Abstract
Primary central nervous system lymphoma (PCNSL) is a rare but highly aggressive non-Hodgkin lymphoma. Treatment-related cardiovascular lesion has become one of the most common complications in patients with tumor. However, very little is known about the cardiovascular death (CVD) of the patients with PCNSL. This study aims at identifying the cardiovascular outcomes of PCNSL patients and making comparison on CVD with extra central nervous system lymphoma (ECNSL). Clinical information of PCNSL and ECNSL was retrieved from the Surveillance, Epidemiology and End Results database. The risk factors of CVD in PCNSL patients and the comparison on the CVD hazard between PCNSL and ECNSL were assessed with the competing risks regression. A 1:2 propensity score matching was used to reduce the imbalanced baseline characteristics between PCNSL and ECNSL. Four thousand thirty-eight PCNSL subjects and 246,760 ECNSL subjects were enrolled in this retrospective study. CVD was the leading cause (41.2%) of non-cancer death in PCNSL patients and mostly occurred within the first year of diagnosis. Age over 60s and diagnosis in 2000–2008 were significantly associated with the elevated risk of CVD in PCNSL patients, while chemotherapy and radiotherapy play no role on the cardiovascular outcomes. Compared with ECNSL patients, the risk of CVD in PCNSL patients were 40% approximately lower. The risk of CVD in the patients with PCNSL still remains unclear currently. Clinicians ought to pay more attention on the risk of CVD in PCNSL patients, especially the elder patients within the first year of diagnosis.
Keywords: primary central nervous system lymphoma, cardiovascular death, SEER database, propensity score matching, risk factor
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and highly aggressive non-Hodgkin Lymphoma (NHL) that involves the brain, eyes, spinal cord, and cerebrospinal fluid. Although the response rate of PCNSL treatment has been ascending over the past decades, the long-term prognosis of PCNSL patients still remains poor due to the delayed treatment-related complications (1).
Cardiovascular death (CVD) has been regarded as one of the most common late complications of cancer therapy (2, 3). Despite a number of studies demonstrating chemotherapeutic drugs and radioactive exposure are related to the elevation on risk of CVD in the patients with NHL (4–6), research specifically on the cardiovascular outcomes in PCNSL patients has rarely been seen. However, the risk of CVD in PCNSL patients requires our further inspections because of the disparate characteristics of PCNSL differing from other NHL.
Firstly, PCNSL originates from intracranial site with blood brain barrier, leading to the poor access and efficacy in the brain for the chemotherapeutic drugs used in NHL (7, 8). As a result, while patients with NHL are typically treated with rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) chemotherapy regimen (9), high-dose methotrexate (HD-MTX) based chemotherapy is recommended as the first-line treatment among the individuals with PCNSL (10). In addition, it has been reported that radiation to head and neck was an independent risk factor of ischemic cerebrovascular disease (11–13). What’s more, the median age at diagnosis of PCNSL is 67 years (14), which is older than 54 years in NHL (15), implying that over half of the patients with PCNSL are the elderly and this proportion seems to be enlarging in recent decades (16).
Therefore, considering the disparities in chemotherapy standard, radiotherapy-induced cerebrovascular risk and median age at diagnosis, patients with PCNSL might be suffering from a greater hazard of dismal cardiovascular prognosis.
To date, the clinical outcomes of CVD in the patients with PCNSL still remains unknown. Hence, there is an urgent necessity to differentiate the risk of CVD in the patients with PCNSL from other with NHL, in order to provide more precise references for clinicians on clinical decision making. This study aims to carry out an analysis on cardiovascular outcomes of CVD in the PCNSL patients and its comparison with the extra central nervous system lymphoma (ECNSL).
Materials and Methods
Data Source
The cases used in this retrospective cohort study were retrieved from the Surveillance, Epidemiology and End Results (SEER) database, consisting of 18 nationwide cancer registries and covering 35% of the population in the United State (17). All the data were downloaded from the SEER*Stat software (version 8.3.6).
