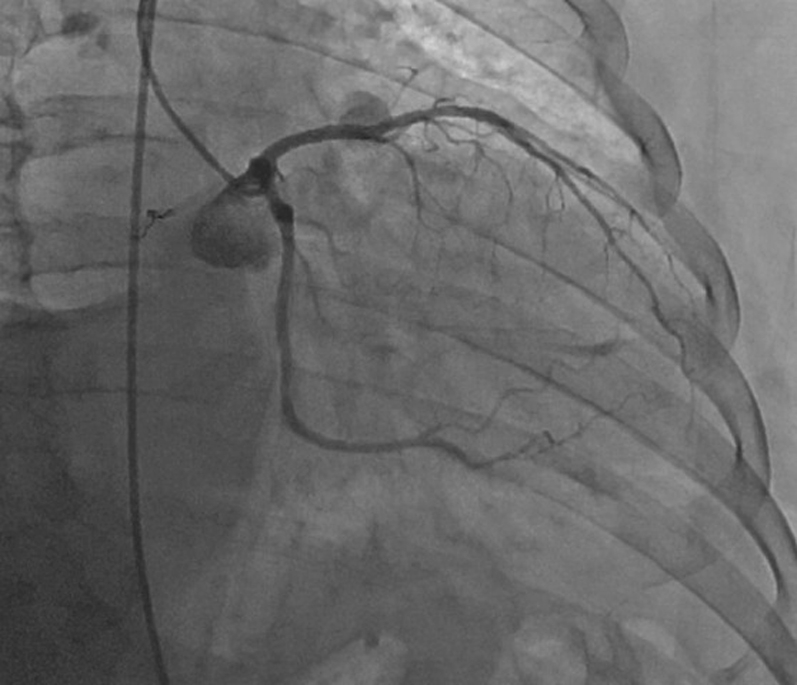
RAO Caudal View of LCA After Alcohol Ablation of Feeder Branch
LCA = left coronary artery; RAO = right anterior oblique.
Figure 12.
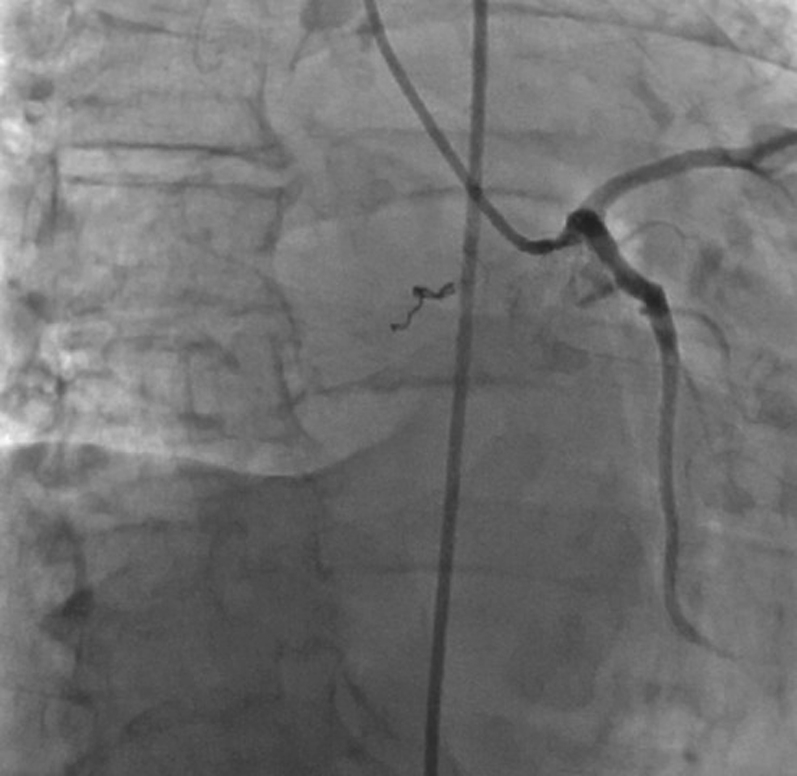
RAO Caudal View of LCA After Alcohol Ablation of Feeder Branch, Focused on Tumor Location
Abbreviations as in Figure 13.
