Abstract
Micro-organisms contribute to Earth’s mineral deposits through a process known as bacteria-induced mineral precipitation (BIMP). It is a complex phenomenon that can occur as a result of a variety of physiological activities that influence the supersaturation state and nucleation catalysis of mineral precipitation in the environment. There is a good understanding of BIMP induced by bacterial metabolism through the control of metal redox states and enzyme-mediated reactions such as ureolysis. However, other forms of BIMP often cannot be attributed to a single pathway but rather appear to be a passive result of bacterial activity, where minerals form as a result of metabolic by-products and surface interactions within the surrounding environment. BIMP from such processes has formed the basis of many new innovative biotechnologies, such as soil consolidation, heavy metal remediation, restoration of historic buildings and even self-healing concrete. However, these applications to date have primarily incorporated BIMP-capable bacteria sampled from the environment, while detailed investigations of the underpinning mechanisms have been lagging behind. This review covers our current mechanistic understanding of bacterial activities that indirectly influence BIMP and highlights the complexity and connectivity between the different cellular and metabolic processes involved. Ultimately, detailed insights will facilitate the rational design of application-specific BIMP technologies and deepen our understanding of how bacteria are shaping our world.
Keywords: biomineralization, organomineralization, biologically induced mineralization, nucleation, biogenic
Introduction
Bacterial activity is evident in our landscapes and throughout the geological record, where it has helped shape Earth’s mineral deposits [1]. This has occurred, to some degree, via a process known as bacteria-induced mineral precipitation (BIMP). The variety of mineral deposits that are formed through bacterial activity can take on the form of stalactites and stalagmites [2], microbialites, stromatolites and thrombolites [3, 4] as well as large-scale sedimentation [4]. More recently, the ability of bacteria to induce mineral formation has gained attention for biotechnological application. In particular, the precipitation of calcium carbonate in the form of calcite, the mineral that forms limestone, has been exploited in innovative technologies in civil engineering. The first patented application is considered to have been by Adolphe and colleagues in 1990 for biological treatment of degrading stone surfaces [5]. Since then, more technologies have been developed, with a lot of attention surrounding the concept of self-healing concrete [6–8]. Other applications of BIMP include soil consolidation or heavy metal bioremediation, and excellent recent reviews exist that cover the spectrum of such technologies in detail [9–14].
For the purposes of this review, BIMP is defined as a process by which bacterial activity indirectly induces mineral formation via the release of metabolic by-products and surface interactions with ions in the open environment [15–17]. This is in contrast to bacteria-controlled biomineralization, e.g. the formation of magnetite by magnetotactic bacteria, which is metabolically and genetically controlled by the bacteria and occurs in defined locations, e.g. magnetosomes [18–20]. The latter has been reviewed in detail elsewhere [15, 21] and will not be covered here. The minerals formed by BIMP generally have no specific function (aside from some potential ecological benefits) and can be considered an unintended and uncontrolled consequence of bacterial activity [22, 23]. Depending on the author, indirect biomineralization is sometimes subdivided further into more nuanced ‘bacteria-induced’ versus ‘bacteria-influenced’ mineral precipitation [13, 20, 24]. The boundaries between the two are, however, not clear cut and in this review no such division is made.
Bacteria-induced mineral precipitation
Precipitation of mineral species in an aqueous system occurs when the ion concentration exceeds solubility and reaches a degree of super-saturation. Once the activation energy barrier is overcome, initial crystal nucleation occurs, in which metastable critical nuclei form that may dissolve back into the bulk phase. Subsequent aggregation of individual nuclei describes the process of crystal growth and precipitation [25–27]. Nucleation can take place either homogeneously, whereby nucleation occurs when critical nuclei form in the absence of foreign particles (via random collisions of ions or atoms in solution), or heterogeneously, whereby nucleation takes place when critical nuclei form on surfaces of foreign particles [25–27]. Such particles lower the activation energy by providing templates with spacing that enhances nucleation and thus, precipitation [25–27]. Furthermore, during the nucleation process foreign particles may aggregate, leading to the formation of mixed precipitates [28].
In BIMP, bacteria can induce biomineralization by modulating precipitation-relevant parameters like local ion concentrations or pH in the environment and/or by bacterial cells themselves providing nucleation sites for crystal formation. In general, this bacterial process involves the attraction of cations to negative charges on the cell surfaces, while metabolic activity provides the appropriate microenvironment and counter-anions so that these cations may precipitate as minerals [29]. The BIMP trait is common amongst bacteria across environments [9, 30–33], and, depending on bacterial species and environment, it can lead to a range of precipitated minerals (Table 1). The bacteria-induced formation of some of these minerals can further lead to co-precipitation of additional divalent metal cations and anions [34–36]. Indirect bacterial influence on precipitation parameters of saturation state and nucleation catalysis can be broadly separated into two contributing areas: cell surface and metabolic activity, and our current understanding of the mechanisms of these will be reviewed here.
Table 1.
Minerals precipitated in association with bacterial activity*
|
Mineral |
Chemical formula |
Reference |
|---|---|---|
|
Carbonates |
|
|
|
Calcite |
CaCO3 |
[30] |
|
Dolomite |
CaMg(CO3)2 |
[111, 112] |
|
Kutnahorite |
CaMn(CO3)2 |
[113] |
|
Siderite |
FeCO3 |
[114] |
|
Magnesite |
MgCO3 |
[54, 115] |
|
Otavite |
CdCO3 |
[116] |
|
Strontianite |
SrCO3 |
[72] |
|
Rhodochrosite |
MnCO3 |
[117] |
|
Cerussite |
PbCO3 |
[118] |
|
Hydrozincite |
Zn5(CO3)2(OH)6 |
[36, 119] |
|
Dypingite |
Mg5(CO3)(OH)2·5H2O |
[120] |
|
Witherite |
BaCO3 |
[121] |
|
Phosphates |
|
|
|
Tricalcium phosphate |
Ca3(PO4)2 |
[78] |
|
Struvite |
NH4MgPO4∙6H2O |
[74, 113] |
|
Bobierrite |
Mg3(PO4)2·8H2O |
[74, 122] |
|
Baricite |
(MgFe)3(PO4)2·8H2O |
[74] |
|
Vivianite |
Fe3(PO4)·2H2O |
[114] |
|
Autunite |
Ca(UO2)2(PO4)2∙10-12H2O |
[44] |
|
Uramphite |
NH4UO2PO4 |
[101, 123] |
|
Apatite |
Ca10(PO4)6(OH)2 |
[124] |
|
Pb-hydroxyapatite |
Ca2.5Pb7.5(OH)2(PO4)6 |
[125] |
|
Strengite |
FePO4·2H2O |
[126, 127] |
|
Variscite |
AlPO4·2H2O |
[97] |
|
Silicates |
|
|
|
Gehlenite |
Ca2Al(AlSiO7) |
[128] |
|
Silica |
SiO2 |
[129] |
|
Nontronite |
Na0.3Fe3+ 2(Si,Al)4O10(OH)2·nH2O |
[130] |
|
Chamosite |
(Fe5Al)(Si3Al)10(OH)8 |
[126] |
|
Kaolinite |
Al4(Si4O10)(OH)4 |
[126] |
|
Sulphides |
|
|
|
Mackinawite |
FeS |
[76] |
|
Greigite |
Fe3S4 |
[76, 131] |
|
Pyrite |
FeS2 |
[132] |
|
Covellite |
CuS |
[133, 134] |
|
Sphalerite |
ZnS |
[135] |
|
Galena |
PbS |
[134] |
|
Digenite |
Cu9S5 |
[136] |
|
Sulphates |
|
|
|
Gypsum |
CaSO4·2H2O |
[41, 54] |
|
Celestite |
SrSO4 |
[72] |
|
Barite |
BaSO4 |
[121, 137] |
|
Oxides |
|
|
|
Magnetite |
Fe3O4 |
[114] |
|
Hematite |
Fe2O3 |
[23, 138] |
|
Ferrihydrite |
Fe2O3·0.5H2O |
[138] |
|
Geothite |
α-FeO(OH) |
[138] |
|
Manganite |
MnOOH |
[139] |
|
Vernadite |
MnO2 |
|
|
Hausmannite |
Mn3O4 |
[142] |
|
Todorokite |
(Ca,Na,K)x(Mn4+,Mn3+)6O10·3.5H2O |
[138] |
|
Birnessite |
(Na,Ca,K)x(Mn4+,Mn3+)2O4·1.5H2O |
[138] |
|
Uraninite |
UO2 |
[143–145] |
|
Calcium Arsenate |
CaHAsO3 |
[146] |
*Note that while these minerals have all been reported to be formed in association with bacterial activity, the mechanisms for their formation are not always known, and some minerals can be formed by multiple different mechanisms. The minerals listed and accompanying sources are non-exhaustive of the examples available in the literature.