Study Population
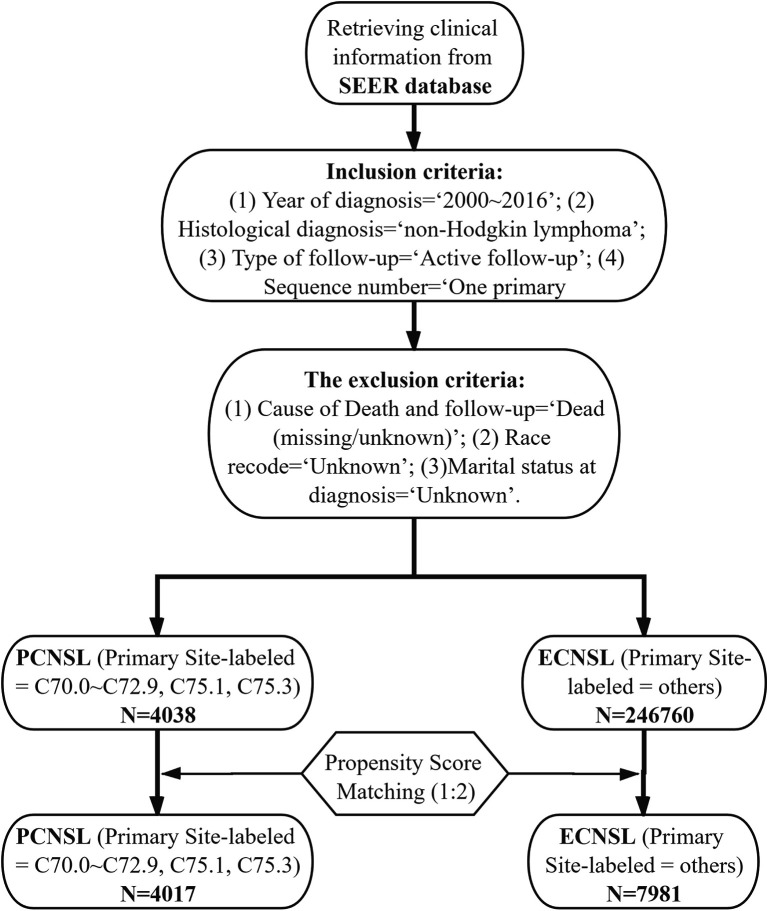
The inclusion criteria were as follows: (1) Year of diagnosis = “2000~2016”; (2) Histological diagnosis = “non-Hodgkin lymphoma”; (3) Type of follow-up = “Active follow-up”; (4) Sequence number = “One primary only”. The exclusion criteria were as follows: (1) Cause of Death and follow-up = “Dead (missing/unknown)”; (2) Race recode = “Unknown”; (3) Marital status at diagnosis = “Unknown”.
Patient Variables and Outcomes
Subjects were classified into two groups by primary site: PCNSL (Primary Site-labeled = C70.0~C72.9, C75.1, C75.3) and ECNSL (Primary Site-labeled = others). Patient variables comprised age at diagnosis (<60, ≥60), sex (male, female), race (white, black, others), marital status (married, unmarried), histology (defuse large B-cell lymphoma, others), chemotherapy (yes, no/unknown), radiotherapy (yes, no/unknown), surgery (yes, no/unknown), and year of diagnosis (2000–2008, 2009–2016). Subject follow-up outcomes included being alive, cancer-related death, CVD, and other death. As recorded in SEER database, CVD includes disease of heart, hypertension without heart disease, cerebrovascular disease, atherosclerosis, aortic aneurysm and dissection, and other diseases of arteries, arterioles, and capillaries. CVD-specific survival was defined as the period from the date of diagnosis to the patient’s death due to CVD.
Statistical Analysis
Baseline characteristics were compared with Chi-square Test for the categorical variables. Fine and Gray’s competing-risks regression was used for univariate and multivariate analyses to assess the risk factors of CVD in the patients with PCNSL. Endpoint was defined as CVD and competing event was defined as non-CVD death.
A 1:2 Propensity Score Matching (PSM) which was computed with logistic regression was to reduce the imbalanced baseline characteristics between PCNSL group and ECNSL group. All the variables were enrolled into the propensity score calculation: age at diagnosis, sex, race, marital status, histology, chemotherapy, radiotherapy, surgery, and year of diagnosis. The match was conducted using nearest-neighbor algorithm with caliper width of 0.02. A P value over 0.05 was regarded as an acceptable balance. Before and after the PSM, we applied the Fine and Gray’s competing-risks regression to the comparison on the hazard of CVD between PCNSL and ECNSL. The CVD-specific cumulative incidence was displayed by using cumulative curves.
Stata/MP software (version 14.0) was used to perform the competing-risks regression. R software (version 3.6.1) was utilized to carried out the PSM. Hazard ratio (HR) were reported within ninety-five percent confidence interval (95% CI). Difference was defined as statistical significance if a two-tailed P value was less than 0.05.
Results
Baseline Characteristics Before and After PSM
A total number of 2,507,898 cases of NHL were retrieved from the SEER database, consisting of 4,038 cases of PCNSL and 246,760 cases of ECNSL. The median follow-up time was 11 months. Among the patients with PCNSL, age over 60 took up the major part (2,387, 59.1%) and most of them were defuse large B-cell lymphoma (DLBCL) (3,214, 79.6%) and white (3,210, 79.5%). The median age of PCNSL patients was 63 years. As for treatments, while 2,715 (67.2%) patients received chemotherapy, only 1,437 (35.6%) patients received radiotherapy. Over a half (2,304, 57.1%) patients did not underwent surgery. After the 1:2 PSM, all the baseline characteristics were well balanced (P > 0.05) between 4,017 cases of PCNSL and 7,981 cases of ECNSL ( Table 1 ), ( Figure 1 ).
Table 1.
