Abstract
Introduction
Feline odontoclastic resorptive lesion (FORL) is one of the most common and painful oral diseases of the cat. It is characterised by tooth resorption due to destructive activity of odontoclasts. FORL can result in tooth loss. While the aetiology of FORL is not clearly understood, it is thought to be multifactorial and bacteria are likely to play a major role.
Hypothesis
Dysbiosis of the normal feline oral microbiota leads to an alteration in commensal bacteria populations, which results in the development of FORL.
Aim
The purpose of the current study was to determine the composition of the microbiomes associated with feline oral health and FORL.
Methodology
Supragingival plaque was collected from 25 cats with a healthy oral cavity and 40 cats with FORL. DNA was extracted from each sample, the V4 region of the 16S rRNA gene amplified by polymerase chain reaction and amplicons sequenced. Diversity and species richness analyses were performed, principal component analysis was used to explore differences between the oral microbiomes of healthy cats and those with FORL, and linear discriminant analysis effect size was used to assess differences between the groups.
Results
The six most abundant bacterial genera identified were Bergeyella , Capnocytophaga, Lampropedia, Morexella, Porphyromonas and Treponema . Two-step cluster analysis of the data identified two FORL sub-groups (FORL-1, FORL-2). The FORL-2 sub-group was very similar to the healthy group, whilst the FORL-1 sub-group was clearly different from both the FORL-2 sub-group and the healthy groups. In this analysis, Capnocytophaga (P <0.001) and Lampropedia (P <0.01) were found at significantly lower levels and Porphyromonas at a slightly higher level in the FORL-1 sub-group compared to the healthy and FORL-2 sub-groups. Microbial diversity was found to be less in the FORL-1 sub-group than in the healthy group. Lampropedia sp., a phosphate-accumulating oral commensal species, was significantly lower in the FORL-1 sub-group.
Conclusion
The oral microbiota associated with the FORL-1 sub-group is distinct from that found in the healthy group and FORL-2 sub-group. Lampropedia species may influence the local calcium-phosphate ratio, which could be a factor in tooth and bone resorption observed in FORL.
Keywords: bacteria, feline, high-throughput sequencing, microbiome, odontoclastic resorptive lesion
Introduction
Feline odontoclastic resorptive lesion (FORL), more commonly known as tooth resorption, is the second most prevalent disease of cats after periodontal disease [1]. It affects over 60 % of cats older than 6 years of age and its incidence increases with age [2, 3]. A general prevalence range of 20–72 % has been reported [3, 4].
Tooth resorption in cats refers to a process by which permanent teeth are progressively destroyed by the activity of odontoclastic cells [5, 6]. It manifests as erosion on the tooth surface at the gingival border that causes destruction of cementum, dentine and the periodontal ligament that can eventually penetrate the pulp cavity and ultimately leads to tooth loss if left untreated. Bone-like tissue replaces resorbed cementum and dentine and tooth fracture may occur due to enamel resorption. Thus, it is a painful disease that causes much distress in affected animals.
FORL can be classified into five stages based on the extent of tooth destruction [3]; stage one is the mildest form (mild dental hard tissue loss, lesions extend into cementum only) whereas stage five is the most severe form (no crown, only root remnants remaining). The disease can also be classified into type one and type two lesions based upon radiographic appearance of the root. Type one lesions occur just above the gingivae at the cervical margin of the tooth with surrounding inflamed gingivae, and display resorption of the crown above the gum line but the root remains intact. Type two lesions commence at the tooth surface, the gingivae at the crown exhibiting granulomatous tissue with the abnormal tooth being replaced by alveolar bone and the periodontal ligament space is either narrow or missing; ankylosis of the root is also apparent. Unfortunately, despite the progressive nature of FORL, its aetiology remains poorly understood.
There are several methods for treating FORL [3]. Fluoride therapy is used if the lesion is early stage (mild), involves enamel only and there is no radiographic abnormality. The tooth is cleaned to remove the plaque and calculus, polished and treated with fluoride gel. Restoration (filling) is recommended for moderately severe lesions involving enamel and dentine but not the root canal, particularly for important teeth. If the lesion is moderate to severe, the crown is missing or there is root pathology present on radiography, root canal therapy may be considered, although extraction is recommended. Unfortunately, none of these treatments can prevent the development or progression of FORL. Removal of teeth is the only treatment to provide a long-term resolution [7] following radiographic examination [8]. Therefore, the development of improved treatment and prevention methods is highly desirable.
The potential role of vitamin D in FORL has been examined but produced conflicting data. Some studies have shown that the prevalence of FORL was greater in cats with lower serum levels of vitamin D [9, 10], while other studies demonstrated that chronic excess levels of vitamin D in the feline diet correlates with the presence of FORL [3, 11].
Although the relationship between FORL and periodontal disease is unclear, the release of cytokines as a result of periodontal disease may lead to FORL due to odontoclast migration. Vitamin D, as well as inflammatory cytokines, have been shown to stimulate osteoclast formation [12]. One study has shown that elevated expression of the pro-inflammatory cytokines IL-1β and IL-6 may play a role in the mediation of osteoclast activity in FORL [13], and elevated expression of IL-1β, IL-6 and an additional pro-inflammatory cytokine (TNF-α) was found in cats with FORL [14]. Inflammation is therefore likely to play a significant role in FORL.
However, it is apparent that FORL is a multifactorial disease and since it is an inflammatory disease it would be prudent to consider this inflammation could be due to bacterial involvement. Indeed, bacteria are known to play a key role in other important oral diseases of animals, including periodontal disease of the cat [15], dog [16] and horse [17], and feline chronic gingivostomatitis (FCGS) [18].
