Abstract
The genera Catabacter (family ‘Catabacteraceae’) and Christensenella (family Christensenellaceae ) are close relatives within the phylum Firmicutes . Members of these genera are strictly anaerobic, non-spore-forming and short straight rods with diverse phenotypes. Phylogenetic analysis of 16S rRNA genes suggest that Catabacter splits Christensenella into a polyphyletic clade. In an effort to ensure that family/genus names represent monophyletic clades, we performed a whole-genome based analysis of the genomes available for the cultured representatives of these genera: four species of Christensenella and two strains of Catabacter hongkongensis . A concatenated alignment of 135 shared protein sequences of single-copy core genes present in the included strains indicates that C. hongkongensis is indeed nested within the Christensenella clade. Based on their evolutionary relationship, we propose the transfer of Catabacter hongkongensis to the genus Christensenella as Christensenella hongkongensis comb. nov.
Keywords: whole genome phylogeny, reclassification, Christensenella, Catabacter
Introduction
Catabacter hongkongensis was first isolated in 2007 from the blood cultures of four patients in Hong Kong and Canada. Based on the phylogenetic positioning of 16S rRNA gene sequences and phenotypic characteristics, it was proposed as a new genus and new family, ‘Catabacteraceae’ [1]. The genus Catabacter comprises just one species, with the type strain Catabacter hongkongensis HKU16T. Based on 16S rRNA gene sequencing surveys, C. hongkongensis has been detected in the blood of patients with diseases such as intestinal obstruction, gastrointestinal malignancy, acute cholecystitis and hypertension in Europe, North America and Asia [1–5]. Although Catabacter hongkongensis was first identified in 2007, the name Catabacter hongkongensis was validly published in 2014 [6].
In 2012, Morotomi and colleagues isolated a novel bacterium from the stool of a healthy male adult. Based on 16S rRNA gene sequence analysis and physiological data, they named it Christensenella minuta DSM 22607T within the novel family Christensenellaceae [7]. In addition to Christensenella minuta DSM 22607T, three other species have been proposed, based on additional isolates from human faeces: ‘Christensenella massiliensis’ Marseille-P2438 [8], ‘Christensenella timonensis’ Marseille-P2437 [9] and ‘ Christensenella intestinihominis ’ AF73-05CM02PP [10]. ‘ Christensenella intestinihominis ’ AF73-05CM02PP is proposed in a pending patent.
16S rRNA gene sequence identity (%ID) has been used to delineate genus (95 %ID) and species (98.7 %ID) cutoffs [11, 12]. The 16S rRNA gene sequence of C. hongkongensis HKU16T has 96–97 %ID with the 16S rRNA genes of the four species of Christensenella , which places them in the range of sharing a genus using that criterion. In addition to sequence similarity, the 16S rRNA gene-based phylogenetic relationships of these taxa indicate they form a monophyletic clade [13].
Whole genome-based analysis with concatenated protein sequences has recently been proposed as a basis for determining the phylogenetic relationships of members of the Bacteria and Archaea [14]. Based on whole genome comparisons, Catabacter and Christensenella were annotated as belonging to the family Christensenellaceae in the order Christensenellales in the Genome Taxonomy Database (GTDB; R05-RS95 17 July 2020) [15]. Twenty-one genomes within the family Christensenellaceae are included in the GTDB R05-RS95 as of 1 August 2020. These include metagenome-assembled genomes and genomes derived from isolates. A formal reclassification of Catabacter as Christensenella would clarify the nomenclature of this taxon.
Here, we used comparative genomics as a basis for proposing the transfer of Catabacter hongkongensis to the genus of Christensenella . Genome sequences of six cultured isolates belonging to the families ‘Catabacteraceae’ and Christensenellaceae and four species from sister clades in the GTDB were selected for phylogenomic analysis. The average nucleotide identity (ANI) of the six genomes was compared, and a phylogeny based on 16S rRNA gene sequences was reconstructed. Based on the resulting phylogeny, we recommend that Catabacter hongkongensis be renamed Christensenella hongkongensis comb. nov.