Baseline characteristics of PCNSL and ECNSL before PSM and after PSM.
| Variable | Before PSM (N/%) | After PSM (N/%) | ||||
|---|---|---|---|---|---|---|
| PCNSL | ECNSL | P value | PCNSL | ECNSL | P value | |
| N | 4038 (1.6%) | 246,760 (98.4%) | 4,017 (33.5%) | 7,981 (66.5%) | ||
| Age at diagnosis | 0.570 | 0.100 | ||||
| <60 | 1,651 (40.9%) | 99,799 (40.4%) | 1,637 (40.8%) | 3,128 (39.2%) | ||
| ≥60 | 2,387 (59.1%) | 146,961 (59.6%) | 2,380 (59.2%) | 4,853 (60.8%) | ||
| Sex | 0.003 | 0.784 | ||||
| Male | 2,125 (52.6%) | 135,728 (55.0%) | 2,112 (52.6%) | 4,175 (52.3%) | ||
| Female | 1,913 (47.4%) | 111,032 (45.0%) | 1,905 (47.4%) | 3,806 (47.7%) | ||
| Race | <0.001 | 0.961 | ||||
| White | 3,210 (79.5%) | 204,478 (82.9%) | 3,207 (79.8%) | 6,378 (79.9%) | ||
| Black | 345 (8.5%) | 25,581 (10.4%) | 335 (8.3%) | 654 (8.2%) | ||
| Others# | 483 (12.0%) | 16,701 (6.8%) | 475 (11.8%) | 949 (11.9%) | ||
| Marital Status | 0.006 | 0.503 | ||||
| Married | 2,357 (58.4%) | 138,678 (56.2%) | 2,351 (58.5%) | 4,620 (57.9%) | ||
| Unmarried | 1,681 (41.6%) | 108,082 (43.8%) | 1,666 (41.5%) | 3,361 (42.1%) | ||
| Histology | <0.001 | 0.849 | ||||
| DLBCL | 3,214 (79.6%) | 56,589 (22.9%) | 3,193 (79.5%) | 6,332 (79.3%) | ||
| Others$ | 824 (20.4%) | 190,171 (77.1%) | 824 (20.5%) | 1,649 (20.7%) | ||
| Chemotherapy | <0.001 | 0.506 | ||||
| Yes | 2,715 (67.2%) | 145,937 (59.1%) | 2,715 (67.6%) | 5,346 (67.0%) | ||
| No/Unknown | 1,323 (32.8%) | 100,823 (40.9%) | 1,302 (32.4%) | 2,635 (33.0%) | ||
| Radiotherapy | <0.001 | 0.234 | ||||
| Yes | 1,437 (35.6%) | 39,315 (15.9%) | 1,416 (35.3%) | 2,726 (34.2%) | ||
| No/Unknown | 2,601 (64.4%) | 207,445 (84.1%) | 2,601 (64.7%) | 5,255 (65.8%) | ||
| Surgery | <0.001 | 0.993 | ||||
| Yes | 1,734 (42.9%) | 49,024 (19.9%) | 1,718 (42.8%) | 3,414 (42.8%) | ||
| No/Unknown | 2,304 (57.1%) | 197,736 (80.1%) | 2,299 (57.2%) | 4,567 (57.2%) | ||
| Year of diagnosis | <0.001 | 0.228 | ||||
| 2000-2008 | 2,046 (50.7%) | 133,840 (54.2%) | 2,037 (50.7%) | 3,954 (49.5%) | ||
| 2009-2016 | 1,992 (49.3%) | 112,920 (45.8%) | 1,980 (49.3%) | 4,027 (50.5%) | ||
#Other races include American Indian/Alaska Native, Asian/Pacific Islander.
$Other histologies include precursor non-Hodgkin lymphoma (B-cell), chronic/small/prolymphocytic/mantle B-cell non-Hodgkin lymphoma, lymphoplasmacytic lymphoma/waldenstrom, Burkitt lymphoma/leukemia, marginal-zone lymphoma, follicular lymphoma, hairy-cell leukemia, plasma cell neoplasms, heavy chain disease, non-Hodgkin lymphoma (B-cell, not otherwise specified), non-Hodgkin lymphoma (T-cell), non-Hodgkin lymphoma (unknown lineage).
PSM, Propensity Score Matching; PCNSL, Primary Central Nerve System Lymphoma; ECNSL, Extra Central Nerve System Lymphoma; DLBCL, Diffuse Large B-Cell Lymphoma.
Bold values mean statistical significance (P < 0.05).
Figure 1.
Flowchart outlining the process of inclusion, exclusion, and PSM.
Cause-Specific Mortality
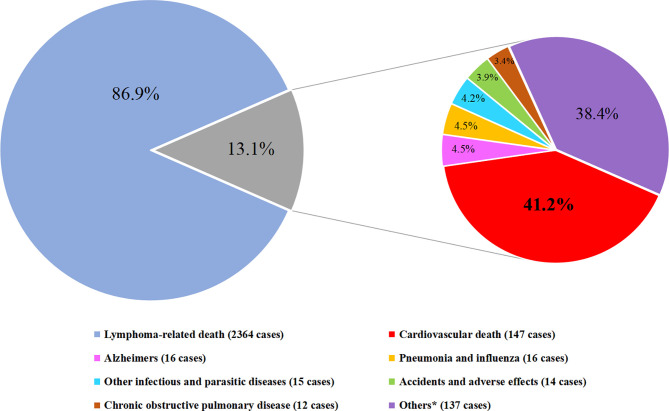
Of those 2,721 PCNSL patients who end with demise, 2,364 (86.9%) were dead due to cancer-related death and the remaining 357 (13.1%) died of non-cancer death, among which CVD accounted for 147 (41.2%) deaths, being the principal cause of non-cancer death ( Figure 2 ).