Cell surface: nucleation catalysis, saturation state and nucleation template
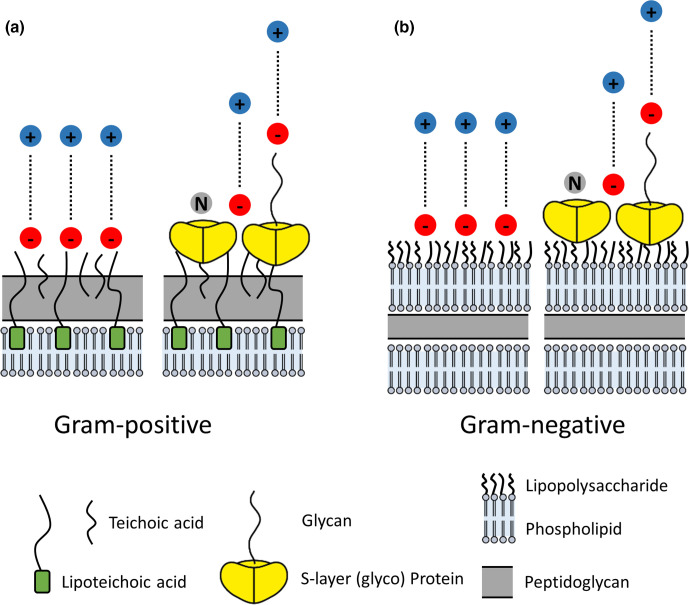
The large surface area to volume ratio of bacteria make them ideal crystal nucleation sites. Covered by functional groups with a net negative charge, their surface acts as a metal cation scavenger concentrating dilute cations attracted from the environment [29, 37, 38]. Net negative surface charge is imparted by carboxyl (R-CO2H) and phosphate groups (R-PO4H2) of teichoic acids in Gram-positive bacteria, and phospholipids and lipopolysaccharides (LPS) in Gram-negative bacteria [39]. Bacterial S-layers further influence net surface charge depending on the presence or absence of S-layer glycol proteins with glycosylated long carbohydrate chains, and depending on the structural groups exposed within their lattice pores [40–42]. These bacterial surface structures are illustrated in Fig. 1. Extracellular polymeric substances (EPS), capsules, sheaths, slimes and biofilm matrices may further surround Gram-positive or Gram-negative bacteria. These are also usually associated with a net negative charge imparted by carboxyl and phosphate groups, which are free to interact with soluble cations [43].
Fig. 1.
Schematic of the major supramolecular structures on the surface architecture of (a) Gram-positive and (b) Gram-negative bacteria, which provide sites for metal cation interaction. The red circles represent sites of negative charge, the grey circle represent sites of neutral charge, the blue circles represent positively charged cations, and dotted lines illustrate the attraction between negative and positive charges. Adapted from [42, 147].
The extent of the surface negative charge is governed by the deprotonation of functional groups with an increase in pH: carboxyl, phosphate, hydroxyl (R-OH) and sulphate (R-SO4) groups increase their negative charge, while amine (R-NH2) groups decrease their positive charge. For bacteria living in environments with neutral pH ranges, this means that surfaces tend to be negatively charged and have a high affinity for cationic species [44, 45]. Carboxyl groups in particular have been found to contribute strongly to the metal-binding capability. Studies on Bacillus subtilis used chemical modification of phosphate and carboxyl functional groups to demonstrate their importance in and relative contribution to metal ion binding [46, 47]. More recent studies of Gram-positive cell walls support this role, with half the binding of calcium and magnesium coming from polyphosphate groups of teichoic acids and half from carboxyl groups of peptidoglycan [48].
Teichoic and teichuronic acids, as well as LPS are natively stabilized by the presence of divalent cations, providing starting nucleation sites for mineral formation [43]. Surface cation binding sites are assumed to form the centre of crystal growth. Mineral precipitation occurs from nucleation of cations to previously adsorbed surface cations. The formation of these critical nuclei is stabilized by the surface functional groups through a reduction of tension between the bulk water phase and mineral nucleus [43]. Once bound, supersaturation is achieved by lowering the free energy necessary for precipitation, often with the help of metabolism-induced changes in pH. Consequently precipitation can then occur faster than in systems without bacteria [49]. For example, in the precipitation of the calcium-magnesium mineral dolomite, the dehydration of the magnesium ion and subsequent carbonation are the rate-limiting step of nucleation [50]. In the presence of carboxyl groups, [Mg(H2O)6]2+ binds and dehydrates to [Mg(H2O)5(R-COO)]+. This lowers the activation energy for subsequent carbonation and attachment of Ca2+ to form dolomite [CaMg(CO3)2] [50–52]. Thus, bacteria provide a mechanism of heterogeneous precipitation, with their surfaces acting as a nucleation catalyst and template, as well as increasing the saturation state through local attraction of cations.
Beyond the direct influence of bacterial surfaces, the microenvironment they create also plays a very important role in influencing ion saturation state. All submerged surfaces, such as those of micro-organisms, are surrounded by a thin-filmed water envelope called the hydrodynamic boundary layer [53]. Bacteria live at an extremely low Reynolds number, that is, the viscous forces of the environment dominate over their ability to move. As a consequence, these bacteria experience greater viscous drag and so struggle to escape their thin water envelope [53]. Within this surrounding water envelope, concentration gradients of ions can form where local concentrations are higher than in the bulk aqueous environment. Supersaturation will vary with ion concentration and so precipitation will be favoured within the cell-surface vicinity where the concentration is highest. The concentration gradient is the combined result of cell surfaces lowering thermodynamic activation energies, sequestering cations, as well as metabolic activity providing anions such as HCO3 -, all of which occurs within the surrounding water layer [29, 54].
This principle can be extended further to other layers surrounding microbial surfaces such as biofilm matrices, slimes, sheaths, filaments, capsules and EPS secretions. These layers can create a microenvironment that favours supersaturation and thus precipitation via local changes in ion mobility, viscosity and nucleation kinetics (Fig. 2) [55]. For example, mineralization has been seen on bacterial sheaths and filaments [56, 57], slimes [58, 59], biofilms and EPS [60]. Some findings even showed that purified EPS alone could contribute to mineral precipitation, while other studies found that EPS production was not always associated with mineral precipitation [61–63]. This emphasizes the complexity of the process dependent on the bacterium, environment, mineral formed and underlying mechanism. In cyanobacterial systems, EPS has been shown to inhibit the precipitation in the bulk phase of the environment by trapping large amounts of divalent cations in its sugars, acidic residues and negatively charged functional groups. Only upon degradation of EPS and liberation of the cations does the saturation index increase, allowing for the precipitation of minerals [24, 64, 65].
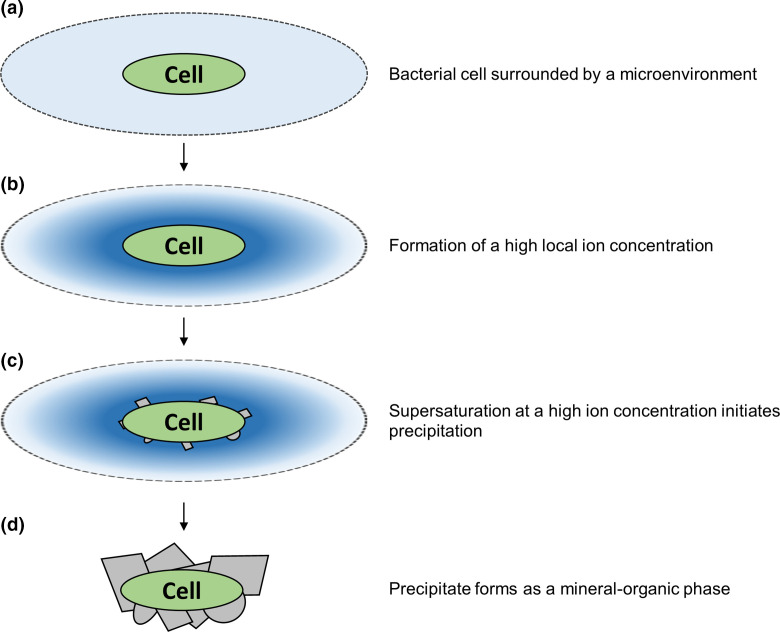
Fig. 2.
Mineral encasement of a bacterial cell. (a) Bacterial cell (green) surrounded by a microenvironment (light blue) created by an extremely low Reynolds number and/or sheaths, capsules, slimes, biofilm matrices or extracellular polymeric substances. (b) Accumulation, stabilization and slow diffusion of ions within the microenvironment close to the cell occurs from metabolism and cell-surface interactions creating a high local ion concentration (dark blue). (c) Within the cell-surface vicinity, at a high ion concentration, the equilibrium is shifted in favour of supersaturation and thus precipitation (grey shapes). (d) Onset of precipitation can lead to the breakdown of the microenvironment and, along with the degradation of some extracellular organic components, leaves behind a mineral-organic phase encasing the cell (grey shapes).
Further to creating favourable conditions, the microenvironment is not subject to the same kinetics as the bulk environment and therefore also protects against inhibiting factors such as ion complexing and cation hydration [66]. Thus, bacterial surfaces and their microenvironments allow precipitation to occur even in unfavourable conditions such as acidic environments [67]. Over the course of precipitation and with the eventual degradation of some extracellular organic components, the microenvironment is broken down and leaves behind a mineral organic phase encasing the cell, illustrated in Fig. 2. Active mechanisms by which the bacteria can avoid or escape such encasement are discussed later in this article.