Methods
Phylogeny based on whole genomes and 16S rRNA gene sequences
We based this analysis on whole genome sequences of six cultured isolates: Catabacter hongkongensis strains HKU16T and ABBA15k, Christensenella minuta DSM 22607T, ‘Christensenella massiliensis’ Marseille-P2438, ‘Christensenella timonensis’ Marseille-P2437 and ‘ Christensenella intestinihominis ’ AF73-05CM02PP. General information about the genomes in this study is listed in Table 1. For the outgroup, we selected the the following species: Clostridium novyi NT (GenBank accession number: GCA_000014125.1), Clostridium butyricum DSM 10702T (GCA_000409755.1), Clostridium thermobutyricum DSM4928T (GCA_002050515.1) and Eubacterium limosum ATCC 8486T (GCA_000807675.2). Whole genome sequences were obtained from NCBI.
Table 1.
Phenotypic characteristics of the strains of Catabacter and Christensenella based on literature review
Data for the strains are from references [1, 7–10, 31]. +, Positive; −, negative; nd, not determined. The G+C contents and N50, contig numbers, genome size and genome coverages were retrieved from the GTDB records of the strains
|
Characteristics |
DSM 22607T |
‘ Christensenella intestinihominis ’ AF73-05CM02PP |
‘Christensenella massiliensis’ Marseille-P2438 |
‘Christensenella timonensis’ Marseille-P2437 |
||
|---|---|---|---|---|---|---|
|
HKU16T |
ABBA15k |
|||||
|
Gram stain |
−/+ |
+ |
− |
− |
+ |
+ |
|
Motility |
− |
− |
− |
− |
+ |
− |
|
Catalase activity |
− |
− |
− |
− |
+ |
nd |
|
Metabolite utilization |
Arabinose, glucose, mannose, rhamnose, salicin, xylose |
Arabinose, Glucose, mannose, rhamnose, xylose, mannitol, maltose, sulphate, pine syrup, raffinose, sorbitol |
nd |
nd |
Arabinose, glucose, mannose xylose |
nd |
|
G+C content (mol%) |
51.48 |
52.07 |
50.38 |
51.71 |
48.53 |
48.79 |
|
Contig number |
45 |
36 |
1 |
2 |
134 |
113 |
|
Protein count |
2776 |
2791 |
2437 |
2430 |
3071 |
2625 |
|
Completeness (contamination) (%) |
98.39 (0.81) |
99.19 (0.81) |
98.79 (0.81) |
97.98 (0.81) |
97.55 (2.97) |
97.9 (3.5) |
|
Genome size (bp) |
2 940 227 |
3 026 655 |
2 560 186 |
2 650 850 |
3 203 641 |
2 797 114 |
|
GenBank assembly accession |
GCA_001678855.1 |
GCA_001678845.1 |
GCA_900155415.1 |
GCA_900087015.1 |
GCA_000981035.1 |
GCA_001507385.1 |
We used Anvi’o version 5.2.0 for reconstructing the whole-genome phylogenomic tree [16]. Briefly, contig databases were created from the genome fasta files. Prodigal version 2.6.3 with default settings [17] was used to identify open reading frames in contigs. Hidden Markov model (HMM) profiles were used to extract the set of single-copy marker genes defined by Campbell et al. [18] . The best HMM hit was selected if a gene was found with multiple copies in a genome. We limited the set of single-copy core genes shared to those present in all analysed genomes and aligned the concatenated protein sequences using muscle [19]. FastTree 2 [20] was used for reconstructing an approximately maximum-likelihood phylogenomic tree with the Jones–Taylor–Thornton model [21]. SH-like local support values [22] are shown on the nodes. 16S rRNA gene sequences were retrieved from NCBI and aligned using mafft. The tree was reconstructed using the maximum-likelihood method by RAxML [23] with a general time reversible model of evolution. The phylogenetic tree was visualized using the online tool iTOL [24].
Average nucleotide identity and phenotype predictions
We used FastANI with default settings [25] to generate a pairwise ANI comparison of the six Christensenella and Catabacter genomes. A heatmap of ANI values was generated and visualized in R [26] with the package ggplot2 [27]. Traitar [28] trait analyzer was used for phenotypic trait prediction based on genome sequences. ABRicate version 1.0.1 (https://github.com/tseemann/ABRicate) was used for the detection of genes involved in antimicrobial resistance (AMR), and the annotation was derived from the default NCBI database AMRFinderPlus.