Figure 2.
Cause of death distribution in PCNSL patients. (Cardiovascular deaths include diseases of heart, cerebrovascular diseases, hypertension without heart disease, atherosclerosis. Others* include nephritis, nephrotic syndrome and nephrosis, diabetes mellitus, septicemia, chronic liver disease and cirrhosis, stomach and duodenal ulcers, congenital anomalies, symptoms, signs and III-defined conditions, suicide and self-inflicted injury, and other cause of death).
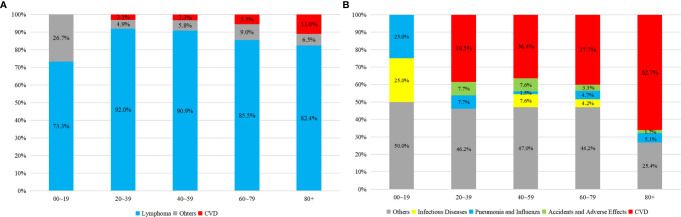
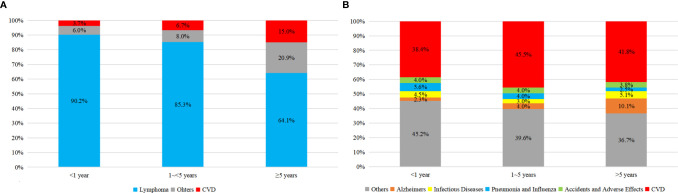
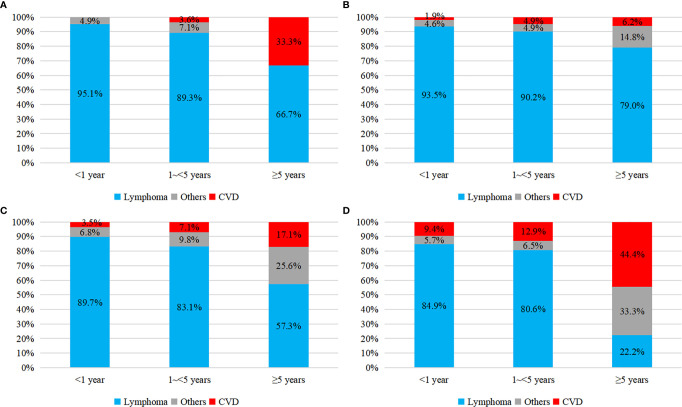
As shown in Figure 3 , there was an obvious growing trend of CVD proportion, from 3.1 to 11.0% among all causes of death and from 38.5 to 62.7% among non-cancer death, as the age of PCNSL patients increasing. Compared to the earlier survival time after the diagnosis of PCNSL, the percentage of CVD was enlarging in latter survival time, from 3.7 to 15% among all causes of death and from 38.4 to 41.8% among non-cancer death, respectively ( Figure 4 ). In different age subgroups (20~39, 40~59, 60~79, 80+), all CVDs demonstrated an increasing tendency. Furthermore, in the subgroup of age 80+, the proportion of CVD overtook that of cancer-related death after 5 years follow-up time ( Figure 5 ).
Figure 3.
Proportion of causes of death in PCNSLs by different age subgroup. (A) all causes of death, (B) non-cancer death.
Figure 4.
Proportion of causes of death in PCNSLs by different survival time. (A) all causes of death, (B) non-cancer death.
Figure 5.
Proportion of all causes of death in PCNSLs by different survival time. (A) age at 20~39, (B) age at 40~59, (C) age at 60~79, (D) age at 80+.
Throughout the analysis period, 68 (46.3%) CVD took place within the first year of diagnosis, 46 (31.3%) CVD occurred between the first and the fifth years, and the remaining 33 (22.4%) CVD arose 5 years later. Heart disease was the most frequent (99, 67.3%) CVD among patients with PCNSL and this proportion kept enlarging slightly, followed by cerebrovascular disease (38, 25.9%) and hypertension (9, 6.1%), with only 1 (0.7%) atherosclerosis death ( Table 2 ).
Table 2.
Cardiovascular specific deaths in PCNSL patients by the year after diagnosis.
| Cause of death | ≤1 year (N, %) | 1–5 years (N, %) | ≥5 years (N, %) |
|---|---|---|---|
| Heart Disease | 44 (64.7%) | 31 (67.4%) | 24 (72.7%) |
| Cerebrovascular Diseases | 18 (26.5%) | 13 (28.3%) | 7 (21.2%) |
| Hypertension without Heart Disease | 5 (7.3%) | 2 (4.3%) | 2 (6.1%) |
| Atherosclerosis | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) |
| Total | 68 (100%) | N = 46 (100%) | N = 33 (100%) |
Bold values mean statistical significance (P < 0.05).