Cell surface: polymorph ratio, crystal morphology, mineral type, and crystal size
In addition to providing nucleation sites and concentrating ions, surface structures can influence mineral polymorph ratio, crystal morphology and the type of minerals precipitated. Polymorphs have the same chemical structure but differ in their crystal structure [68]. Calcium carbonate mainly encompasses the polymorphs' calcite, vaterite and aragonite, and their ratios can be affected by cell-surface chemistry. For example, the presence of carboxylic groups, phosphonates, sulfonates and amino acids has been found to promote formation of vaterite [69]. The morphology of calcium carbonate crystals has been reported to be influenced through the presence of organic matter, e.g. by an increase in acidity of l-amino acids and xanthan content, where calcite crystals transitioned from rhombohedra to fibro-radial spherulites, and the monocrystals that make up the typical vaterite crystal spheres evolved from clustered short needles to clustered large hexagons [58].
The type of mineral precipitated is in part determined by the selective adsorption of metals to certain functional groups. Different metals were found to bind cell-surface components with different affinities. For example, it was reported that Mg2+ bound with a higher affinity than Ca2+ to cell walls of the Gram-positive B. subtilis [46, 47, 70] as well as to cell envelopes of the Gram-negative Escherichia coli [71]. The selective adsorption of calcium and strontium cations versus that of magnesium to pores within S-layers of Synechococcus sp. governed the preferred precipitation of the sulphate minerals gypsum and celestite [72].
Cell surface or metabolism: are precipitating bacteria dead or alive?
Different observations have been reported regarding whether BIMP is strictly dependent on bacterial activity, specifically whether dead cells may be able to facilitate biomineralization. This leads to different interpretations of how important cell-surface structures are for the process of mineral precipitation. While materials science studies showed that precipitation can occur on functional group monolayers [73], absence of precipitation on dead cells suggests that the organic material is not simply a nucleation seed, but that metabolic activity also plays a key role [74, 75]. In contrast, other work found that minerals do form on dead cells and their debris [61, 76, 77]. This discrepancy may simply be a result of the differences between bacterial species, environmental conditions or methodologies used to prepare the dead cells, as this may affect structural properties [2]. Systematic studies would be required to determine how much of this variability in mineral precipitation on live versus dead cells is genuinely due to specific properties of the particular species investigated, or if other factors of experimental design or conditions are the main drivers of the outcome.
While an unequivocal answer to the question is currently lacking, considerations of the implications of BIMP in bacterial communities may shed some light. As described above, mineral precipitation on the cell surface leads to encasement of the cell (Fig. 2). Therefore, if only living cells precipitated minerals, the whole population could run the risk of entombment and death. To allow for the continued growth of a population, precipitation might therefore be assumed to occur only on dead cells and/or a restricted number of live cells [78]. On the other hand, mechanisms exist for active evasion of entombment by shedding encrusted S-layers [41], forming mineral sheaths/capsules [56], forming nanoglobules to act as decoy precipitation targets [79, 80], or even controlling surface functional group distribution to control precipitation occurrence [81].
The role of metabolism in evading entombment is also unclear. One observation has been that induction of a proton motive force by metabolic activity of live cells reduced the cell-wall metal-binding ability [82]. Metabolism as an active mechanism against entombment also has been proposed in cyanobacteria and suggested that dead cells could potentially be better at mineral precipitation because they retained more of their negative surface charge [83]. Zeta potential analysis was used to approximate the net surface charge of the bacteria by measuring the potential differences between the cell and fluid interface [84]. In these studies, metabolic activity was found to contribute to a more positive surface charge, likely regulated to attract anions for metabolism. On the other hand, dead cells retained a constant negative charge on their surface structures [84]. At a community level, i.e. a mixture of live and dead bacteria, one explanation for the ability of these bacteria to precipitate minerals on their surface may be as a result of cations binding to negatively charged surfaces of dead or inactive cells. Alternatively, a somewhat counterintuitive explanation might be the attraction of carbonate anions to metabolically active cells and letting these act as the seed for nucleation rather than the typical cations [84]. Evidence of changes in cell-surface charge between dead and live cells is still limited, and there is likely to be variability among bacterial species depending on their surface structures. Taking into account these observations, the more likely explanation is that most often both surface structure and bacterial metabolism are required as catalysts to modulate precipitation parameters by influencing saturation state and nucleation ability. Precipitation should occur under conditions of supersaturation, when cations attracted to the bacterial surface react with counter anions in the environment. Anion concentration is in turn environment dependent or may be supplemented by metabolism, suggesting both live and dead bacteria may be needed.
Bacterial metabolism: pH and anions, including dissolved inorganic carbon (DIC)
Apart from the availability of nucleation sites, mineral precipitation also depends on (i) availability of anions, (ii) availability of cations and (iii) pH [85]. Bacterial metabolism plays an integral role in BIMP whereby it chemically alters the environment through the production of metabolites and by-products that influence the local pH and ion concentrations (e.g. carbonate, phosphate or metal cations). Modulation of these parameters ultimately affects supersaturation conditions and thus precipitation.
A key parameter to consider in mineral precipitation is the ion activity product (IAP), which for low-solubility minerals can be approximated as the product of the concentrations of the anion and cation composing the mineral, as exemplified for calcium carbonate in Equation 1. Supersaturation is achieved when the IAP of the mineral exceeds its solubility product constant (K sp), as defined in the saturation index (SI) (Equation 2). A system is considered supersaturated when SI>0 [13, 86, 87].
| (Equation 1) |
| (Equation 2) |
While K sp is a constant for a given system, IAP depends on effective concentrations and can be influenced by environmental factors such as bacterial metabolism. The precise value of SI at which precipitation occurs spontaneously for a given system can vary, depending, for example, on the presence of organics that can promote precipitation or even inhibit it despite high saturation states [69, 86, 88–90]. In that regard, SI only predicts the point at which precipitation is thermodynamically favoured but not when it actually begins. In BIMP, the point at which precipitation is observed can in part depend on cell density and nucleation points [13, 60, 91–93], but it also critically depends on the effects of bacterial metabolism on IAP.
Metabolic activity is furthermore accompanied by changes in pH due to the production of various metabolic by-products. This in turn affects precipitation potential, with a higher pH directly contributing to the availability of anions through deprotonation and supersaturation. For example, in the case of mineral carbonates, the precipitation potential is dependent on both the pH and the carbonate anion concentration, known as the total dissolved inorganic carbon (DIC), which is the sum of the dissolved forms of CO2, HCO3 - and CO3 2-. Moreover, the concentration of anions is directly related to the pH through the dissociation constants as seen in the carbonate equilibrium (Equation 3) [94]. At higher pH, the carbonate equilibrium is shifted to the right and carbonate species are deprotonated. As a result, more bicarbonate (HCO3 -) and carbonate (CO3 2-) ions are available for precipitation. Similarly, phosphate groups will be subject to changes in protonation state, depending on environmental pH (Equation 4). Sulphate groups will typically be present in their deprotonated state due to their low pKa values (usually below 2.5) (Equation 5), which will generally be exceeded by environmental pH [60]. Precipitation at low pH is possible in theory, but mostly applies to phosphate and sulphate-containing minerals where the anion component has a lower pKa. However, in practice, low pH often leads to dissolution of minerals.
| (Equation 3) |
| (Equation 4) |
| (Equation 5) |
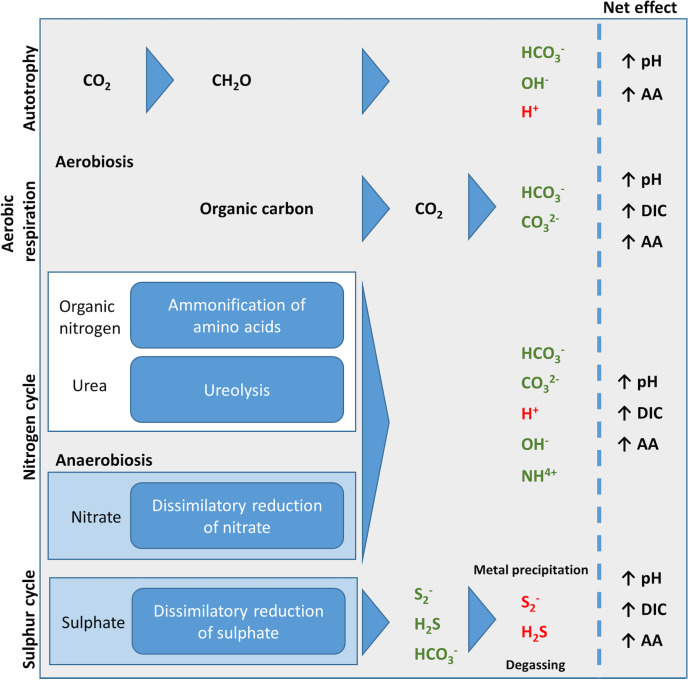
Bacterial metabolism, through a modulation in pH and the production of anions such as phosphates, sulphates and carbonates, therefore has a direct influence on IAP and can increase the likelihood of anions and cations precipitating together as minerals [87]. Which anions are produced ultimately also depends on the availability of nutrients and metabolic capabilities of the specific bacteria present. For example, bacteria capable of reducing sulphate can produce sulphide ions that can directly precipitate as minerals, while bacteria that break down urea or amino acids increase the local pH, which in turn favours formation of carbonates for mineral precipitation (Fig. 3). For reasons of brevity, however, only the key contributing factors in terms of net ion production and pH effects created by different metabolic pathways contributing to mineral precipitation are discussed here. The specific physicochemical details of the various individual metabolic pathways that can induce mineral precipitation have been reviewed elsewhere [33, 95].