Results and discussion
The genome sizes of the six Catabacter and Christensenella species/strains range from 2.5 Mbp to 3.3 Mbp and the G+C content of genomic DNA from 48.53 to 52.07 mol%. Based on the pairwise comparison of the six genomes in the families ‘Catabacteraceae’ and Christensenellaceae , we observed that the ANI values of the two Catabacter hongkongensis strains (HKU16T and ABBA15k) were >98.97 % (Fig. 1), confirming that the two strains belong to the same species. Moreover, the ANI values for the six genomes were between 77.56–83.48 %, which corresponds to the accepted ANI cut-off 95–96 % used to designate the same species [29, 30] and <83 % for inter-species ANI values [25]. ‘ Christensenella intestinihominis ’ AF73-05CM02PP and C. minuta DSM 22607T showed the highest ANI similarity values (83.48 %) between different species.
Fig. 1.
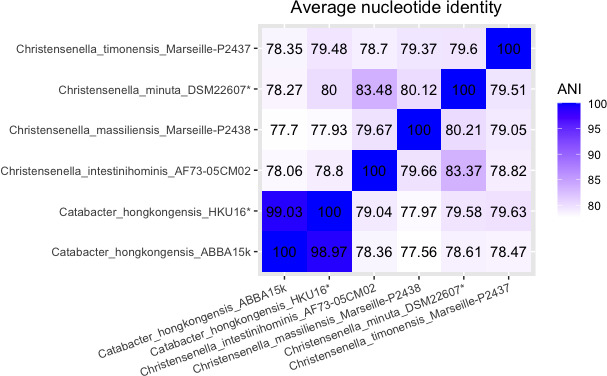
Heatmap of ANI values amongst the genomes of Catabacter hongkongensis strains and Christensenella species in this study. Type strains are marked with an asterisk.
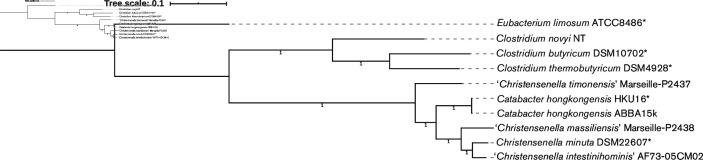
The16S rRNA gene phylogeny shows Catabacter is nested within the Christensenella clade with 100 % bootstrap support (Fig. 2). The two strains of Catabacter ( C. hongkongensis HKU16T and ABBA15k) have identical 16S rRNA gene sequences. The 16S rRNA gene sequence identities between Catabacter hongkongensis and Christensenella species were between 96–97 %. Both 16S rRNA gene sequence similarity and 16S rRNA gene-based phylogenetic relationships of these taxa support that Catabacter and Christensenella belong to the same genus.
Fig. 2.
Phylogenetic tree showing the relationship of Catabacter hongkongensis to Christensenella species based on 16S rRNA gene sequence analysis. GenBank accession numbers are provided in parentheses. The gene ID number of JGI IMG Integrated Microbial Genomes and Microbiomes is provided for the16S rRNA sequence of Catabacter honkongensis ABBA15K. Type strains are marked with an asterisk. Bootstrap values are expressed as a percentage for 100 iterations. Clostridium and Eubacterium are used for the outgroup. The tree is rooted by Eubacterium limosum . Scale bar indicates 0.1 nucleotide substitutions per site.
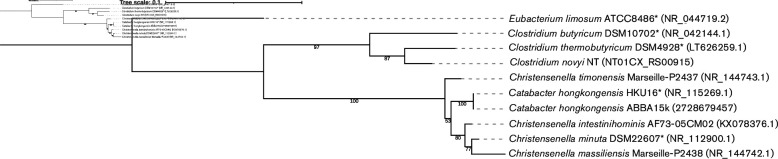
We identified 135 protein-encoding single-copy core genes present in the genomes of Christensenella , Catabacter and the outgroup taxa. We used these 135 genes in a concatenated alignment resulting in a total of 51 813 aligned amino acid sites. In the resulting phylogenetic tree (Fig. 3), the Catabacter and Christensenella species and strains formed a monophyletic clade with high bootstrap support, indicating a shared common ancestor. The species ‘C. timonensis’ Marseille-P2437 is basal and forms a sister clade to the rest of the taxa in the phylogeny. The two strains of Catabacter hongkongensis (HKU16T and ABBA15k) are, as expected based on their high ANI, on the same branch of the phylogeny. The Catabacter branch is a sister taxon to the remaining Christensenella species ( C. minuta DSM 22607T, ‘C. massiliensis’ Marseille-P2438, ‘ C. intestinihominis ’ AF73-05CM02PP).