Competing-Risks Regression Analyses of CVD in PCNSL Patients
As shown in Table 3 , PCNSL patients over 60 were at 2.8-fold higher risk of CVD (HR = 2.833, 95% CI = 1.857–4.323, P < 0.001) and those diagnosed in 2009–2016 were related to a lower risk of CVD (HR = 0.592, 95% CI = 0.409–0.858, P = 0.006). Specifically, a significant decreased risk of CVD was observed in the patients with chemotherapy (HR = 0.662, 95% CI = 0.464–0.944, P = 0.023) in univariate analysis, but not in multivariate analysis (HR = 0.751, 95% CI = 0.525–1.074, P = 0.117). Other variables, including marital status, radiotherapy, surgery, histology, race, and sex, played no role on the risk of CVD.
Table 3.
Competing-risks regression analyses of CVD in PCNSL patients.
| Variate | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis | <0.001 | <0.001 | ||
| <60 | Reference | Reference | ||
| ≥60 | 2.759 (1.810–4.208) | 2.833 (1.857–4.323) | ||
| Sex | 0.463 | |||
| Male | Reference | —— | ||
| Female | 1.137 (0.807–1.603) | —— | ||
| Race | 0.507 | |||
| White | Reference | —— | ||
| Black | 0.635 (0.297–1.356) | 0.240 | —— | |
| Others# | 0.935 (0.544–1.605) | 0.807 | —— | |
| Marital Status | 0.512 | |||
| Unmarried | Reference | —— | ||
| Married | 0.890 (0.630–1.259) | —— | ||
| Histology | 0.800 | |||
| DLBCL | Reference | —— | ||
| Others$ | 1.055 (0.699–1.591) | —— | ||
| Chemotherapy | 0.023 | 0.117 | ||
| Yes | Reference | Reference | ||
| No/Unknown | 0.662 (0.464–0.944) | 0.751 (0.525–1.074) | ||
| Radiotherapy | 0.692 | |||
| Yes | Reference | —— | ||
| No/Unknown | 1.073 (0.758–1.519) | —— | ||
| Surgery | 0.563 | |||
| Yes | Reference | —— | ||
| No/Unknown | 0.903 (0.638–1.277) | —— | ||
| Year of diagnosis | 0.015 | 0.006 | ||
| 2000–2008 | Reference | Reference | ||
| 2009–2016 | 0.634 (0.438–0.916) | 0.592 (0.409–0.858) | ||
#Other races include American Indian/Alaska Native, Asian/Pacific Islander.
$Other histologies include precursor non-Hodgkin lymphoma (B-cell), chronic/small/prolymphocytic/mantle B-cell non-Hodgkin lymphoma, lymphoplasmacytic lymphoma/waldenstrom, Burkitt lymphoma/leukemia, marginal-zone lymphoma, follicular lymphoma, hairy-cell leukemia, plasma cell neoplasms, heavy chain disease, non-Hodgkin lymphoma (B-cell, not otherwise specified), non-Hodgkin lymphoma (T-cell), non-Hodgkin lymphoma (unknown lineage).
HR, Hazard ratio; CI, Confidence Interval; DLBCL, Diffuse Large B-Cell Lymphoma.
Bold values mean statistical significance (P < 0.05).
Comparative Hazard of CVD Between PCNSL and ECNSL
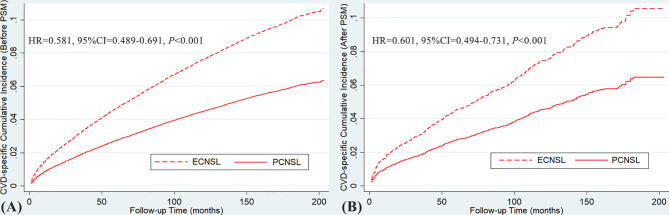
Before and after the 1:2 PSM, the risk of CVD among PCNSL patients were both approximately 40% lower compared with ECNSL patients (before PSM: HR = 0.581, 95% CI = 0.489–0.691, P < 0.001; after PSM: HR = 0.601, 95% CI = 0.494–0.731, P < 0.001). As illustrated in Figure 6 , the cumulative incidence curve of CVD in PCNSL patients was beneath the curve for ECNSL patients, indicating minor incidence of CVD within the analysis period.
Figure 6.
(A) CVD-specific cumulative incidence over time in PCNSL and ECNSL before PSM. (B) CVD-specific cumulative incidence over time in PCNSL and ECNSL after PSM. (Before PSM: HR = 0.581, 95% CI = 0.489–0.691, P < 0.001; after PSM: HR = 0.601, 95% CI = 0.494–0.731, P < 0.001).
Discussion
In our perspective, this study offers insight into the cardiovascular outcomes in the PCNSL patients.