Fig. 3.
Metabolic pathways associated with bacteria-induced mineral precipitation. Various products of metabolism result in a net effect, shown on the right, that primes the environment for mineral precipitation. AA refers to anion availability, typically bicarbonate and carbonate. Products in green are increased and those in red are decreased as a result of metabolic activity. Adapted from [98].
Autotrophic metabolic pathways
Autotrophic metabolic pathways such as non-methylotrophic methanogenesis or oxygenic and anoxygenic photosynthesis utilize CO2 to produce organic matter. This causes a depletion in CO2 that alters the bicarbonate equilibrium through a shift to the left (Equation 3), leading to removal of H+ as bicarbonate concentration increases, as well as dissociation of bicarbonate ions to CO2 and OH-. The resulting increase in pH favours precipitation under conditions of low DIC but high concentrations of suitable cations (Fig. 3) [24, 85].
Aerobic heterotrophic metabolism
Aerobic heterotrophic metabolism can cause local increases in anion concentration and pH. As mentioned above, aerobic heterotrophs break down organic carbon to produce CO2 that partially converts to carbonate and bicarbonate and increases DIC and pH in the bulk phase [64, 96].
Nitrogen cycle
Dissimilatory reduction of nitrate under anoxic conditions and deamination of amino acids for their catabolic use both lead to production of ammonium and hydroxide ions and consumption of H+ ions. This causes an increase in pH and thus shifts dissociation equilibria of anions that are relevant for mineral formation (Fig. 3) [96]. The role of ureolysis in mineral precipitation is explained below.
Sulphur cycle
Dissimilatory reduction of sulphate, carried out in anoxic conditions by sulphate-reducing bacteria, results in the production of carbonate, bicarbonate and hydrogen sulphide (H2S) (Fig. 3). Whether this leads to biomineralization depends on the fate of the H2S produced. Excreted sulphide can lead to authigenic precipitation in the bulk phase by directly reacting with metal cations in the environment to precipitate sulphide minerals [97]. Alternatively, loss of H2S can occur through degassing or consumption by anoxygenic sulphide phototrophic bacteria that oxidize H2S to elemental sulphur and form intra- or -extracellular deposits. The removal of H2S increases the pH and thus favours precipitation (Fig. 3) [98]. On the other hand, autotrophic sulphide-oxidizing aerobic bacteria use H2S (and other reduced sulphur compounds, S0 and S2O3 2-) to produce sulphate ions that form sulphuric acid, decreasing the pH and dissolving precipitates [64, 98]. The balance between precipitation and dissolution therefore will be dependent on environmental conditions such as oxygen availability, light and pH, which serve to decouple the different metabolic processes in time and space and establish local conditions where net precipitation can occur [99].
Single enzyme-mediated reactions
Aside from broader metabolic pathways, specific enzymes can also contribute to precipitation. Acid phosphatases liberate phosphoryl groups, thus accelerating formation of phosphate mineral species, and strains overproducing this enzyme were shown to precipitate uranium phosphate species [34, 100–102]. However, not all bacteria with phosphatase activity can precipitate minerals, lending weight to the idea that specific cell-surface structures are likely required to provide nucleation sites for precipitation [103].
Carbonic anhydrase, catalysing the interconversion of CO2 to HCO3 - and H+, has been suggested as a key enzyme in precipitation due to its effect on local HCO3 - concentration. The presence of extracellular carbonic anhydrase was found to govern the location of crystal precipitates in biofilms of Alcanivorax borkumensis [96]. Indeed, carbonate precipitation was restricted to areas with high extracellular concentration and activity of carbonic anhydrase.
Ureolysis as part of the nitrogen cycle is also an enzymatically driven process. This enzymatic activity may potentially be strong enough to increase supersaturation to such high levels that precipitation can occur without the need for nucleation sites provided by bacterial cell surfaces [104]. Indeed, it was observed that some strongly ureolytic bacteria could induce calcite precipitation at a considerable distance to the bacterial colony [32].
While the processes described in this review, i.e. the complex interplay between physical properties of bacterial cells and their metabolic activity, explain why in BIMP one is more likely to encounter heterogenous precipitation, strongly ureolytic bacteria may, in fact, be an exception and capable of driving homogenous nucleation.
Cell metabolism: provision of cations
Apart from the generation of anion species needed for precipitation, cation availability can also be influenced through metabolic activity. As defined within the IAP, the concentration of the metal cation is also important for the precipitation of a mineral species (Equation 1). Bacteria, often via enzymatic activities, may reduce a mineral compound to produce divalent cations that can then react with anions to precipitate as a different mineral [105, 106]. Some bacteria utilize metal ions as terminal electron acceptors in microaerobic or anaerobic conditions to produce cations, for example Fe2+ through reduction of oxidized iron (Fe3+), usually from dissolution of other iron oxides, as reviewed in detail elsewhere [106–108]. The resulting Fe2+ can subsequently interact with various anions to form a variety of iron minerals (Table 1). Many iron-reducing bacteria are also capable of reduction of manganese (Mn4+ to Mn2+), providing Mn2+ cations for mineral formation [59]. Metal oxidation can also occur under anoxic conditions through the activity of some phototrophic bacteria and some nitrate-respiring bacteria [59].
Local cation concentration can also fluctuate due to active bacterial processes such as intracellular metal ion homeostasis via ionic pumps and channels. In high-calcium environments, such as calcareous caves and limestone soils, the need to maintain a low intracellular calcium concentration is essential to ensure bacterial survival and growth [109]. Microbes can achieve this through active efflux of intracellular calcium by ATP-dependent antiporters, increasing the local calcium availability and pH near the cell surface and thus contributing to precipitation [85, 110]. Thus, active calcium efflux could be seen to influence biomineralization in two ways: it ensures bacterial growth to provide nucleation sites while simultaneously increasing the local cation concentration. Indeed, active processes of ion excretion may precede the passive precipitation discussed previously and allow microbes to act as nuclei for subsequent crystal growth [98].
Prospects
In exploring the underlying processes enabling BIMP, a lot of benefit has been gained from research across multiple disciplines investigating the different aspects of organic-mineral interphases. While there are mechanistic differences in the way bacteria induce mineralization dependent on their surface architecture and metabolism, understanding the contributing components is important for biotechnological application. An additional layer of complexity is introduced when considering that bacteria do not occur in isolation, and that metabolic processes of one group of organisms are often interdependent with the activities of other groups. Indeed, in nature precipitation results from the activities of mixed populations, which often grow as biofilms rather than planktonic cells [24, 98]. This could possibly be exploited in utilizing communities and biofilm growth of micro-organisms to maximize precipitation potential. BIMP has seen increased applications in civil engineering and biotechnology over recent years, as extensively reviewed elsewhere [10, 12–14, 87].
In brief, mineral precipitation mainly has two different roles in these technologies. For applications that include soil consolidation, heritage conservation and self-healing concrete, precipitated minerals and embedded cells and organic components become the ‘glue’ that binds and/or seals the surrounding matrix. For applications of bioremediation such as of toxic heavy metals or radionuclides or in carbon dioxide sequestration, the elements in question are directly precipitated or co-precipitated, rendering them bio-unavailable [13, 14]. Fundamental mechanistic insight will therefore be important in making more informed decisions in choosing the appropriate bacteria for a specific application in terms of strain characteristics and minerals precipitated. This could allow for selective mineral precipitation, dependent on preferential surface binding and metabolic anion production of the chosen bacterium. Additionally, one could even modulate the speed of precipitation through the choice of metabolic capability, depending on application need. In the future, detailed mechanistic insights may inform rational directed evolution or genetic engineering approaches for application-driven strain development. The complexity of BIMP and its dependency on precise bacterial properties may therefore even be viewed as a benefit. Nature may reveal a useful and versatile toolbox of different bacteria, supplemented by systematic strain engineering to meet future needs for sustainable BIMP technologies.
Funding information
This work was funded through the Engineering and Physical Sciences Research Council (EPSRC; EP/PO2081X/1) Resilient Materials for Life (RM4L) project with support from industrial collaborators/partners. TDH was supported by a University of Bath Research Studentship.
Acknowledgements
The authors would like to thank Kevin Paine for valuable discussions and insights into the use of bacteria in construction materials.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: BIMP, bacteria-induced mineral precipitation; DIC, dissolved inorganic carbon; EPS, extracellular polymeric substance; IAP, ion activity product; LPS, lipopolysaccharide.