We hypothesise that, in FORL, the normal oral polymicrobial microbiota becomes dysbiotic and leads to a disturbance of the microbial synergy within the bacterial community in which they thrive. In turn, this causes alteration of commensal bacteria that results in the initiation and / or propagation of FORL. Therefore, the aim of this study was to compare the bacteria associated with the microbiomes of FORL and feline oral health, using high-throughput sequencing of bacterial 16S rRNA genes. Such knowledge will aid understanding of the bacteria associated with FORL and aid in developing strategies to prevent or treat this important and common feline disease.
Methods
Ethical approval
Study protocols were reviewed and approved by the Nestlé Purina PetCare Animal Care and Use Committee and complied with all regulations set forth in the United States Department of Agriculture (USDA) SDA Animal Welfare Act: https://www.aphis.usda.gov/animal_welfare/downloads/bluebook-ac-awa.pdf.
Collection of clinical samples
Oral samples (25 orally healthy and 40 FORL) were collected from cats at the Nestlé Purina PetCare facility (St. Joseph, MO, USA). Dental examinations involved visual and tactile dental examination by two trained veterinarians as part of the standard veterinary oral evaluation of cats, and samples for the study were collected by the same personnel.
The healthy group comprised 14 males and 11 females with a mean age of 4.9 years (range 1.7 to 7.5 years). In the FORL group, there were 28 males and 12 females with a mean age of 7.2 years (range 2.9 to 11.4 years). All cats in both groups were domestic shorthair.
Clinical classification of FORL was based on appearance of the tooth by radiography and defined as two distinct types (I and II) [3]. Cats with obvious erosion of enamel and dentine at the base of the tooth with localised gingival inflammation were deemed to be suffering from FORL; orally healthy control cats exhibited no signs of any oral disease. All cats were free of systemic illnesses and had not received antibiotic therapy in the preceding 6 months.
Supragingival plaque was collected from each cat using a sterile swab, sampling the upper left, upper right, lower left and lower right teeth, as appropriate, to give maximal sampling coverage. Each swab was placed in a 1.5 ml centrifuge tube and then totally immersed in 1 ml of RNAlater (Sigma Aldrich, St Louis, MO, USA), stored for 24 h at 4 °C then stored at −80 °C until required for processing.
Processing of clinical samples and isolation of DNA
Swabs were centrifuged for 15 min at 13 000 g, supernatant removed, sample resuspended in 150 µl TE buffer and sample swabs were then sonicated for 30 s. The sonicate was transferred to an Eppendorf 96×2000 µl DeepWell plate (Merck, Kenilworth, NJ, USA), centrifuged for 15 min at 13 000 g and the precipitate resuspended in 150 µl TE buffer. Samples were then transferred to a plate containing 0.25 ml lysis buffer (AGOWA mag Mini DNA Isolation Kit, AGOWA, Berlin, Germany), 0.3 g zirconium beads (diameter, 0.1 mm; Biospec Products, Bartlesville, OK, USA) and 0.2 ml phenol in each well. Samples were homogenised at 2100 oscillations per min for 2 min using a Mini-Beadbeater (Biospec Products). Finally, DNA was isolated using the AGOWA mag Mini DNA Isolation Kit in accordance with the manufacturer’s instructions.
High-throughput sequencing of bacterial 16S rRNA genes
Amplicon libraries of the V4 hypervariable region of the 16S rRNA gene were produced for each sample using the forward primer 515F (GTGCCAGCMGCCGCGGTAA) and the reverse primer 806R (GGACTACHVGGGTWTCTAAT). These primers contained Illumina adaptors and a unique 8-nucleotide sample index sequence key [19]. Amplicon libraries were pooled in equimolar amounts and purified using the Illustra GFXTM PCR DNA and Gel Band Purification Kit (GE Healthcare, Eindhoven, The Netherlands). The quality and size of amplicons were analysed on an Agilent 2100 Bioanalyzer (Santa Clara, CA, USA). Amplicons were pair-end sequenced using the Illumina MiSeq platform v2 kit which generated 2×251 nucleotide reads (Illumina, San Diego, USA).
Sequencing output analysis
Sequence reads were quality filtered using Trimmomatic version 0.32 and then merged using fastq-join implemented in QIIME version 1.8.0 [20]. Sequences were clustered into operational taxonomic units (OTUs) using USEARCH version 8.0.1623 [21] after being quality filtered with USEARCH (maxee 0.5). Using QIIME version v 1.8.0 [22], the most abundant sequence of each OTU was selected. The representative sequence of each cluster was assigned a taxonomy using the RDP classifier [23] with a minimum confidence of 0.8 and the 97 % representative sequence based on the silva rRNA database (release 119) for QIIME [24]. Raw sequencing reads are available under NCBI BioProject number PRJNA720563.
Statistical analysis
An a priori power calculation with an alpha of 0.05 would require a minimum of 17 samples per group in a parametric analysis, such as a t-test. However, it was likely that a non-parametric test would be more appropriate and that therefore the minimum number of samples required per group would be at least 20. To accommodate for multiple analyses with sufficient statistical power, more than 20 samples were analysed in each group.
Diagnosis was cross tabulated against sex and the Fisher’s exact test was used to determine significant differences and the age difference between groups was determined by a Mann-Whitney U test in SPSS (version 21.0). The 65 cats analysed (25 healthy and 40 FORL) were separated into five bins of 13 animals based on age. The expected number of cats per bin for health and FORL was five and eight, respectively. Frequency weights were calculated by dividing the expected number of cats per group per bin by the actual number of cats per group per bin. Weights for gender were determined in a similar manner.
Analysis of sequencing data
Normalisation of sequencing depth was carried out by random sub-sampling of the dataset to 50 % of the reads per sample. Sequencing data were analysed using several statistical programmes. The OTU dataset was reduced by log-2 transformation in order to conduct principal component analysis (PCA).
PCA, diversity analyses (species richness, Chao-1 index, Simpson index, Shannon diversity index) and assessment of differences between microbial profiles of the two groups (health and FORL) by one-way PERMANOVA were performed using PAleontological STatistics (PAST; v3.02) software [25]. The Mann-Whitney test in SPSS (version 21.0) was used to compare the diversity and species richness output. Taxa and OTUs that determined differences between the two groups were identified using linear discriminant analysis (LDA) effect size (LEfSe) [26].