Principal Findings
In this multi-registry based cohort study consisting of 4,038 subjects, we found that age over 60 and diagnosed year in 2000–2008 were in correlation with the higher risk of CVD. Among all the causes of non-cancer death in PCNSL patients, CVD was the leading causation, accounting for 147 (41.2%) demises. The majority of CVD occurred within the first year and heart disease was the most frequent CVD. In addition, in the subgroup by no matter age period or survival time, CVD all demonstrated an ascending trend. It is worth noting that in the subgroup of age 80+, the proportion of CVD even exceeded that of cancer-related death after 5 years follow-up time. This result is similar to the previous study. A SEER-based research published on European Heart Journal showed that after 12 years of diagnosis, the cumulative mortality of heart disease even exceeded that of cancer itself in the breast patients with age 75+ (18). Besides, the patients with PCNSL were at a 40% lower risk of CVD compared with those with ECNSL. These results are demanding for more attention to the risk of CVD in the patients with PCNSL, especially in the elder with poor cardiac condition within the first year. With these information, clinical workers can be better aware of cardiovascular risk and consciously detect the early changes in the PCNSL patients’ cardiovascular condition in time, especially those in poor cardiac condition within the first year of diagnosis. Applying these information into clinical practice might greatly reduce the non-cancer death in PCNSL patients and improve the quality of their life.
Risk Factors of CVD
In the investigation of risk factors, we utilized the competing-risk regression, which is considered as a more suitable and accurate method when estimating the incidence of competing events, rather than using the Kaplan-Meier survival function (19).
Age and Year of Diagnosis
The median age of PCNSL patients was 63, which is close to the result of the previous study (20). Our result showed that the patients over 60 were suffering from more than twice as risk of CVD as their counterpart. This result conformed with the findings by Husam et al. who demonstrated that cardiovascular mortality was higher in older breast cancer patients (21). Study from Soisson et al. also showed that endometrial cancer survivors older than 60 years had an increased risk of cardiovascular diseases (22). These results call for the adequate attention to cardiovascular prevention and surveillance for the elder patients. The PCNSL patients diagnosed in latter period (2009–2016) was significantly related to lower risk of CVD compared with previous period (2000–2008) and this might be attributed to the advancement of cardiovascular detection and management over the years (23–25).
Chemotherapy
Chemotherapy seems to be a favorable factor of CVD in the univariate analysis, whereas not in the multivariate analysis after enrolling age at diagnosis and year of diagnosis. This inconsistency might be due to the treatment bias on ages: patients younger than 60 were more likely to receive and tolerate the chemotherapeutic regimen because of their better cardiovascular condition, while those over 60 presumably had more contraindications to chemotherapy as a result of dismal cardiovascular comorbidity. Thus, after the adjustment of age of diagnosis, the treatment bias was amended in multivariate analysis.
HD-MTX has been recommended as an indispensable agent in the chemotherapeutic regimen by the clinical guideline (26). Interestingly, some studies indicated that methotrexate was in correlation with reduced cardiovascular risks (27), while some others demonstrated it was not (28). In our study, the cardiovascular risks of chemotherapy in PCNSL patients might be subjected to methotrexate to a large extent, suggesting that methotrexate has no impact on the cardiovascular risks. Nonetheless, it should be notable that the specific chemotherapeutic agent, the dosage, and the courses were not recorded in the database. The effect of methotrexate on the cardiovascular risk still requires more clinical trials.
Radiotherapy
In the previous studies, radiation to head and neck was found to be an independent risk factor of ischemic cerebrovascular disease (11–13), whereas the increased cardiovascular risks in the individuals receiving radiotherapy were not observed in our results. This discrepancy might be attributed to the various distribution of radiotherapy dosage across the brain. As reported by some studies (29, 30), only when radiation in the vicinity of the Willis circle arteries will it be a significant risk factor of radiation-associated CVD, otherwise the radiation to other brain structures appeared to play no significant role in the risk of CVD. Similarly, according to N. Haddy et al. (31) studying on the relationship between brain radiation dose and cerebrovascular mortality, the only risk factor was the radiation dose (>30 Gy) received by prepontine cistern, which is an area where Willis circle arteries locates. Nevertheless, information on radiation dosage and method were absent in SEER data and the exact reason of this results still remains unknown.
Comparison on the Risk of CVD Between PCNSL and ECNSL
After the 1:2 PSM, all the clinical variables, including age, sex, histology, chemotherapy, radiotherapy, and surgery, were well-balanced, which avoided the selection biases as much as possible and allowed the data to be more comparable. The relative risk of CVD in PCNSL patients was 40% lower compared with that in ECNSL patients before and after the PSM. We hypothesized that this disparity might be caused by the different chemotherapeutic drugs for PCNSL and ECNSL. R-CHOP regimen, standard treatment for ECNSL, were confirmed that was significantly associated with elevated risk of congestive heart failure and other cardiovascular diseases (4, 5). Apart from chemotherapy, some confounding variables might potentially also account for the relative risk of CVD, such as cardiovascular comorbidity, diabetes, long-term medicine taking, smoking, alcoholism, etc. Unfortunately, these information were not available in the SEER database and therefore a further analysis was restricted. Such intriguing result calls for more detailed clinical studies.