Fig. 3.
Phylogeny tree reconstructed by the approximately maximum-likelihood method showing the position of Catabacter relative to Christensenella based on 135 concatenated. core protein sequences with 51 813 aligned amino acid sites. All the nodes are strongly supported with SH-like support values of 1. Type strains are marked with asterisk. Clostridium and Eubacterium are used for the outgroup. The tree is rooted by Eubacterium limosum ATCC 8486. Scale bar indicates 0.1 amino acid substitutions per site.
The position of Catabacter (and its family 'Catabacteraceae'), nested within the Christensenella clade, splits the Christensenellaceae family and genus, such that neither are monophyletic. For the family and genus names to represent monophyletic groups, the renaming of Catabacter hongkongensis to Christensenella hongkongensis would be required. As a consequence, the genus name Catabacter should be reclassified as Christensenella .
The cultured strains of the species of Catabacter ( C. hongkongensis HKU16T and ABBA15k) and Christensenella ( C. minuta DSM 22607T, ‘C. massiliensis’ Marseille-P2438, ‘C. timonensis’ Marseille-P2437 and ‘C. intestinihominis’ AF73-05CM02PP) have been shown to be strictly anaerobic and non-spore-forming rods with varied motility, Gram stain reaction and the catalase reaction [1, 7–10]. The different phenotypic characteristics of the species compared in this study are summarized in Table 1. Catabacter hongkongensis HKU16T and ABBA15k strains are reported to be Gram-positive, while the four species of Christensenella are reported as either Gram-positive or Gram-negative. Morotomi and colleagues reported that C. minuta DSM 22607T is Gram-negative [7], while Alonso and colleagues reported C. minuta stains consistently as Gram-positive [31]. Based on our Gram staining, C. minuta cell membranes also stained as Gram-positive, which is consistent with the observation of Alonso and colleagues. Moreover, the phenotype predictions obtained from Traitar indicate these taxa should stain Gram-positive. The Gram-variable reaction might be due to the age of the culture for staining [32].
C. hongkongensis strains (HKU16T, HKU17, CA1, CA2) and most clinical-derived isolates are reported to be motile and resistant to cefotaxime [1, 2, 5, 33] except for C. hongkongensis ABBA15k, which was isolated in 2016 from the blood of a patient with a fever in Sweden [34]. Strain ABBA15k showed 100 % pairwise 16S rRNA gene identity with Catabacter hongkongensis HKU16T. However, the genome of C. hongkongensis ABBA15k is smaller than C. hongkongensis HKU16T, and the genes coding for chemotaxin (cheA) and flagellar assembly (flhA and MotA) were not present in the genome of C. hongkongensis ABBA15k [34]. The tetracycline resistance gene tet was detected in the genome of C. hongkongensis HKU16T, but no resistance genes were detected in the genome of C. hongkongensis ABBA15k [34].
Screening for AMR genes of the genomes with ABRicate in this study showed that the tet gene was also present in the genomes of Christensenella minuta DSM 22607T, ‘Christensenella massiliensis’ Marseille-P2438, ‘Christensenella timonensis’ Marseille-P2437 and Catabacter hongkongensis HKU16T but not in ‘ Christensenella intestinihominis ’ AF73-05CM02PP and Catabacter hongkongensis ABBA15k. A streptomycin resistance gene (aadE) was also detected in the genome of ‘Christensenella massiliensis’ Marseille-P2438. Detailed information about AMR genes is listed in Table 2. ‘ Christensenella intestinihominis ’ AF73-05CM02PP and Catabacter hongkongensis HKU16T were predicted to be motile by Traitar. However, ‘ Christensenella intestinihominis ’ AF73-05CM02PP was classified as non-motile in the original phenotypic characterization [10], which might be attributable to the growth conditions used. It is also possible that the genome of the strain may not contain all genes required for flagellar formation.
Table 2.