Two-step cluster analysis
As the complex clinical data were not collected from the cats at the time of sampling, a two-step cluster analysis was undertaken. Categorical variables were the diagnosis (healthy or FORL) and the sex of the cats. Continuous variables were cytokines (IL-1β, TNF-α) and chemokines (IL-8, RANTES, KC and MCP-1) analysed by a Luminex multiplex assay in saliva samples collected from the cats (data not shown), the 20 most abundant bacteria and species richness. The clusters determined by the analysis were analysed further for statistical differences in sex (Fisher’s exact test) and age (Kruskal-Wallis test). Frequency weights for the clusters were determined and applied. The diversity indices and microbial profiles in the clusters were analysed with the Kruskal-Wallis test and Dunn’s post-test.
Results
Effect of age, sex and dentition
There were no statistically significant differences in sex between the healthy and FORL group (P=0.15), but the age was significantly lower in the healthy group (P <0.001). All the healthy cats had full dentition. Although radiographic data was only available for 18 cats of the 40 cats with FORL, these animals had between one and eight teeth affected by the disease.
Sequencing data output
Following quality processing of the data, 794 064 reads were obtained, with 441 distinct OTUs comprising 21 phyla, 39 classes, 78 orders; 125 families and 136 genera. Reads per sample ranged from 5888 to 19 292 (median 11 902, mean 12 216, SD 2926). The mean number of OTUs in the healthy and FORL samples was similar: 12 459±2622 (healthy), 12 065±3124 (FORL).
Composition of the healthy and FORL samples
At the genus or higher taxonomical level, 441 OTUs were identified, of which 317 were found in the healthy group and 404 were found in the FORL group. Of these, nine were unique to cats with healthy oral cavities and six of these were identified to genus level (Table 1). In cats with FORL, 41 unique OTUs were identified of which 28 were identified to genus level (Table 1).
Table 1.
Genera unique to feline oral health and FORL
|
Oral health |
FORL |
|---|---|
|
Bacteroides (2)* |
|
|
|
|
|
|
Fusibacter (2)* |
|
|
Helicobacter (2)* |
|
|
Leptotrichia (2)* |
|
|
|
|
|
Moraxella (2)* |
|
|
|
|
|
|
|
|
Rothia (2)* |
|
|
|
|
|
Staphylococcus (3)* |
|
|
Treponema (3)* |
*Values in brackets indicate the number of different OTUs that were assigned to that genus.
Genera identified by analysis of microbiome composition as being unique to either oral health or FORL.
Linear discriminant analysis (LDA) effect size (LEfSe) was used to assess the differences between the two groups at the genus level and at the OTU level (Fig. 1). Fifteen OTUs showed a statistically significant difference between the healthy and FORL groups (P <0.05, LDA score of at least 3), of which 14 OTUs were associated with FORL (LDA score of at least 3.0) and one OTU was associated with health (LDA score of at least 3.0).
Fig. 1.
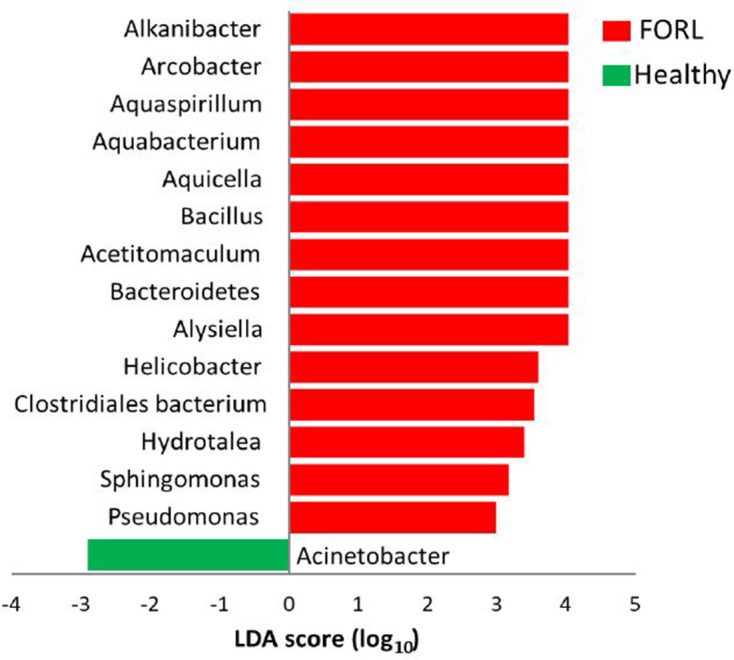
Most significant taxa associated with feline oral health and FORL. Taxa (at genus or higher level) that differentiate between the microbiomes of feline oral health (green) and FORL (red). Fifteen taxa were significantly different between the two groups. Only taxa with an LDA score of three or above are shown. Taxa are ranked by the effect size in LEfSe.