Limitations
Several limitations should be noted due to the inherent drawbacks of a retrospective study. The incomplete information of chemotherapy and radiotherapy, such as dosage, drugs, and courses, limited us from a further investigation into these therapeutic risks of CVD. In addition, the SEER database lacked the cardiovascular comorbidity of patients at baseline characteristics, which might result in the potential biases on cardiovascular outcomes. Moreover, we had no access to the profile of other recognized risk factors of CVD, encompassing smoking, hypertension, diabetes, etc. Despite these shortcomings mentioned above, the methods applied in the analyses were rigorously designed and the results still provide certain referential value on cardiovascular outcomes.
Conclusion
In conclusion, this is a study providing insights into the risk of CVD in the patients with PCNSL. CVD was the leading cause of non-cancer death in PCNSL patients. The proportion of CVD demonstrated an increasing trend among PCNSL patients by different age period and survival time. Thus, clinicians ought to pay more attention on the risk of CVD in PCNSL patients, especially the elder patients within the first year of diagnosis. Chemotherapy and radiotherapy were not relevant with the cardiovascular outcomes of PCNSL patients, which might mitigate the concern of the treatment-induced cardiovascular risk. Compared with ECNSL patients, a more favorable cardiovascular outcome was demonstrated in PCNSL patients. More clinical trials are required to further inspect the risk of CVD in the patients with PCNSL.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data-software/.
Ethics Statement
Ethical approval was not required for publicly available information.
Author Contributions
ZQ, YC, and XX designed the study. ZQ, MS, and XW downloaded, analyzed, and interpreted the data. ZQ, YT, and YJ prepared the draft manuscript. YC and XX provided the critical revisions. All authors contributed to the article and approved the submitted version and agreed with the order of presentation of the authors.
Funding
This study was funded by the Special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovation. (“Climbing Program” Special Funds) (pdjh2021a0407 and pdjh2021b0416).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.691038/full#supplementary-material
References
- 1. Grommes C, DeAngelis LM. Primary CNS Lymphoma. J Clin Oncol (2017) 35(21):2410–8. 10.1200/JCO.2017.72.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of Death Among Cancer Patients. Ann Oncol (2017) 28(2):400–7. 10.1093/annonc/mdw604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cautela J, Lalevée N, Ammar C, Ederhy S, Peyrol M, Debourdeau P, et al. Management and Research in Cancer Treatment-Related Cardiovascular Toxicity: Challenges and Perspectives. Int J Cardiol (2016) 224:366–75. 10.1016/j.ijcard.2016.09.046 [DOI] [PubMed] [Google Scholar]
- 4. Baech J, Hansen SM, Lund PE, Soegaard P, Brown PN, Haaber J, et al. Cumulative Anthracycline Exposure and Risk of Cardiotoxicity; a Danish Nationwide Cohort Study of 2440 Lymphoma Patients Treated With or Without Anthracyclines. Br J Haematol (2018) 183(5):717–26. 10.1111/bjh.15603 [DOI] [PubMed] [Google Scholar]
- 5. Hershman DL, McBride RB, Eisenberger A, Tsai WY, Grann VR, Jacobson JS, et al. Doxorubicin, Cardiac Risk Factors, and Cardiac Toxicity in Elderly Patients With Diffuse B-Cell Non-Hodgkin’s Lymphoma. J Clin Oncol (2008) 26(19):3159–65. 10.1200/JCO.2007.14.1242 [DOI] [PubMed] [Google Scholar]
- 6. Lee SF, Luque–Fernandez MA, Chen YH, Catalano PJ, Chiang CL, Wan EY, et al. Doxorubicin and Subsequent Risk of Cardiovascular Diseases Among Survivors of Diffuse Large B-cell Lymphoma in Hong Kong. Blood Adv (2020) 4(20):5107–17. 10.1182/bloodadvances.2020002737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mead GM, Bleehen NM, Gregor A, Bullimore J, Shirley D, Rampling RP, et al. A Medical Research Council Randomized Trial in Patients With Primary Cerebral Non-Hodgkin Lymphoma: Cerebral Radiotherapy With and Without Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Chemotherapy. Cancer (2000) 89(6):1359–70. [DOI] [PubMed] [Google Scholar]
- 8. Schultz C, Bleehen NM, Gregor A, Bullimore J, Shirley D, Rampling RP, et al. Preirradiation Chemotherapy With Cyclophosphamide, Doxorubicin, Vincristine, and Dexamethasone for Primary CNS Lymphomas: Initial Report of Radiation Therapy Oncology Group Protocol 88-06. J Clin Oncol (1996) 14(2):556–64. 10.1200/JCO.1996.14.2.556 [DOI] [PubMed] [Google Scholar]
- 9. Ansell SM. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc (2015) 90(8):1152–63. 10.1016/j.mayocp.2015.04.025 [DOI] [PubMed] [Google Scholar]