Antimicrobial resistance (AMR) genes detected for the genomes of Catabacter hongkongensis strains and Christensenella species
Coverage refers to the proportion of the gene in the reference gene sequence.
|
Strain |
Contig (position strand) |
Reference gene (accession) |
Coverage |
Identity (%) |
Gene product |
Resistance |
|---|---|---|---|---|---|---|
|
‘Christensenella timonensis’ Marseille-P2437 |
FLKP01000002.1 (1477797–1479716 +) |
tet(W) (NG_048299.1) |
1-1920/1920 |
99.53 |
Tetracycline resistance ribosomal protection protein Tet(W) |
Tetracycline |
|
FLKP01000002.1 (1480702–1481922 +) |
tet(40) (NG_048141.1) |
1-1221/1221 |
99.67 |
Tetracycline efflux MFS transporter Tet(40) |
Tetracycline |
|
|
‘Christensenella massiliensis’ Marseille-P2438 |
(142755–144674 −) |
tet(W) |
1-1920/1920 |
100 |
Tetracycline resistance ribosomal protection protein Tet(W) |
Tetracycline |
|
(1980989–1981855 +) |
aadE (NG_047378.1) |
1-867/867 |
99.77 |
Aminoglycoside 6-adenylyltransferase AadE |
Streptomycin |
|
|
Catabacter hongkongensis HKU16T |
(37275–39194 +) |
tet(32) (NG_048125.1) |
1-1920/1920 |
100 |
Tetracycline resistance ribosomal protection protein Tet(32) |
Tetracycline |
|
DSM 22607T |
(54376–56295 +) |
tet(W) (NG_048281.1) |
1-1920/1920 |
100 |
Tetracycline resistance ribosomal protection protein Tet(W) |
Tetracycline |
|
Catabacter hongkongensis ABBA15k |
No AMR genes have been detected in the genome |
|||||
|
‘ Christensenella intestinihominis ’ AF73-05CM02PP |
No AMR genes have been detected in the genome |
|||||
In conclusion, both Catabacter and Christensenella include species and strains that are strictly anaerobic, non-spore forming, short straight rods and have diverse phenotypes regarding motility, Gram-staining and antibiotic resistance. The name Christensenella was validly published earlier than Catabacter . Only one species exists within the genus of Catabacter , while four species have been proposed for the genus Christensenella and the family Christensenellaceae . Based on our 16S rRNA gene sequences phylogeny and the genome-based phylogenomic analysis, we propose that transfer of Catabacter hongkongensis to the genus Christensenella and the species Catabacter hongkongensis be renamed Christensenella hongkongensis comb. nov.
Description of Christensenella hongkongensis comb. nov.
Christensenella hongkongensis (hong.kong.en’sis. N.L. fem. adj. hongkongensis pertaining to Hong Kong, SAR, PR China).
Basonym: Catabacter hongkongensis Lau et al. 2014.
The description of Christensenella hongkongensis is identical to that proposed for Catabacter hongkongensis [1].
The type strain is HKU16T (=DSM 18959T=JCM 17853T=CCUG 54229T).
Funding information
This work was supported by the Max Planck Society.
Acknowledgements
This research was supported by the Max Planck Society.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: AMR, antimicrobial resistance; ANI, average nucleotide identity; GTDB, Genome Taxonomy Database; HMM, Hidden Markov model.