References
- 1.Zavarzin GA. [Microbial geochemical calcium cycle] Mikrobiologiia. 2002;71:1–17. doi: 10.1023/A:1017945329951. [DOI] [PubMed] [Google Scholar]
- 2.Banks ED, Taylor NM, Gulley J, Lubbers BR, Giarrizo JG, et al. Bacterial calcium carbonate precipitation in cave environments: a function of calcium homeostasis. Geomicrobiol J. 2010;27:444–454. doi: 10.1080/01490450903485136. [DOI] [Google Scholar]
- 3.Kaźmierczak J, Fenchel T, Kühl M, Kempe S, Kremer B, et al. Caco3 precipitation in multilayered cyanobacterial mats: clues to explain the alternation of micrite and sparite layers in calcareous stromatolites. Life. 2015;5:744–769. doi: 10.3390/life5010744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riding R. Microbial carbonates: the geological record of calcified bacterial-algal mats and biofilms. Sedimentology. 2000;47:179–214. doi: 10.1046/j.1365-3091.2000.00003.x. [DOI] [Google Scholar]
- 5.Adolphe JP, Loubière JF, Paradas J, Soleilhavoup F. Procédé de traitement biologique d’une surface artificielle. European Patent. 1990;8903517 [Google Scholar]
- 6.Jonkers HM. Self Healing Concrete: A Biological Approach. In: van der Zwaag S, editor. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science. Dordrecht: Springer Netherlands; 2007. pp. 195–204. editor. [Google Scholar]
- 7.Sharma TK, Alazhari M, Heath A, Paine K, Cooper RM. Alkaliphilic Bacillus species show potential application in concrete crack repair by virtue of rapid spore production and germination then extracellular calcite formation. J Appl Microbiol. 2017;122:1233–1244. doi: 10.1111/jam.13421. [DOI] [PubMed] [Google Scholar]
- 8.Tan L, Reeksting B, Ferrandiz-Mas V, Heath A, Gebhard S, et al. Effect of carbonation on bacteria-based self-healing of cementitious composites. Constr Build Mater. 2020;257:119501. doi: 10.1016/j.conbuildmat.2020.119501. [DOI] [Google Scholar]
- 9.Anbu P, Kang C-H, Shin Y-J, So J-S. Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus. 2016;5:250. doi: 10.1186/s40064-016-1869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias D, Cisternas L, Rivas M. Biomineralization mediated by ureolytic bacteria applied to water treatment: a review. Crystals. 2017;7:345. [Google Scholar]
- 11.Dhami NK, Reddy MS, Mukherjee A. Bacillus megaterium mediated mineralization of calcium carbonate as biogenic surface treatment of green building materials. World J Microbiol Biotechnol. 2013;29:2397–2406. doi: 10.1007/s11274-013-1408-z. [DOI] [PubMed] [Google Scholar]
- 12.De Muynck W, De Belie N, Verstraete W. Microbial carbonate precipitation in construction materials: a review. Ecological Engineering. 2010;36:118–136. [Google Scholar]
- 13.Phillips AJ, Gerlach R, Lauchnor E, Mitchell AC, Cunningham AB, et al. Engineered applications of ureolytic biomineralization: a review. Biofouling. 2013;29:715–733. doi: 10.1080/08927014.2013.796550. [DOI] [PubMed] [Google Scholar]
- 14.Zhu T, Dittrich M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol. 2016;4:1–21. doi: 10.3389/fbioe.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazylinski DA, Frankel RB, Konhauser KO. Modes of biomineralization of magnetite by microbes. Geomicrobiol J. 2007;24:465–475. doi: 10.1080/01490450701572259. [DOI] [Google Scholar]
- 16.Konhauser K, Riding R. Fundamentals of Geobiology. Chichester, UK: John Wiley & Sons, Ltd; 2012. Bacterial Biomineralization; pp. 105–130. [Google Scholar]
- 17.Marvasi M, Visscher PT, Perito B, Mastromei G, Casillas-Martínez L. Physiological requirements for carbonate precipitation during biofilm development of Bacillus subtilis etfA mutant. FEMS Microbiol Ecol. 2010;71:341–350. doi: 10.1111/j.1574-6941.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner J, Morin G, Menguy N, Perez Gonzalez T, Widdrat M, et al. Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr)oxide intermediates. Proc Natl Acad Sci U S A. 2013;110:14883–14888. doi: 10.1073/pnas.1307119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagishi A, Tanaka M, Lenders JJM, Thiesbrummel J, Sommerdijk NAJM, et al. Control of magnetite nanocrystal morphology in magnetotactic bacteria by regulation of mms7 gene expression. Sci Rep. 2016;6:1–11. doi: 10.1038/srep29785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arias D, Cisternas LA, Rivas M. Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile) [Online] Desalination. 2017;405:1–9. [Google Scholar]
- 21.Prozorov T. Magnetic microbes: bacterial magnetite biomineralization. Semin Cell Dev Biol. 2015;46:36–43. doi: 10.1016/j.semcdb.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Bazylinski DA, Frankel RB. Biologically controlled mineralization in prokaryotes. Reviews in Mineralogy and Geochemistry. 2003;54:217–247. doi: 10.2113/0540217. [DOI] [Google Scholar]
- 23.Konhauser K. Introduction to Geomicrobiology. Blackwell: Oxford; 2007. Cell surface reactivity and metal sorption. [Google Scholar]
- 24.Dupraz C, Reid RP, Braissant O, Decho AW, Norman RS, et al. Processes of carbonate precipitation in modern microbial mats. Earth-Science Reviews. 2009;96:141–162. doi: 10.1016/j.earscirev.2008.10.005. [DOI] [Google Scholar]
- 25.Benning LG, Waychunas GA. Nucleation, Growth, and Aggregation of Mineral Phases: Mechanisms and Kinetic Controls. In: Brantley SL, Kubicki JD, White AF, editors. Kinetics of Water-Rock Interaction. New York, NY: Springer New York; 2008. pp. 259–333. [Google Scholar]
- 26.Cubillas P, Anderson MW. Synthesis Mechanism: Crystal Growth and Nucleation. In: Čejka J, Corma A, Zones S, editors. Zeolites and Catalysis: Synthesis, Reactions and Applications. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2010. pp. 1–55. [Google Scholar]
- 27.Fortin D, Ferris FG, Beveridge TJ. Surface-Mediated mineral development by bacteria. Reviews in Mineralogy and Geochemistry. 1997;35:161–180. [Google Scholar]
- 28.Reeder RJ, Lamble GM, Northrup PA. XAFS study of the coordination and local relaxation around Co2+, Zn2+, Pb2+, and Ba2+ trace elements in calcite. American Mineralogist. 1999;84:1049–1060. doi: 10.2138/am-1999-7-807. [DOI] [Google Scholar]
- 29.Schultze-Lam S, Fortin D, Davis BS, Beveridge TJ. Mineralization of bacterial surfaces. Chem Geol. 1996;132:171–181. doi: 10.1016/S0009-2541(96)00053-8. [DOI] [Google Scholar]
- 30.Boquet E, Boronat A, Ramos-Cormenzana A. Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature. 1973;246:527–529. [Google Scholar]
- 31.Krajewska B. Urease-aided calcium carbonate mineralization for engineering applications: a review. J Adv Res. 2018;13:59-67. doi: 10.1016/j.jare.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeksting BJ, Hoffmann TD, Tan L, Paine K, Gebhard S. In-Depth profiling of calcite precipitation by environmental bacteria reveals fundamental mechanistic differences with relevance to application. Appl Environ Microbiol. 2020;86:e02739–19. doi: 10.1128/AEM.02739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seifan M, Berenjian A. Microbially induced calcium carbonate precipitation: a widespread phenomenon in the biological world. Appl Microbiol Biotechnol. 2019;103:4693–4708. doi: 10.1007/s00253-019-09861-5. [DOI] [PubMed] [Google Scholar]
- 34.Basnakova G, Stephens ER, Thaller MC, Rossolini GM, Macaskie LE. The use of Escherichia coli bearing a phoN gene for the removal of uranium and nickel from aqueous flows. Appl Microbiol Biotechnol. 1998;50:266–272. doi: 10.1007/s002530051288. [DOI] [PubMed] [Google Scholar]
- 35.Lauchnor EG, Schultz LN, Bugni S, Mitchell AC, Cunningham AB, et al. Bacterially induced calcium carbonate precipitation and strontium Coprecipitation in a porous media flow system. Environ Sci Technol. 2013;47:1557–1564. doi: 10.1021/es304240y. [DOI] [PubMed] [Google Scholar]
- 36.Podda F, Zuddas P, Minacci A, Pepi M, Baldi F. Heavy metal coprecipitation with hydrozincite [Zn5(CO3)2(OH)6] from mine waters caused by photosynthetic microorganisms. Appl Environ Microbiol. 2000;66:5092–5098. doi: 10.1128/aem.66.11.5092-5098.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beveridge TJ, Fyfe WS. Metal fixation by bacterial cell walls. Can J Earth Sci. 1985;22:1893–1898. doi: 10.1139/e85-204. [DOI] [Google Scholar]