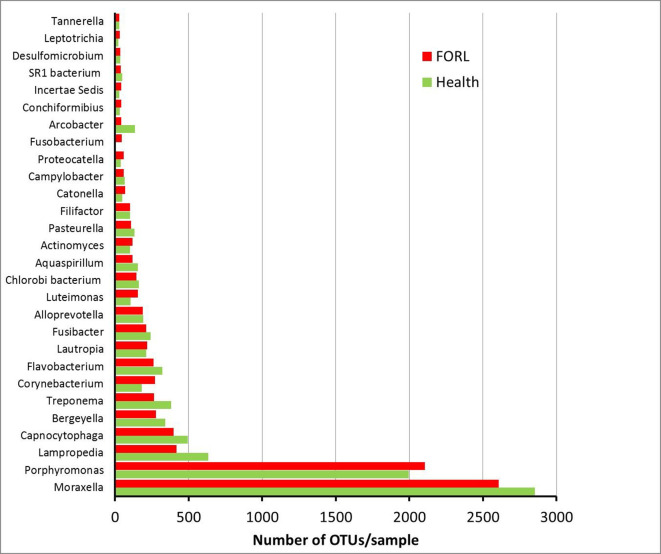
The most abundant OTUs identified in the samples are shown in Fig. 2. The genus Moraxella was the most abundant OTU identified in both health and FORL. The next most abundant OTUs represented the genera Porphyromonas, Lampropedia, Capnocytophaga, Bergeyella and Treponema . The number of OTUs for the genera Moraxella, Lampropedia, Capnocytophaga, Bergeyella and Treponema were slightly higher in health compared to FORL, whereas the number of OTUs for Porphyromonas was slightly higher in FORL. These differences were statistically significant for Capnocytophaga and Lampropedia (both P <0.05).
Fig. 2.
Most abundant genera or higher taxa identified in feline oral health and FORL. Distribution of the most prevalent genera or higher taxa in feline oral health and FORL. The average number of OTUs for each taxon are shown for feline oral health (green) and FORL (red).
Analysis of microbial profiles
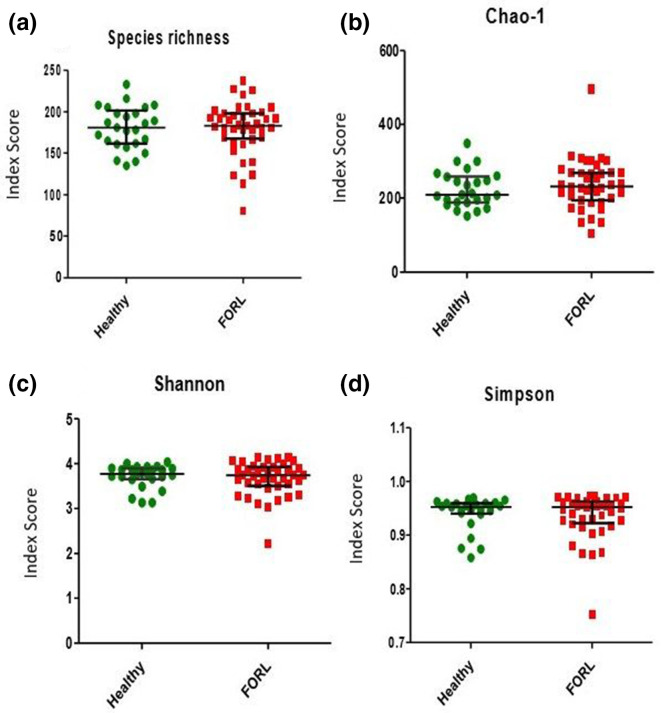
PCA was used to explore differences between the oral microbiomes in health and FORL (Fig. 3). PCA showed significant overlap of both the healthy and FORL groups, with no distinct clustering observed between the groups. The difference between the microbial profiles in health and FORL was not statistically significant (P=0.077). No significant difference in species richness or diversity was observed between the healthy and FORL groups (Fig. 4).
Fig. 3.
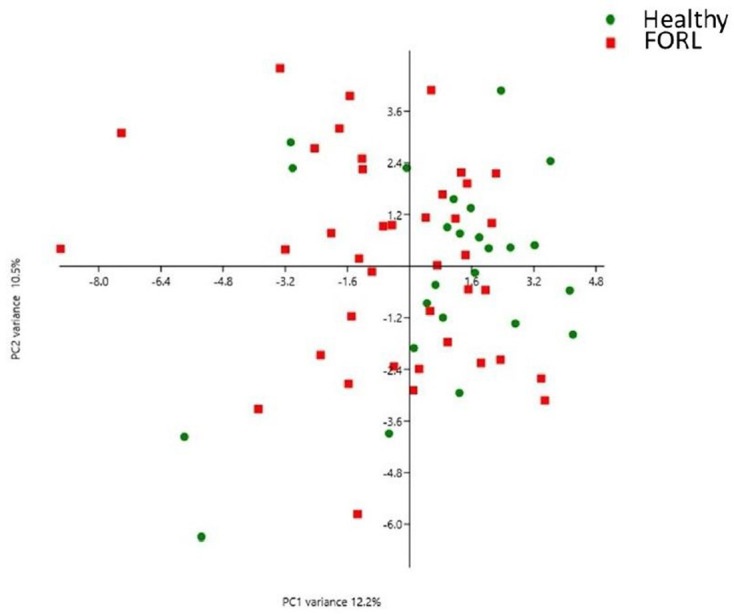
Two-dimensional ordination of feline microbial profiles in oral health and FORL by principal component analysis (PCA). Identified OTUs were randomly subsampled to 50 % and log2-transformed prior to the PCA. Green dots represent the microbiomes of feline oral health (n=25) and the red squares represent the microbiomes associated with FORL (n=40). Significant overlap was observed between the two groups with no clustering evident, indicating that the microbiomes within and between each group were not significantly different in composition.
Fig. 4.
Diversity analysis of microbiomes in feline oral health and FORL. (a) Observed species richness (number of OTUs per sample). (b) Chao-1 index. (c) Shannon diversity index. (d) Simpson index. Samples from feline oral health (n=25) are shown in green and those from FORL (n=40) are shown in red. No statistically significant differences in species richness or species diversity were observed between oral health and FORL.
Two-step cluster analysis
Following cluster analysis three clusters were identified, and two cats were identified as outliers. The major predictors in cluster formation were FORL, salivary biomarkers (chemokines, IL-1β) and species richness. Cluster one was the healthy group and comprised 25 cats. Two distinct sub-groups within the FORL group were identified, FORL-1 (cluster two; 12 cats) and FORL-2 (cluster three; 28 cats).