References
- 1.Lau SKP, McNabb A, Woo GKS, Hoang L, Fung AMY, et al. Catabacter hongkongensis gen. nov., sp. nov., isolated from blood cultures of patients from Hong Kong and Canada. J Clin Microbiol. 2007;45:395–401. doi: 10.1128/JCM.01831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau SKP, Fan RYY, Lo H-W, Ng RHY, Wong SSY, et al. High mortality associated with Catabacter hongkongensis bacteremia. J Clin Microbiol. 2012;50:2239–2243. doi: 10.1128/JCM.00128-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsendoorn A, Robert R, Culos A, Roblot F, Burucoa C. Catabacter hongkongensis Bacteremia with fatal septic shock. Emerg Infect Dis. 2011;17:1330–1331. doi: 10.3201/eid1707.101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torri A, Delbianco F, Baccarini FD, Fusari M, Bertini S, et al. First report of sepsis due to Catabacter hongkongensis in an Italian patient. New Microbes New Infect. 2016;9:54–55. doi: 10.1016/j.nmni.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YJ, Won EJ, Kim SH, Shin MG, Shin JH, et al. First case report of bacteremia due to Catabacter hongkongensis in a Korean patient. Ann Lab Med. 2017;37:84–87. doi: 10.3343/alm.2017.37.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oren A, Garrity GM. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol. 2014;64:2927–2929. doi: 10.1099/ijs.0.068759-0. [DOI] [PubMed] [Google Scholar]
- 7.Morotomi M, Nagai F, Watanabe Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol. 2012;62:144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 8.Ndongo S, Khelaifia S, Fournier P-E, Raoult D. Christensenella massiliensis, a new bacterial species isolated from the human gut. New Microbes New Infect. 2016;12:69–70. doi: 10.1016/j.nmni.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndongo S, Dubourg G, Khelaifia S, Fournier P-E, Raoult D. Christensenella timonensis, a new bacterial species isolated from the human gut. New Microbes New Infect. 2016;13:32–33. doi: 10.1016/j.nmni.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou Y, Xue W, Lv M, Xiao L, Li X. Christensenella intestinihominis and application thereof. US20190282633A1. 2019 Sep 19
- 11.Stackebrandt E, Goebel BM. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Evol Microbiol. 1994;44:846–849. doi: 10.1099/00207713-44-4-846. [DOI] [Google Scholar]
- 12.Stackebrandt E, Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol Today. 2006;33:152–155. [Google Scholar]
- 13.Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019;17:83. doi: 10.1186/s12915-019-0699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang JM, Darling AE, Eisen JA. Phylogeny of bacterial and archaeal genomes using conserved genes: supertrees and supermatrices. PLoS One. 2013;8:e62510. doi: 10.1371/journal.pone.0062510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parks DH, Chuvochina M, Chaumeil P-A, Rinke C, Mussig AJ. A complete domain-to-species taxonomy for bacteria and archaea. Nature Biotechnology. 2020 doi: 10.1038/s41587-020-0501-8. [DOI] [PubMed] [Google Scholar]
- 16.Eren AM, Esen Özcan C, Quince C, Vineis JH, Morrison HG, et al. Anvi'o: an advanced analysis and visualization platform for 'omics data. PeerJ. 2015;3:e1319. doi: 10.7717/peerj.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JH, O'Donoghue P, Campbell AG, Schwientek P, Sczyrba A, et al. UGA is an additional glycine codon in uncultured SR1 bacteria from the human microbiota. Proc Natl Acad Sci U S A. 2013;110:5540–5545. doi: 10.1073/pnas.1303090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 22.Guindon S, Delsuc F, Dufayard J-F, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. In: Posada D, editor. Bioinformatics for DNA Sequence Analysis. Totowa, NJ: Humana Press; 2009. pp. 113–137. editor. [DOI] [PubMed] [Google Scholar]
- 23.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letunic I, Bork P. Interactive tree of life (iTOL) V3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 27.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer Publishing Company, Incorporated; 2009. [Google Scholar]
- 28.Weimann A, Mooren K, Frank J, Pope PB, Bremges A. From genomes to phenotypes: Traitar. the Microbial Trait Analyzer. mSystems. 2016;1 doi: 10.1128/mSystems.00101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richter M, Rosselló-Móra R. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A. 2009;106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- 31.Alonso BL, Irigoyen von Sierakowski A, Sáez Nieto JA, Rosel AB. First report of human infection by Christensenella minuta, a gram-negative, strickly anaerobic rod that inhabits the human intestine. Anaerobe. 2017;44:124–125. doi: 10.1016/j.anaerobe.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Rand KH, Tillan M. Errors in interpretation of Gram stains from positive blood cultures. Am J Clin Pathol. 2006;126:686–690. doi: 10.1309/V4KE2FPM5T8V4552. [DOI] [PubMed] [Google Scholar]
- 33.Ryu J, Kim Y, Lee J, Cho SY, Park TS, et al. A Case of Catabacter hongkongensis and Alistipes indistinctus Isolated from Blood Cultures of a Patient with Acute Appendicitis. Lab Med Online. 2019;9:177–180. doi: 10.3343/lmo.2019.9.3.177. [DOI] [Google Scholar]
- 34.Kaden R, Thelander M, Engstrand L, Herrmann B. First case of human bacteraemia by Catabacter hongkongensis in Scandinavia. New Microbes New Infect. 2017;15:6–8. doi: 10.1016/j.nmni.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]