- 10. Grommes C, Rubenstein JL, DeAngelis LM, Ferreri AJM, Batchelor TT, et al. Comprehensive Approach to Diagnosis and Treatment of Newly Diagnosed Primary CNS Lymphoma. Neuro Oncol (2019) 21(3):296–305. 10.1093/neuonc/noy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Bruin ML, Dorresteijn LD, van't Veer MB, Krol AD, van der Pal HJ, Kappelle AC, et al. Increased Risk of Stroke and Transient Ischemic Attack in 5-Year Survivors of Hodgkin Lymphoma. J Natl Cancer Inst (2009) 101(13):928–37. 10.1093/jnci/djp147 [DOI] [PubMed] [Google Scholar]
- 12. Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-Occurring Stroke Among Long-Term Survivors of Childhood Leukemia and Brain Tumors: A Report From the Childhood Cancer Survivor Study. J Clin Oncol (2006) 24(33):5277–82. 10.1200/JCO.2006.07.2884 [DOI] [PubMed] [Google Scholar]
- 13. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke Among Cancer Patients. Nat Commun (2019) 10(1):5172. 10.1038/s41467-019-13120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiels MS, Pfeiffer RM, Besson C, Clarke CA, Morton LM, Nogueira L, et al. Trends in Primary Central Nervous System Lymphoma Incidence and Survival in the U.S. Br J Haematol (2016) 174(3):417–24. 10.1111/bjh.14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nair R, Arora N, Mallath MK. Epidemiology of Non-Hodgkin’s Lymphoma in India. Oncology (2016) 91 Suppl 1:18–25. 10.1159/000447577 [DOI] [PubMed] [Google Scholar]
- 16. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ, et al. Age, Gender, and Racial Differences in Incidence and Survival in Primary CNS Lymphoma. Br J Cancer (2011) 105(9):1414–8. 10.1038/bjc.2011.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. https://seer.cancer.gov/about/overview.html.
- 18. Weberpals J, Jansen L, Müller OJ, Brenner H. Long-Term Heart-Specific Mortality Among 347 476 Breast Cancer Patients Treated With Radiotherapy or Chemotherapy: A Registry-Based Cohort Study. Eur Heart J (2018) 39(43):3896–903. 10.1093/eurheartj/ehy167 [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation (2016) 133(6):601–9. 10.1161/CIRCULATIONAHA.115.017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niparuck P, Boonsakan P, Sutthippingkiat T, Pukiat S, Chantrathammachart P, Phusanti S, et al. Treatment Outcome and Prognostic Factors in PCNSL. Diagn Pathol (2019) 14(1):56. 10.1186/s13000-019-0833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abdel-Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol (2017) 2(1):88–93. 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 22. Soisson S, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, et al. Long-Term Cardiovascular Outcomes Among Endometrial Cancer Survivors in a Large, Population-Based Cohort Study. J Natl Cancer Inst (2018) 110(12):1342–51. 10.1093/jnci/djy070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yousuf O, Chrispin J, Tomaselli GF, Berger RD. Clinical Management and Prevention of Sudden Cardiac Death. Circ Res (2015) 116(12):2020–40. 10.1161/CIRCRESAHA.116.304555 [DOI] [PubMed] [Google Scholar]
- 24. McCullough PA. Coronary Artery Disease. Clin J Am Soc Nephrol (2007) 2(3):611–6. 10.2215/CJN.03871106 [DOI] [PubMed] [Google Scholar]
- 25. Lancellotti P, Suter TM, López-Fernández T, Galderisi M, Lyon AR, Van der Meer P, et al. Cardio-Oncology Services: Rationale, Organization, and Implementation. Eur Heart J (2019) 40(22):1756–63. 10.1093/eurheartj/ehy453 [DOI] [PubMed] [Google Scholar]
- 26. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, et al. Diagnosis and Treatment of Primary CNS Lymphoma in Immunocompetent Patients: Guidelines From the European Association for Neuro-Oncology. Lancet Oncol (2015) 16(7):e322–32. 10.1016/S1470-2045(15)00076-5 [DOI] [PubMed] [Google Scholar]
- 27. Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, et al. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am J Cardiol (2011) 108(9):1362–70. 10.1016/j.amjcard.2011.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med (2019) 380(8):752–62. 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aizer AA, Du R, Wen PY, Arvold ND. Radiotherapy and Death From Cerebrovascular Disease in Patients With Primary Brain Tumors. J Neurooncol (2015) 124(2):291–7. 10.1007/s11060-015-1839-5 [DOI] [PubMed] [Google Scholar]
- 30. El-Fayech C, Haddy N, Allodji RS, Veres C, Diop F, Kahlouche A, et al. Cerebrovascular Diseases in Childhood Cancer Survivors: Role of the Radiation Dose to Willis Circle Arteries. Int J Radiat Oncol Biol Phys (2017) 97(2):278–86. 10.1016/j.ijrobp.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 31. Haddy N, Mousannif A, Tukenova M, Guibout C, Grill J, Dhermain F, et al. Relationship Between the Brain Radiation Dose for the Treatment of Childhood Cancer and the Risk of Long-Term Cerebrovascular Mortality. Brain (2011) 134(Pt 5):1362–72. 10.1093/brain/awr071 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://seer.cancer.gov/data-software/.