There were no statistically significant differences in age between the FORL-1 and FORL-2 sub-groups (P=0.198), but there was a significant difference between the healthy group and each of the two FORL sub-groups (P <0.01). There were no statistically significant differences in sex of the cats between any of the healthy, FORL-1 and FORL-2 groups, and although male cats were predominant in FORL-1 this was not statistically significant (P=0.279).
One cat belonging to FORL-1 sub-group had the highest number of teeth extracted (five) due to FORL. Cats in the FORL-1 sub-group had an average of 3±1.6 teeth affected as compared to cats from the FORL-2 sub-group who had an average of 1.5±0.7 affected teeth.
Principal component analysis of clusters
PCA showed that the FORL-2 sub-group possessed a very similar microbiome composition to the healthy group, with these two groups clustering close together and distinctly from the FORL-1 sub-group (Fig. 5). Following data reduction using PCA there was a highly significant difference in the PERMANOVA (P=0.0001) in the post-test analysis between the following groups of cats: healthy and FORL-1 (P <0.0001), FORL-1 and FORL-2 (P <0.0001). There was no significant difference between healthy and FORL-2 (P=0.238).
Fig. 5.
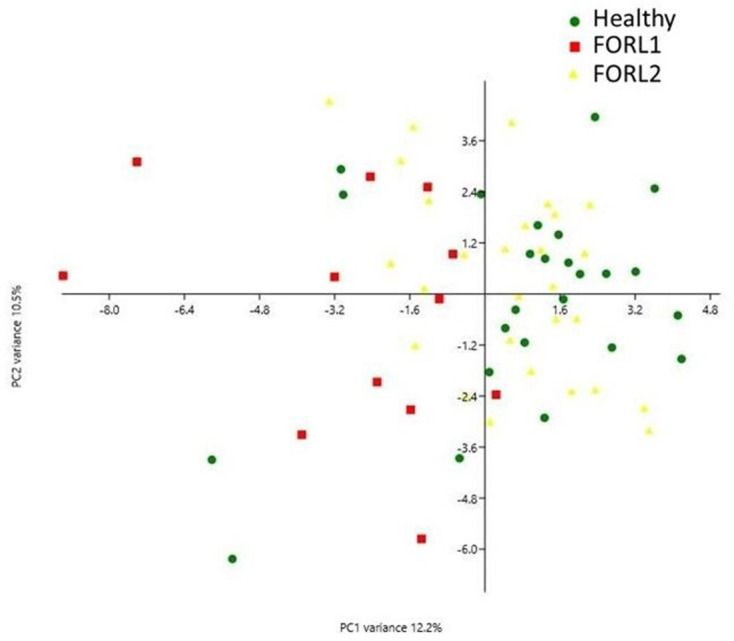
PCA plot of sub-groups identified following two-step cluster analysis. Green dots represent the microbiomes of feline oral health (n=25), red squares represent the microbiomes of the FORL-1 sub-group (n=12) and yellow triangles represent the microbiomes of the FORL-2 sub-group (n=28). Following two-step cluster analysis, the oral health group and FORL-2 sub-group clustered together (but were distinct from the FORL-1 sub-group), indicating similarity in their microbiome composition.
OTU distribution between clusters
Three OTUs were unique to the FORL-1 sub-group in comparison to the FORL-2 sub-group and none of these unique OTUs were identified to the genus level. The FORL-1 sub-group contained 337 OTUs and 401 OTUs were found in the FORL-2 sub-group; there were 70 differences in the OTUs between the FORL-1 and FORL-2 sub-groups, of which 49 were identified to the genus level. Sixty-five OTUs were found only in healthy cats, of which 47 were identified to the genus level (Table 2). Fifteen OTUs were unique to the FORL-1 sub-group in comparison with the healthy group, of which 12 were identified to the genus level (Table 2).
Table 2.
Comparison of genera unique to feline oral health and the FORL-1 sub-group
|
Genera (OTUs) found in feline oral health but not in FORL-1 |
Genera (OTUs) found in FORL-1 but not in feline oral health |
|---|---|
|
Acinetobacter (2)* |
|
|
Clostridiales bacterium (3)* |
|
|
Bergeyella (2)* |
Peptosteptococcus (2)* |
|
|
|
|
|
|
|
|
|
|
|
|
|
Incertae sedis |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Methylobacterium (2)* |
|
|
|
|
|
Moraxella (3)* |
|
|
|
|
|
|
|
|
|
|
|
Prevotella (2)* |
|
|
Pseudomonas (2)* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Uncultured species (3)* |
|
|
Uncultured Roseobacter |
|
|
|
Comparison of the genera (OTUs) identified by analysis of microbiome composition in the most closely related healthy group and FORL-1 sub-group, that are unique to either health or FORL-1.
*Values in brackets indicate the number of different OTUs that were assigned to that genus.
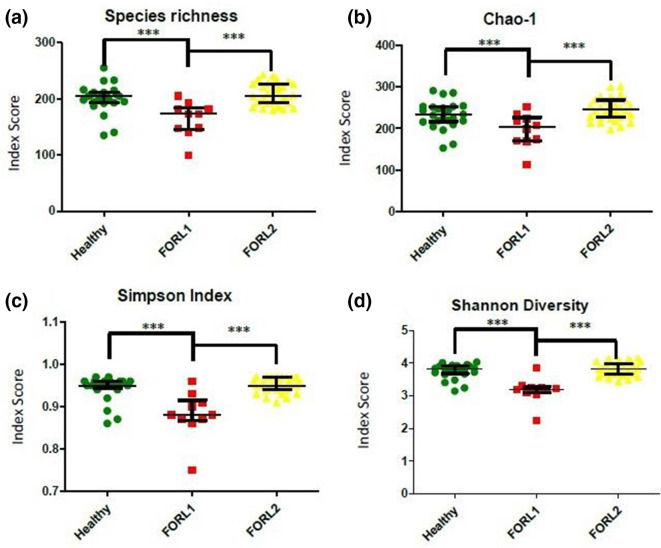
Diversity analyses of clusters
Statistically significant differences (P <0.001) were observed among all three groups (healthy, FORL-1, FORL-2) for each of the four analyses (Fig. 6). The FORL-1 sub-group appeared to differ in several respects, with lower values for microbial species diversity and lower average numbers of OTUs when compared to both the healthy group and the FORL-2 sub-group.