- 38.Douglas S, Beveridge TJ. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol Ecol. 1998;26:79–88. doi: 10.1111/j.1574-6941.1998.tb00494.x. [DOI] [Google Scholar]
- 39.Madigan MT, Bender KS, Buckley DH, Sattley MW, Stahl DA. Brock Biology of Microorganisms. 15th ed. New York, USA: Pearson Higher Education; 2018. [Google Scholar]
- 40.Merroun ML, Raff J, Rossberg A, Hennig C, Reich T, et al. Complexation of uranium by cells and S-layer sheets of Bacillus sphaericus JG-A12. Appl Environ Microbiol. 2005;71:5532–5543. doi: 10.1128/AEM.71.9.5532-5543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultze-Lam S, Harauz G, Beveridge TJ. Participation of a cyanobacterial S layer in fine-grain mineral formation. J Bacteriol. 1992;174:7971–7981. doi: 10.1128/JB.174.24.7971-7981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sleytr UB, Schuster B, Egelseer E-M, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014;38:823–864. doi: 10.1111/1574-6976.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Southam G. Bacterial surface-mediated mineral formation. In: Lovley DR, editor. Environmental Microbe-Metal Interactions. American Society of Microbiology; 2000. pp. 257–276. editor. [Google Scholar]
- 44.Merroun ML, Nedelkova M, Ojeda JJ, Reitz T, Fernández ML, et al. Bio-precipitation of uranium by two bacterial isolates recovered from extreme environments as estimated by potentiometric titration, TEM and X-ray absorption spectroscopic analyses. J Hazard Mater. 2011;197:1–10. doi: 10.1016/j.jhazmat.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 45.Warren LA, Haack EA. Biogeochemical controls on metal behaviour in freshwater environments. Earth-Science Reviews. 2001;54:261–320. doi: 10.1016/S0012-8252(01)00032-0. [DOI] [Google Scholar]
- 46.Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–887. doi: 10.1128/JB.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doyle RJ, Matthews TH, Streips UN. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980;143:471–480. doi: 10.1128/JB.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas KJ, Rice CV. Revised model of calcium and magnesium binding to the bacterial cell wall. Biometals. 2014;27:1361–1370. doi: 10.1007/s10534-014-9797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bäuerlein E. Biomineralization of unicellular organisms: an unusual membrane biochemistry for the production of inorganic nano- and microstructures. Angewandte Chemie - International Edition. 2003;42:614–641. doi: 10.1002/anie.200390176. [DOI] [PubMed] [Google Scholar]
- 50.Roberts JA, Kenward PA, Fowle DA, Goldstein RH, González LA, et al. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc Natl Acad Sci U S A. 2013;110:14540–14545. doi: 10.1073/pnas.1305403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz AK, Glusker JP, Markham GD, Bock CW. Deprotonation of water in the presence of carboxylate and magnesium ions. J Phys Chem B. 1998;102:6342–6350. doi: 10.1021/jp9815412. [DOI] [Google Scholar]
- 52.Kluge S, Weston J. Can a hydroxide ligand trigger a change in the coordination number of magnesium ions in biological systems? Biochemistry. 2005;44:4877–4885. doi: 10.1021/bi047454j. [DOI] [PubMed] [Google Scholar]
- 53.Purcell EM. Life at low Reynolds number. Am J Phys. 1977;45:3–11. doi: 10.1119/1.10903. [DOI] [Google Scholar]
- 54.Thompson JB, Ferris FG. Cyanobacterial precipitation of gypsum, calcite, and magnesite from natural alkaline lake water. Geology. 1990;18:995–998. doi: 10.1130/0091-7613(1990)018<0995:CPOGCA>2.3.CO;2. [DOI] [Google Scholar]
- 55.Buczynski C, Chafetz HS. Habit of bacterially induced precipitates of calcium carbonate and the influence of medium viscosity on mineralogy. Journal of Sedimentary Research. 1991;61:226–233. doi: 10.1306/D42676DB-2B26-11D7-8648000102C1865D. [DOI] [Google Scholar]
- 56.Gilbert PUPA, Albrecht M, Frazer BH. The Organic-Mineral interface in Biominerals. Reviews in Mineralogy and Geochemistry. 2005;59:157–185. doi: 10.2138/rmg.2005.59.7. [DOI] [Google Scholar]
- 57.Jürgensen A, Widmeyer JR, Gordon RA, Bendell-Young LI, Moore MM, et al. The structure of the manganese oxide on the sheath of the bacterium Leptothrix discophora : An XAFS study. American Mineralogist. 2004;89:1110–1118. doi: 10.2138/am-2004-0724. [DOI] [Google Scholar]
- 58.Braissant O, Cailleau G, Dupraz C, Verrecchia EP. Bacterially induced mineralization of calcium carbonate in terrestrial environments: the role of exopolysaccharides and amino acids. Journal of Sedimentary Research. 2003;73:485–490. [Google Scholar]
- 59.Frankel RB, Bazylinski DA. Biologically induced mineralization by bacteria. Reviews in Mineralogy and Geochemistry. 2003;54:95–114. doi: 10.2113/0540095. [DOI] [Google Scholar]
- 60.Braissant O, Decho AW, Dupraz C, Glunk C, Przekop KM, et al. Exopolymeric substances of sulfate-reducing bacteria: interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology. 2007;5:401–411. [Google Scholar]
- 61.Ercole C, Cacchio P, Botta AL, Centi V, Lepidi A. Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharides and capsular polysaccharides. Microsc Microanal. 2007;13:42–50. doi: 10.1017/S1431927607070122. [DOI] [PubMed] [Google Scholar]
- 62.Fishman MR, Giglio K, Fay D, Filiatrault MJ. Physiological and genetic characterization of calcium phosphate precipitation by Pseudomonas species. Scientific Reports. 2018;8:1–14. doi: 10.1038/s41598-018-28525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim HJ, Shin B, Lee YS, Park W. Modulation of calcium carbonate precipitation by exopolysaccharide in Bacillus sp. JH7. Appl Microbiol Biotechnol. 2017;101:6551–6561. doi: 10.1007/s00253-017-8372-8. [DOI] [PubMed] [Google Scholar]
- 64.Dupraz C, Visscher PT. Microbial lithification in marine stromatolites and hypersaline mats. Trends Microbiol. 2005;13:429–438. doi: 10.1016/j.tim.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 65.Kremer B, Kazmierczak J, Stal LJ. Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North sea. Geobiology. 2008;6:46–56. doi: 10.1111/j.1472-4669.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 66.Wright DT, Oren A. Nonphotosynthetic bacteria and the formation of carbonates and evaporites through time. Geomicrobiol J. 2005;22:27–53. doi: 10.1080/01490450590922532. [DOI] [Google Scholar]
- 67.Fernández-Remolar DC, Preston LJ, Sánchez-Román M, Izawa MRM, Huang L, et al. Carbonate precipitation under bulk acidic conditions as a potential biosignature for searching life on Mars. Earth and Planetary Science Letters. 2012:351–352. [Google Scholar]
- 68.Tegethoff FW. Calcium Carbonate: From the Cretaceous Period into the 21st Century. Basel: Springer; 2001. [Google Scholar]
- 69.Rodriguez-Navarro C, Jimenez-Lopez C, Rodriguez-Navarro A, Gonzalez-Muñoz MT, Rodriguez-Gallego M. Bacterially mediated mineralization of vaterite. Geochim Cosmochim Acta. 2007;71:1197–1213. doi: 10.1016/j.gca.2006.11.031. [DOI] [Google Scholar]
- 70.Beveridge TJ, Murray RG. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976;127:1502–1518. doi: 10.1128/JB.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beveridge TJ, Koval SF. Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol. 1981;42:325–335. doi: 10.1128/AEM.42.2.325-335.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultze-Lam S, Beveridge TJ. Nucleation of celestite and strontianite on a cyanobacterial S-layer. Appl Environ Microbiol. 1994;60:447–453. doi: 10.1128/AEM.60.2.447-453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng H, Shen XC, Wang X-M, Du C. Calcium carbonate crystallization controlled by functional groups: a mini-review. Front Mater Sci. 2013;7:62–68. doi: 10.1007/s11706-013-0191-y. [DOI] [Google Scholar]
- 74.Rivadeneyra A, Gonzalez-Martinez A, Gonzalez-Lopez J, Martin-Ramos D, Martinez-Toledo M, et al. Precipitation of phosphate minerals by microorganisms isolated from a fixed-biofilm reactor used for the treatment of domestic wastewater. Int J Environ Res Public Health. 2014;11:3689–3704. doi: 10.3390/ijerph110403689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yates KK, Robbins LL. Production of carbonate sediments by a unicellular green alga. American Mineralogist. 1998;83:1503–1509. doi: 10.2138/am-1998-11-1238. [DOI] [Google Scholar]