Online Video 14.
Final Post-Ablation Angiogram RAO Caudal View
Online Video 15.
Final Post-Ablation Angiogram LAO Caudal View
Online Video 16.
Final Post-Ablation Angiogram RAO Caudal View
Discussion
Alcohol ablation has been used in multiple disciplines of medicine, and it has several indications. For instance, alcohol septal ablation is used in hypertrophic cardiomyopathy, where it relieves left ventricular outflow tract obstruction by creating a localized myocardial infarction in the area of the basal septal muscle where systolic anterior motion–septal contact is occurring (4,5). Transarterial chemoembolization, that is, the use of drug-eluting polyvinyl alcohol microspheres (“beads”), has been used in treatment of hepatocellular carcinoma as a complementary procedure before portal vein embolization procedure because it eliminates the arterial blood supply to the tumor. Percutaneous ethanol injection may be considered for patients with small hepatocellular carcinomas who are not candidates for resection due to their poor functional hepatic reserve (6). Ethanol has also been used as a nonsurgical method in treatment of toxic thyroid adenomas with percutaneous injection using ultrasound guidance at weekly intervals for 5 to 8 weeks. This form of therapy is not widely used in the United States. However, where available, it can be used when Iodine-131 or surgery is not desirable and when the nodule is not too large (<5 ml in volume) (7,8). Finally, pre-operative embolization of hypervascular thoracic, lumbar, and sacral spinal column tumors with polyvinyl alcohol particles has been linked to reduced intraoperative blood loss and improved qualitative variables such as visibility and resectability of the tumor (9,10). Alcohol ablation of the extracardiac tumor through a coronary artery branch has not been described in the published reports.
Follow-Up
After alcohol ablation, follow-up CMR revealed a reduction in tumor size (3.1 × 2.7 × 1.8 cm and later, 2.2 × 1.9 × 1.3 cm from the initial 4.1 × 3.4 × 2.8 cm) and no identifiable perfusion of this tumor (Figures 13 and 14). Follow-up cardiac CMR every 3 months thus far has shown a steady reduction in tumor size.
Figure 13.
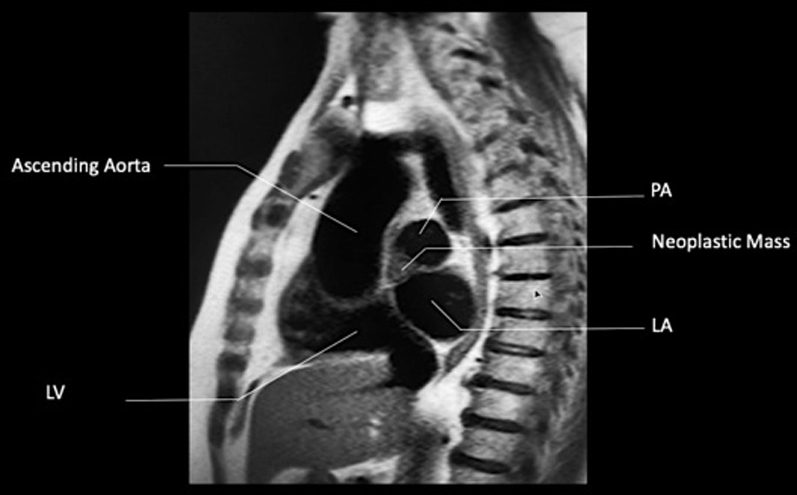
CMR, T1-Weighted SSFP Sequence (Sagittal Plane)
Figure 14.