Fig. 6.
Diversity analysis of microbiomes in feline oral health and FORL following two-step cluster analysis. (a) Observed species richness (number of OTUs per sample). (b) Chao-1 index. (c) Shannon diversity index. (d) Simpson index. Samples from feline oral health (n=25) are shown in green, those from FORL-1 sub-group (n=12) are shown in red and those from FORL-2 sub-group (n=28) are shown in yellow. Following two-step cluster analysis, statistically significant differences in species richness and diversity were observed between all three groups ***P <0.001.
Most abundant genera identified in clusters
Following two-step cluster analysis, the six most abundant genera found in both the healthy and the FORL-1 and FORL-2 sub-groups of cats were Bergeyella, Capnocytophaga, Lampropedia, Moraxella, Porphyromonas, and Treponema . These were the same genera identified prior to two-step cluster analysis. Significant differences were observed for Capnocytophaga (P <0.001) and Lampropedia (P <0.01) between the FORL-1 sub-group and the healthy group, and between the FORL-1 and FORL-2 sub-groups.
Discussion
FORL is an extremely prevalent inflammatory oral disease of cats. Despite this, its aetiology remains largely unknown. The current study is the first to investigate the possible involvement of bacteria in the aetiology of FORL, and to also compare the microbiome of FORL with that of orally healthy cats. High throughput 16S rRNA gene sequencing identified 441 OTUs across the healthy and FORL group (353 OTUs in the healthy group, 396 OTUs in the FORL group). No association between FORL and any specific bacterial species was found. Furthermore, species richness and diversity indices and PCA of the microbiome data failed to demonstrate differences between the healthy and FORL groups. Two-step cluster analysis was then applied and successfully identified two sub-groups of FORL (FORL-1 and FORL-2). The FORL-1 sub-group was clearly distinct from the FORL-2 sub-group and healthy group as shown by PCA and species diversity and richness indices. The FORL-2 sub-group possessed microbiome profiles most similar to the healthy group. A limitation of this study is that the severity of FORL in clinical specimens was not graded according to the previously described five clinical stages [3]. It would logically be expected that cases in the FORL-2 sub-group would be less severe, since their microbiome data most closely aligns with the samples within the healthy group, and by the same token one would expect the FORL-1 sub-group to be comprised of more severe cases. Despite this, it is evident that cats in the FORL-1 sub-group had an altered microbiome compared to the FORL-2 sub-group and the healthy group. Since the oral microbiome is polymicrobial in nature, a slight change in this polybiotic synergy could lead to dysbiosis which results in inflammation that may exacerbate FORL.
The healthy feline oral microbiota has recently been characterised in both health and disease. Using high throughput 16S rRNA gene sequencing, the most prevalent genera in the oral microbiota of cats with a healthy oral cavity were identified [27]. These included unclassified Pasteurellaceae (18.7 %), Moraxella (10.9 %), Thermomonas (6.9 %), an unclassified Comamonadaceae (5.6 %), Neisseria (4.9 %), an unclassified Moraxellaceae (4.4 %) and Pasteurella (4.3 %). Of these genera only Moraxella was one of the six most abundant genera identified in the current study.
Differences in the healthy feline microbiome between cats fed wet and dry diets have been reported [28]. Greater bacterial diversity was observed in cats fed a dry diet, and these cats had a higher abundance of Porphyromonas sp. (P <0.01) and Treponema sp. (P <0.01) compared to those fed a wet diet; the most commonly found phyla in both groups were Bacteroidetes (31 %), Firmicutes (24 %) and Proteobacteria (21%). Similarly, the current study identified the most dominant phyla as Bacteroidetes (50 %) and Proteobacteria (33.3 %) (data not shown). Interestingly, one of the genera that Adler et al. [28] showed to be significantly different between the wet and dry diets was Lampropedia , which was found at higher levels in cats fed a wet diet. However, in the current study all cats were fed a carefully controlled and monitored mixed wet and dry diet, therefore the type of diet is highly unlikely to be a confounding factor that could have affected oral microbiome composition. The current study identified Lampropedia in both the healthy and FORL groups. Adler et al. [28] also found Porphyromonas sp. (P <0.01) and Treponema sp. (P <0.01) at significantly different prevalence between the groups, but the present study found these two species in both the healthy and FORL groups without any significant differences in prevalence between them.
Harris et al. [29] investigated the microbiota associated with feline oral health, gingivitis and mild periodontitis. The most abundant genera in healthy gingivae were Porphyromonas , Moraxella and Fusobacteria , whereas the family Peptostreptococcaceae were most abundant in gingivitis and mild periodontitis. PCA demonstrated distinct differences between the microbiomes of health and gingivitis, and an overlap between the healthy and mild periodontitis microbiomes [29]. Similar findings were evident in the current study, where the microbiomes of health and the FORL-1 sub-group were distinct whereas an overlap was observed between the healthy group and the FORL-2 sub-group. Most of the bacteria identified in the healthy group and FORL-2 sub-group were similar to those found by Harris et al. [29]. In the current study Capnocytophaga was one of the most abundant genera found in both health and FORL but was found at a significantly lower level in the FORL-1 sub-group (P <0.001) when compared to healthy and FORL-2 sub-group. However, Harris et al. [29] found this genus to be significantly more abundant in health than in either gingivitis or mild periodontitis, but no significant difference was observed between the latter two groups. Further differences were evident in the abundance of the genera Morexella, Bergeyella and Treponema between the two studies. Whilst Harris et al. [29] noted that Moraxella and Bergeyella zoohelcum were more abundant in health than disease and Treponema was associated with mild periodontitis, the current study demonstrated that Morexella, Bergeyella and Treponema were some of the most abundant genera in health and disease without any significant statistical differences between the cohorts.