- 76.Picard A, Gartman A, Clarke DR, Girguis PR. Sulfate-reducing bacteria influence the nucleation and growth of mackinawite and greigite. Geochimica et Cosmochimica Acta. 2018;220:367–384. [Google Scholar]
- 77.Velásquez L, Dussan J. Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus . J Hazard Mater. 2009;167:713–716. doi: 10.1016/j.jhazmat.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 78.Benzerara K, Menguy N, Guyot F, Skouri F, de Luca G, et al. Biologically controlled precipitation of calcium phosphate by Ramlibacter tataouinensis. Earth Planet Sci Lett. 2004;228:439–449. doi: 10.1016/j.epsl.2004.09.030. [DOI] [Google Scholar]
- 79.Aloisi G, Gloter A, Krüger M, Wallmann K, Guyot F, et al. Nucleation of calcium carbonate on bacterial nanoglobules. Geology. 2006;34:1017. doi: 10.1130/G22986A.1. [DOI] [Google Scholar]
- 80.Bontognali TRR, Vasconcelos C, Warthmann RJ, Dupraz C, Bernasconi SM, et al. Microbes produce nanobacteria-like structures, avoiding cell entombment. Geology. 2008;36:663–666. [Google Scholar]
- 81.Asada R, Tazaki K. Silica biomineralization of unicellular microbes under strongly acidic conditions. The Canadian Mineralogist. 2001;39:1–16. doi: 10.2113/gscanmin.39.1.1. [DOI] [Google Scholar]
- 82.Urrutia Mera M, Kemper M, Doyle R, Beveridge TJ. The membrane-induced proton motive force influences the metal binding ability of Bacillus subtilis cell walls. Appl Environ Microbiol. 1992;58:3837–3844. doi: 10.1128/AEM.58.12.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martinez RE, Gardés E, Pokrovsky OS, Schott J, Oelkers EH. Do photosynthetic bacteria have a protective mechanism against carbonate precipitation at their surfaces? Geochimica et Cosmochimica Acta. 2010;74:1329–1337. [Google Scholar]
- 84.Martinez RE, Pokrovsky OS, Schott J, Oelkers EH. Surface charge and zeta-potential of metabolically active and dead cyanobacteria. J Colloid Interface Sci. 2008;323:317–325. doi: 10.1016/j.jcis.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 85.Hammes F, Verstraete* W. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol. 2002;1:3–7. doi: 10.1023/A:1015135629155. [DOI] [Google Scholar]
- 86.Silva-Castro GA, Uad I, Gonzalez-Martinez A, Rivadeneyra A, Gonzalez-Lopez J, et al. Bioprecipitation of calcium carbonate crystals by bacteria isolated from saline environments grown in culture media amended with seawater and real brine. Biomed Res Int. 2015;2015:1–12. doi: 10.1155/2015/816102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dhami NK, Reddy MS, Mukherjee A. Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol. 2013;4:1–13. doi: 10.3389/fmicb.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mitchell AC, Ferris FG, Grant Ferris F. The influence of Bacillus pasteurii on the nucleation and growth of calcium carbonate. Geomicrobiol J. 2006;23:213–226. doi: 10.1080/01490450600724233. [DOI] [Google Scholar]
- 89.Sánchez-Román M, Rivadeneyra MA, Vasconcelos C, McKenzie JA. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol Ecol. 2007;61:273–284. doi: 10.1111/j.1574-6941.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 90.Silva-Castro GA, Uad I, Rivadeneyra A, Vilchez JI, Martin-Ramos D, et al. Carbonate precipitation of bacterial strains isolated from sediments and seawater: formation mechanisms. Geomicrobiol J. 2013;30:840–850. doi: 10.1080/01490451.2013.777492. [DOI] [Google Scholar]
- 91.Bosak T, Newman DK. Microbial kinetic controls on calcite morphology in supersaturated solutions. J Sed Res. 2005;75:190–199. doi: 10.2110/jsr.2005.015. [DOI] [Google Scholar]
- 92.Gat D, Tsesarsky M, Shamir D, Ronen Z. Accelerated microbial-induced CaCO3precipitation in a defined coculture of ureolytic and non-ureolytic bacteria. Biogeosciences. 2014;11:2561–2569. [Google Scholar]
- 93.Rodriguez-Navarro C, Jroundi F, Schiro M, Ruiz-Agudo E, González-Muñoz MT. Influence of substrate mineralogy on bacterial mineralization of calcium carbonate: implications for stone conservation. Appl Environ Microbiol. 2012;78:4017–4029. doi: 10.1128/AEM.07044-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeebe RE, Wolf-Gladrow D. CO2 in Seawater: Equilibrium, Kinetics, Isotopes. Amsterdam; New York: Elsevier Science Publishers B.V.; 2001. [Google Scholar]
- 95.Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Macias Franco MR, Narayanasamy R, et al. Microbially induced calcium carbonate precipitation (MICP) and its potential in Bioconcrete: microbiological and molecular concepts. Frontiers in Materials. 2019;6:1–15. [Google Scholar]
- 96.Krause S, Liebetrau V, Löscher CR, Böhm F, Gorb S, et al. Marine ammonification and carbonic anhydrase activity induce rapid calcium carbonate precipitation. Geochim Cosmochim Acta. 2018;243:116–132. doi: 10.1016/j.gca.2018.09.018. [DOI] [Google Scholar]
- 97.Ehrlich HL. Microbes as geologic agents: their role in mineral formation. Geomicrobiol J. 1999;16:135–153. doi: 10.1080/014904599270659. [DOI] [Google Scholar]
- 98.Castanier S, Le Métayer-Levrel G, Perthuisot J-P. Ca-carbonates precipitation and limestone genesis — the microbiogeologist point of view. Sedimentary Geology. 1999;126:9–23. doi: 10.1016/S0037-0738(99)00028-7. [DOI] [Google Scholar]
- 99.Visscher PT, Reid RP, Bebout BM, Hoeft SE, Macintyre IG, et al. Formation of lithified micritic laminae in modern marine stromatolites (Bahamas); the role of sulfur cycling. American Mineralogist. 1998;83:1482–1493. doi: 10.2138/am-1998-11-1236. [DOI] [Google Scholar]
- 100.Martinez RJ, Beazley MJ, Taillefert M, Arakaki AK, Skolnick J, et al. Aerobic uranium (VI) bioprecipitation by metal-resistant bacteria isolated from radionuclide- and metal-contaminated subsurface soils. Environ Microbiol. 2007;9:3122–3133. doi: 10.1111/j.1462-2920.2007.01422.x. [DOI] [PubMed] [Google Scholar]
- 101.Newsome L, Morris K, Lloyd JR. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chemical Geology. 2014;363:164–184. [Google Scholar]
- 102.Powers LG, Mills HJ, Palumbo A, V, Zhang C, Delaney K, et al. Introduction of a plasmid-encoded phoA gene for constitutive overproduction of alkaline phosphatase in three subsurface Pseudomonas isolates. FEMS Microbiology Ecology. 2002;41:115–123. doi: 10.1111/j.1574-6941.2002.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 103.Macaskie LE, Bonthrone KM, Rouch DA. Phosphatase-mediated heavy metal accumulation by a Citrobacter sp. and related enterobacteria. FEMS Microbiol Lett. 1994;121:141–146. doi: 10.1111/j.1574-6968.1994.tb07090.x. [DOI] [PubMed] [Google Scholar]
- 104.Dittrich M, Obst M. Are picoplankton responsible for calcite precipitation in lakes? Ambio. 2004;33:559–564. doi: 10.1579/0044-7447-33.8.559. [DOI] [PubMed] [Google Scholar]
- 105.Dick GJ, Torpey JW, Beveridge TJ, Tebo BM. Direct identification of a bacterial manganese(II) oxidase, the multicopper oxidase MnxG, from spores of several different marine Bacillus species. Appl Environ Microbiol. 2008;74:1527. doi: 10.1128/AEM.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ehrlich HL, Newman DK, Kappler A. Ehrlich’s Geomicrobiology. CRC Press.; 2015. [Google Scholar]
- 107.Lovley DR. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/MR.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reguera G, Kashefi K. The electrifying physiology of Geobacter bacteria, 30 years on. Adv Microb Physiol. 2019;74:1–96. doi: 10.1016/bs.ampbs.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 109.Anderson S, Appanna VD, Huang J, Viswanatha T. A novel role for calcite in calcium homeostasis. FEBS Lett. 1992;308:94–96. doi: 10.1016/0014-5793(92)81059-U. [DOI] [PubMed] [Google Scholar]
- 110.Yates KK, Robbins LL. Radioisotope tracer studies of inorganic carbon and Ca in microbially derived CaCO3. Geochim Cosmochim Acta. 1999;63:129–136. doi: 10.1016/S0016-7037(98)00297-X. [DOI] [Google Scholar]
- 111.Deng S, Dong H, Lv G, Jiang H, Yu B, et al. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: results from Qinghai lake, Tibetan Plateau, NW China. Chemical Geology. 2010;278:151–159. [Google Scholar]
- 112.Wright DT, Wacey D. Precipitation of dolomite using sulphate-reducing bacteria from the Coorong region, South Australia: significance and implications. Sedimentology. 2005;52:987–1008. doi: 10.1111/j.1365-3091.2005.00732.x. [DOI] [Google Scholar]