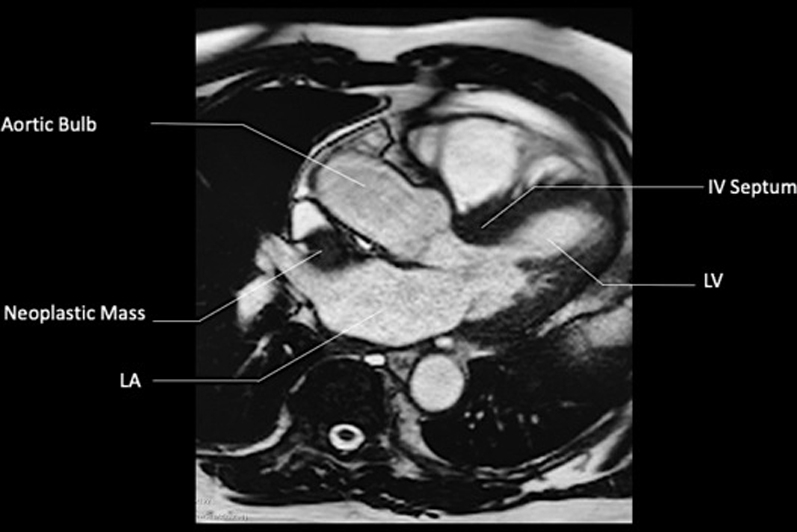
CMR, T1-Weighted SSFP Sequence With Contrast (Axial Plane)
IV = interventricular; other abbreviations as in Figures 4 and 6.
Conclusions
Although a rare condition, certain vascular extracardiac masses may be safely and successfully treated with percutaneous coil embolization and/or alcohol ablation should arterial supply come from a coronary feeder branch.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose. John W. Hirshfeld, Jr., MD, served as the Guest Associate Editor for this article.
Informed consent was obtained for this case.
Appendix
For supplemental videos, please see the online version of this paper.
References
- 1.Shields T.W. Overview of primary mediastinal tumors and cysts. In: Shields T.W., LoCicero J. III, Ponn R.B., Rusch V.W., editors. Volume 2. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 2489–2493. (General Thoracic Surgery, 6th edition). [Google Scholar]
- 2.Strollo D.C., Rosado-de-Christenson M.L., Jett J.R. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest. 1997;112:1344–1357. doi: 10.1378/chest.112.5.1344. [DOI] [PubMed] [Google Scholar]
- 3.De Palma A., Lorusso M., Di Gennaro F. Pulmonary and mediastinal paragangliomas: rare endothoracic malignancies with challenging diagnosis and treatment. J Thorac Dis. 2018;10:5318–5327. doi: 10.21037/jtd.2018.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura R.A., Seggewiss H., Schaff H.V. Hypertrophic obstructive cardiomyopathy: surgical myectomy and septal ablation. Circ Res. 2017;121:771–783. doi: 10.1161/CIRCRESAHA.116.309348. [DOI] [PubMed] [Google Scholar]
- 5.Pelliccia F., Niccoli G., Gragnano F. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a contemporary reappraisal. EuroIntervention. 2019;15:411–417. doi: 10.4244/EIJ-D-18-00959. [DOI] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Guglielmi R., Pacella C.M., Bianchini A. Percutaneous ethanol injection treatment in benign thyroid lesions: role and efficacy. Thyroid. 2004;14:125–131. doi: 10.1089/105072504322880364. [DOI] [PubMed] [Google Scholar]
- 8.Papini E., Panunzi C., Pacella C.M. Percutaneous ultrasound-guided ethanol injection: a new treatment of toxic autonomously functioning thyroid nodules? J Clin Endocrinol Metab. 1993;76:411–416. doi: 10.1210/jcem.76.2.8432784. [DOI] [PubMed] [Google Scholar]
- 9.Nair S., Gobin Y.P., Leng L.Z. Preoperative embolization of hypervascular thoracic, lumbar, and sacral spinal column tumors: technique and outcomes from a single center. Interv Neuroradiol. 2013;19:377–385. doi: 10.1177/159101991301900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loftus T.J., Pipkin M., Machuca T., Oduntan O. Angiographic embolization followed by piecemeal resection of giant posterior mediastinal schwannoma: case report and concise review. Int J Surg Case Rep. 2018;53:250–253. doi: 10.1016/j.ijscr.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]