The bacteria associated with feline chronic gingivostomatitis (FCGS), another important feline oral disease, has been investigated [18]. The genus Capnocytophaga was predominant in health and Pastuerella multocida subsp. multocida was most strongly associated with FCGS, as determined by 16S rRNA gene sequencing. Culture-dependent methods confirmed Pastuerella multocida subsp. multocida as the predominant species in FCGS, whereas Pasteurella pneumotropica was most prevalent in oral health.
A provisional curated taxonomy and 16S rRNA gene reference sequence bank for the feline oral microbiome has been established. Representing 171 feline oral taxa, this resource will be an important tool and reference database to further our understanding of feline bacterial taxa associated with oral health and disease [30].
No specific bacterial genera or species were found to be associated with either health or disease in the current study. The six most abundant genera identified in the current study were Lampropedia (54.3 %), Capnocytophaga (47.2 %), Treponema (31.1 %), Bergeyella (30.8 %), Moraxella (29.3 %) and Porphyromonas (25.5 %), and are regarded as being part of the normal feline oral microbiota [18, 27, 29, 30]. Capnocytophaga and Lampropedia were found at significantly lower levels in the FORL-1 sub-group compared to the FORL-2 sub-group and healthy group. Bergeyella and Porphyromonas were also found to be amongst the most abundant genera isolated and identified by culture-dependent methods in the same sample set used in the current study (unpublished data).
While it was not possible to associate specific bacteria with FORL in the current study, an altered microbiota was identified in a sub-group of cats with the disease. Since clear differences in the microbiomes of the healthy and FORL groups were not observed, a two-step cluster analysis was performed. This analysis identified two sub-groups of FORL, namely FORL-1 and FORL-2. The FORL-2 sub-group was very similar in microbiome composition to the healthy group whilst the FORL-1 sub-group microbiomes were distinct from those found in both the healthy group and FORL-2 sub-group. This may reflect the severity of disease, with cats in the FORL-1 sub-group having more advanced disease compared to those in the FORL-2 sub-group. Unfortunately, radiographic data was only available for 18 of the 40 cats with FORL. Despite this, it was still possible to demonstrate that cats in the FORL-1 sub-group had a greater number of affected teeth (3±1.6) as compared to cats from the FORL-2 sub-group (1.5±0.7). The limited clinical data regarding FORL-affected tooth numbers appears to support this view, because the number of teeth affected could be an indication of disease severity in the cats belonging to the FORL-1 sub-group as compared to the cats belonging to the FORL-2 sub-group. However, since only limited radiographic data for cats with FORL were available due to resource constraints, further studies are required to confirm this observation.
Although there are no reports of Lampropedia being associated with FORL, they generally exist as part of the normal feline oral microbiota [28]. Certain species of Lampropedia are associated with phosphate accumulation [31] and are found in phosphate rich environments [32]. In a study investigating the microbiome in cats with nasal neoplasia, Lampropedia was identified in the nasal cavity but this did not indicate any association with the disease [33]. The current study appears to be the first to associate Lampropedia with a feline disease. Significantly lower mean serum Ca/PO4 ratio levels have been demonstrated in cats with FORL when compared to cats without FORL [34]. It is unknown whether Lampropedia has any role in the lowered Ca/PO4 ratio noted in FORL. It is possible that changes in the microenvironment of the oral cavity may alter the levels of Lampropedia species and may possibly influence the oral calcium / phosphate ratio, which in turn may cause bone resorption. The Ca/PO4 ratio was not estimated in the current study. Since not all species of Lampropedia can accumulate phosphate, further studies are required to identify specific Lampropedia species in the feline oral cavity and their association with phosphate metabolism, as well as a possible role in FORL.
In conclusion, this study suggests that the feline oral microbiota exists in complex polymicrobial communities and slight changes in this polymicrobial environment may lead to enhanced communal virulence and inflammation, which may result in the development of FORL in susceptible cats.
Funding information
This study was funded by Nestlé Purina PetCare.
Author contributions
M. P. R., C. J. N. and D. B., conceived and designed the experiments; J. S., was responsible for sample collection; S. T., performed the experiments; M. R., S. T., D. F. L., C. J. N. and B. B., were responsible for data analysis; M. P. R. and S. T., were responsible for preparing the manuscript.
Conflicts of interest
JS is an employee of Nestlé Purina PetCare. The authors declare that there are no other conflicts of interest.
Ethical statement
The study was conducted in strict accordance with guidelines established by the Nestlé Purina PetCare (NPPC) Advisory Committee for Ethics, who approved the work.
Footnotes
Abbreviations: FCGS, feline chronic gingivostomatitis; FORL, feline odontoclastic resorptive lesion; LDA, linear discriminant analysis; LEfSe, linear discriminant analysis effect size; OTU, operational taxonomic unit; PAST, PAleontological Statistics; PCA, principal component analysis.