- 113.González-Muñoz MT, De Linares C, Martínez-Ruiz F, Morcillo F, Martín-Ramos D, et al. Ca-Mg kutnahorite and struvite production by Idiomarina strains at modern seawater salinities. Chemosphere. 2008;72:465–472. doi: 10.1016/j.chemosphere.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 114.Kooli WM, Comensoli L, Maillard J, Albini M, Gelb A, et al. Bacterial iron reduction and biogenic mineral formation for the stabilisation of corroded iron objects. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-017-19020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.González-Muñoz M, Chekroun K. Bacterially induced Mg-calcite formation: role of Mg2+ in development of crystal morphology. J Sed Res. 2000;70:559–564. [Google Scholar]
- 116.Li L, Qian C, Cheng L, Wang R. A laboratory investigation of microbe-inducing CdCO3 precipitate treatment in Cd2+ contaminated soil. J Soils Sediments. 2010;10:248–254. [Google Scholar]
- 117.Naik-Samant S, Furtado I. Formation of rhodochrosite by haloferax alexandrinus GUSF-1. J Clust Sci. 2019;30:1435–1441. [Google Scholar]
- 118.Kang C-H, Oh SJ, Shin Y, Han S-H, Nam I-H, et al. Bioremediation of lead by ureolytic bacteria isolated from soil at abandoned metal mines in South Korea. Ecol Eng. 2015;74:402–407. doi: 10.1016/j.ecoleng.2014.10.009. [DOI] [Google Scholar]
- 119.Ngwenya BT, Magennis M, Podda F, Gromov A. Self-preservation strategies during bacterial biomineralization with reference to hydrozincite and implications for fossilization of bacteria. J R Soc Interface. 2014;11:20140845. doi: 10.1098/rsif.2014.0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Power IM, Wilson SA, Thom JM, Dipple GM, Southam G. Biologically induced mineralization of dypingite by cyanobacteria from an alkaline wetland near Atlin, British Columbia, Canada. Geochemical Transactions. 2007;8:1–16. doi: 10.1186/1467-4866-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanchez-Moral S, Luque L, Cañaveras JC, Laiz L, Jurado V, et al. Bioinduced barium precipitation in St. Callixtus and Domitilla catacombs. Annals of Microbiology. 2004;54:1–12. [Google Scholar]
- 122.Rivadeneyra MA, Ramos-Cormenzana A, Garcia-Cervigon A. Formation of bobierrite (magnesium phosphate) crystal aggregates by Acinetobacter sp. Mineral J. 1987;13:443–447. doi: 10.2465/minerj.13.443. [DOI] [PubMed] [Google Scholar]
- 123.Macaskie L, Empson R, Cheetham A, Grey C, Skarnulis A. Uranium bioaccumulation by a Citrobacter sp. as a result of enzymically mediated growth of polycrystalline HUO2PO4. Science. 1992;257:782–784. doi: 10.1126/science.1496397. [DOI] [PubMed] [Google Scholar]
- 124.Crosby CH, Bailey JV. The role of microbes in the formation of modern and ancient phosphatic mineral deposits. Front Microbiol. 2012;3:1–7. doi: 10.3389/fmicb.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen Z, Pan X, Chen H, Guan X, Lin Z. Biomineralization of Pb(II) into Pb-hydroxyapatite induced by Bacillus cereus 12-2 isolated from Lead-Zinc mine tailings. J Hazard Mater. 2016;301:531–537. doi: 10.1016/j.jhazmat.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 126.Konhauser KO. Bacterial iron biomineralisation in nature. FEMS Microbiol Rev. 1997;20:315–326. doi: 10.1111/j.1574-6976.1997.tb00317.x. [DOI] [Google Scholar]
- 127.Konhauser KO, Fyfe WS, Schultze-Lam S, Ferris FG, Beveridge TJ. Iron phosphate precipitation by epilithic microbial biofilms in Arctic Canada. Can J Earth Sci. 1994;31:1320–1324. doi: 10.1139/e94-114. [DOI] [Google Scholar]
- 128.Biswas M, Majumdar S, Chowdhury T, Chattopadhyay B, Mandal S, et al. Bioremediase a unique protein from a novel bacterium BKH1, ushering a new hope in concrete technology. Enzyme Microb Technol. 2010;46:581–587. doi: 10.1016/j.enzmictec.2010.03.005. [DOI] [Google Scholar]
- 129.Yee N, Phoenix VR, Konhauser KO, Benning LG, Ferris FG. The effect of cyanobacteria on silica precipitation at neutral pH: implications for bacterial silicification in geothermal hot springs. Chem Geol. 2003;199:83–90. doi: 10.1016/S0009-2541(03)00120-7. [DOI] [Google Scholar]
- 130.Ta K, Peng X, Chen S, Xu H, Li J, et al. Hydrothermal nontronite formation associated with microbes from low-temperature diffuse hydrothermal vents at the South Mid-Atlantic Ridge. J Geophys Res. 2017;122:2375–2392. doi: 10.1002/2017JG003852. [DOI] [Google Scholar]
- 131.Lefèvre CT, Menguy N, Abreu F, Lins U, Pósfai M, et al. A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science. 2011;334:1720–1723. doi: 10.1126/science.1212596. [DOI] [PubMed] [Google Scholar]
- 132.Thiel J, Byrne JM, Kappler A, Schink B, Pester M. Pyrite formation from FeS and H 2 S is mediated through microbial redox activity. Proceedings of the National Academy of Sciences of the United States of America . 2019. [DOI] [PMC free article] [PubMed]
- 133.Gramp JP, Sasaki K, Bigham JM, Karnachuk OV, Tuovinen OH. Formation of covellite (cus) under biological sulfate-reducing conditions. Geomicrobiol J. 2006;23:613. doi: 10.1080/01490450600964383. [DOI] [Google Scholar]
- 134.Karnachuk OV, Sasaki K, Gerasimchuk AL, Sukhanova O, Ivasenko DA, et al. Precipitation of Cu-sulfides by copper-tolerant Desulfovibrio isolates. Geomicrobiol J. 2008;25:219. doi: 10.1080/01490450802153124. [DOI] [Google Scholar]
- 135.Labrenz M, Druschel GK, Thomsen-Ebert T, Gilbert B, Welch SA, et al. Formation of sphalerite (ZnS) deposits in natural biofilms of sulfate-reducing bacteria. Science. 2000 doi: 10.1126/science.290.5497.1744. [DOI] [PubMed] [Google Scholar]
- 136.Wolicka D, Borkowski A. Influence of electron donors and copper concentration on geochemical and mineralogical processes under conditions of biological sulphate reduction. Acta Geologica Polonica. 2014;64:138–146. doi: 10.2478/agp-2014-0007. [DOI] [Google Scholar]
- 137.Keren R, Mayzel B, Lavy A, Polishchuk I, Levy D, et al. Sponge-associated bacteria mineralize arsenic and barium on intracellular vesicles. Nat Commun. 2017;8:1–12. doi: 10.1038/ncomms14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Polgári M, Gyollai I, Fintor K, Horváth H, Pál-Molnár E, et al. Microbially mediated ore-forming processes and cell mineralization. Front Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Greene AC, Madgwick JC. Microbial formation of manganese oxides. Appl Environ Microbiol. 1991;57:1114–1120. doi: 10.1128/AEM.57.4.1114-1120.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Villalobos M, Toner B, Bargar J, Sposito G. Characterization of the manganese oxide produced by Pseudomonas putida strain MnB1. Geochim Cosmochim Acta. 2003;67:2649–2662. doi: 10.1016/S0016-7037(03)00217-5. [DOI] [Google Scholar]
- 141.Webb SM, Tebo BM, Bargar JR. Structural characterization of biogenic Mn oxides produced in seawater by the marine Bacillus sp. strain SG-1. American Mineralogist. 2005;90:1342–1357. doi: 10.2138/am.2005.1669. [DOI] [Google Scholar]
- 142.Mandernack KW, Post J, Tebo BM. Manganese mineral formation by bacterial spores of the marine Bacillus, strain SG-1: Evidence for the direct oxidation of Mn(II) to Mn(IV) Geochimica et Cosmochimica Acta. 1995 [Google Scholar]
- 143.Lovley DR, Phillips EJ. Reduction of uranium by Desulfovibrio desulfuricans . Appl Environ Microbiol. 1992;58:850–856. doi: 10.1128/AEM.58.3.850-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roh Y, Liu SV, Li G, Huang H, Phelps TJ, et al. Isolation and characterization of metal-reducing Thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl Environ Microbiol. 2002;68:6013–6020. doi: 10.1128/AEM.68.12.6013-6020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki Y, Kelly SD, Kemner KM, Banfield JF. Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment. Appl Environ Microbiol. 2003;69:1337–1346. doi: 10.1128/AEM.69.3.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Achal V, Pan X, Fu Q, Zhang D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J Hazard Mater. 2012;201-202:178–184. doi: 10.1016/j.jhazmat.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 147.Seifan M, Berenjian A. Application of microbially induced calcium carbonate precipitation in designing bio self-healing concrete. World J Microbiol Biotechnol. 2018;34:168. doi: 10.1007/s11274-018-2552-2. [DOI] [PubMed] [Google Scholar]