References
- 1.Lyon KF. Subgingival odontoclastic resorptive lesions. classification, treatment, and results in 58 cats. Vet Clin N Am Small Anim Pract. 1992;22:1417–1432. doi: 10.1016/s0195-5616(92)50135-8. [DOI] [PubMed] [Google Scholar]
- 2.Reiter AM, Mendoza KA. Feline odontoclastic resorptive lesions an unsolved enigma in veterinary dentistry. Vet Clin N Am Small Anim Pract. 2002;32:791–837. doi: 10.1016/s0195-5616(02)00027-x. [DOI] [PubMed] [Google Scholar]
- 3.Mestrinho LA, Runhau J, Bragança M, Niza MM. Risk assessment of feline tooth resorption: a Portuguese clinical case control study. J Vet Dent. 2013;30:78–83. doi: 10.1177/089875641303000202. [DOI] [PubMed] [Google Scholar]
- 4.Girard N, Servet E, Biourge V, Hennet P. Feline tooth resorption in a colony of 109 cats. J Vet Dent. 2008;25:166–174. doi: 10.1177/089875640802500302. [DOI] [PubMed] [Google Scholar]
- 5.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Kanagasingam S, Pitt Ford T. External cervical resorption: a review. J Endod. 2009;35:616–625. doi: 10.1016/j.joen.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 7.DuPont GA. Radiographic evaluation and treatment of feline dental resorptive lesions. Vet Clin North Am Small Anim Pract. 2005;35:943–962. doi: 10.1016/j.cvsm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Gorrel C. Tooth resorption in cats: pathophysiology and treatment options. J Feline Med Surg. 2015;17:37–43. doi: 10.1177/1098612X14560098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booij-Vrieling HE. PhD thesis. Utrecht University, The Netherlands; Tooth resorption in cats: contribution of vitamin D and inflammation. [Google Scholar]
- 10.Girard N, Servet E, Hennet P, Biourge V. Tooth resorption and vitamin D3 status in cats fed premium dry diets. J Vet Dent. 2010;27:142–147. doi: 10.1177/089875641002700301. [DOI] [PubMed] [Google Scholar]
- 11.Reiter AM, Lewis JR, Okuda A. Update on the etiology of tooth resorption in domestic cats. Vet Clin North Am Small Anim Pract. 2005;35:913–942. doi: 10.1016/j.cvsm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Zupan J, Jeras M, Marc J. Osteoimmunology and the influence of pro-inflammatory cytokines on osteoclasts. Biochem Med. 2013;23:43–63. doi: 10.11613/BM.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLaurier A, Allen S, deFlandre C, Horton MA, Price JS. Cytokine expression in feline osteoclastic resorptive lesions. J Comp Pathol. 2002;127:169–177. doi: 10.1053/jcpa.2002.0577. [DOI] [PubMed] [Google Scholar]
- 14.Booij-Vrieling HE, Tryfonidou MA, Riemers FM, Penning LC, Hazewinkel HAW. Inflammatory cytokines and the nuclear vitamin D receptor are implicated in the pathophysiology of dental resorptive lesions in cats. Vet Immunol Immunopathol. 2009;132:160–166. doi: 10.1016/j.vetimm.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallonee DH, Harvey CE, Venner M, Hammond BF. Bacteriology of periodontal disease in the cat. Arch Oral Biol. 1988;33:677–683. doi: 10.1016/0003-9969(88)90123-9. [DOI] [PubMed] [Google Scholar]
- 16.Davis IJ, Wallis C, Deusch O, Colyer A, Milella L, et al. A cross-sectional survey of bacterial species in plaque from client owned dogs with healthy gingiva, gingivitis or mild periodontitis. PLoS One. 2013;8:e83158. doi: 10.1371/journal.pone.0083158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy R, Lappin DF, Dixon PM, Buijs MJ, Zaura E, et al. The microbiome associated with equine periodontitis and oral health. Vet Res. 2016;47:49. doi: 10.1186/s13567-016-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolieslager SMJ, Riggio MP, Lennon A, Lappin DF, Johnston N, et al. Identification of bacteria associated with feline chronic gingivostomatitis using culture-dependent and culture-independent methods. Vet Microbiol. 2011;148:93–98. doi: 10.1016/j.vetmic.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 22.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, et al. The Silva ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer Ø, Harper DAT, Ryan PD. PAST: PAleontological STatistics software package for education and data analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- 26.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturgeon A, Pinder SL, Costa MC, Weese JS. Characterization of the oral microbiota of healthy cats using next-generation sequencing. Vet J. 2014;201:223–229. doi: 10.1016/j.tvjl.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Adler CJ, Malik R, Browne GV, Norris JM. Diet may influence the oral microbiome composition in cats. Microbiome. 2016;4:23. doi: 10.1186/s40168-016-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris S, Croft J, O'Flynn C, Deusch O, Colyer A, et al. A pyrosequencing investigation of differences in the feline subgingival microbiota in health, gingivitis and mild periodontitis. PLoS One. 2015;10:e0136986. doi: 10.1371/journal.pone.0136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewhirst FE, Klein EA, Bennett ML, Croft JM, Harris SJ, et al. The feline oral microbiome: a provisional 16S rRNA gene based taxonomy with full-length reference sequences. Vet Microbiol. 2015;175:294–303. doi: 10.1016/j.vetmic.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stante L, Cellamare CM, Malaspina F, Bortone G, Tilche A. Biological phosphorus removal by pure culture of Lampropedia spp. Water Res. 1997;31:1317–1324. doi: 10.1016/S0043-1354(96)00351-X. [DOI] [Google Scholar]
- 32.Tripathi C, Mahato NK, Singh AK, Kamra K, Korpole S, et al. Lampropedia cohaerens sp. nov., a biofilm-forming bacterium isolated from microbial mats of a hot water spring, and emended description of the genus Lampropedia . Int J Syst Evol Microbiol. 2016;66:1156–1162. doi: 10.1099/ijsem.0.000853. [DOI] [PubMed] [Google Scholar]
- 33.Dorn ES, Tress DB, Suchodolski JS, Nisar T, Ravindran P, et al. Bacterial microbiome in the nose of healthy cats and in cats with nasal disease. PLoS One. 2017;12:e0180299. doi: 10.1371/journal.pone.0180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiter AM, Lyon KF, Nachreiner RF, Shofer FS. Evaluation of calciotropic hormones in cats with odontoclastic resorptive lesions. Am J Vet Res. 2005;66:1446–1452. doi: 10.2460/ajvr.2005.66.1446. [DOI] [PubMed] [Google Scholar]