Abstract
STUDY QUESTION
Are reproductive, metabolic or psychological health profiles of women with clinically diagnosed polycystic ovary syndrome (PCOS) different from those with undiagnosed PCOS?
SUMMARY ANSWER
Obtaining a clinical diagnosis of PCOS is strongly linked to the experience of fertility problems, but not clinical depression or poor metabolic health, although these were highly prevalent in women with PCOS irrespective of when they were diagnosed.
WHAT IS KNOWN ALREADY
PCOS is an endocrine disorder that is relative common, but heterogeneous in presentation. This may impact on the pathways to diagnosis and timely treatment.
STUDY DESIGN, SIZE, DURATION
A cross-sectional analysis of a community-based cohort of 974 women, established retrospectively when women were around 30 years of age.
PARTICIPANTS/MATERIALS, SETTING, METHODS
In this cohort of women born in Adelaide, South Australia, half of women who met the Rotterdam criteria for PCOS were previously undiagnosed. We compared women with prior clinical diagnosis of PCOS, those diagnosed through participation in this research, and the remainder in the cohort. Sociodemographic characteristics, reproductive, metabolic and psychological health, including medical conditions and medications were considered. Logistic regression was undertaken to identify independent predictors of prior clinical diagnosis.
MAIN RESULTS AND THE ROLE OF CHANCE
There were 56 women with a prior clinical diagnosis of PCOS (5.7%) and a further 64 (6.6%) were undiagnosed until study entry. The great majority of women with a prior diagnosis of PCOS reported having had problems with periods (95%) and excess body hair (63%). Corresponding proportions for women undiagnosed until study participation were slightly lower (81% and 45%, respectively). Although the proportion of women attempting or achieving pregnancy was similar across all groups, those with a prior diagnosis of PCOS were four times more likely to have reported difficulties becoming pregnant than those undiagnosed (odds ratio = 4.05, 95% CI 1.74–9.45) and frequently sought medical assistance. Metabolic problems were higher in both PCOS groups compared to women without PCOS. In both PCOS groups, the prevalence of clinical depression was 50% higher than in those with no PCOS (P = 0.021).
LIMITATIONS, REASONS FOR CAUTION
The number of women who were diagnosed with PCOS both prior to and during the study limited statistical power available to detect modest differences between the PCOS groups. Some women in the group classified as not having PCOS may have remained undiagnosed, but any bias from this source would contribute to more conservative findings.
WIDER IMPLICATIONS OF THE FINDINGS
Findings reinforce the need for early detection of PCOS symptoms from adolescence, ensuring timely diagnosis and appropriate health care. The high prevalence of depression among clinically diagnosed and undiagnosed women with PCOS suggests this is a feature of the condition and supports recent recommendations in the international PCOS guidelines to screen all women with PCOS for depression and anxiety.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by a project grant (2017) from the National Health and Medical Research Council of Australia (NHMRC) Centre for Research Excellence in Polycystic Ovary Syndrome (Grant ID APP1078444). R.C.F. and J.C.A. were supported by Robinson Research Institute Lloyd Cox Career Development Fellowships (2018). Establishment of the cohort was funded by an NHMRC Strategic Award No. 465455, a Career Development Award in Population Health (No. 349548) and the Australian Research Council (Future Fellowship FT100101018) awarded to M.J.D. All authors declared no conflict of interest.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: depression, diagnosis, infertility, polycystic ovary syndrome, reproductive health
Introduction
Polycystic ovary syndrome (PCOS) is a complex endocrine condition, with metabolic, reproductive and psychological sequelae (Escobar-Morreale, 2018). It may affect 6–18% of reproductive-aged women, although prevalence estimates vary by the source population and diagnostic criteria applied (March et al., 2010; Escobar-Morreale, 2018; Witchel et al., 2020).
Cardinal symptoms of PCOS include irregular or absent periods, excessive hair growth and difficulty becoming pregnant (Escobar-Morreale, 2018). These reflect the underlying hormonal imbalance (hyperandrogenaemia) and ovarian dysfunction that are hallmarks of the syndrome. As well, some women with PCOS are susceptible to weight gain and have increased risks of metabolic complications including insulin resistance, diabetes, hypertension and dyslipidaemia (Cooney and Dokras, 2018). Elevated anxiety and depression are consistently reported in women with PCOS (Barry et al., 2011; Karjula et al., 2017), although it is uncertain whether this reflects perturbed endocrinology or distress created by PCOS symptoms, or the stigma of receiving a diagnosis (Karjula et al., 2017; Fernandez et al., 2018).
Historically, most women have not received a diagnosis of PCOS until they sought fertility treatment (Homburg, 1996; Lujan et al., 2008). The development of international diagnostic criteria for PCOS and evidence-based guidelines has led to increased awareness of the syndrome among healthcare providers and women. However, gaining a diagnosis still seems to be problematic as women report that multiple consultations with several different health professionals were often required (Gibson-Helm et al., 2017).
Timely diagnosis of PCOS may enable women to receive support for managing aspects of the condition that affect health, slowing progression of comorbidities. Knowledge of the condition could also be useful for family planning, and promote lifestyle modifications that may reduce the recourse to fertility treatment (Huber-Buchholz et al., 1999; Crosignani et al., 2003). Conversely, it has been argued that diagnosis of women with mild symptoms could constitute unnecessary disease labelling and result in psychological harms through stigmatisation (Copp et al., 2017, 2019).
It is not known whether women who receive a diagnosis of PCOS have a specific symptomology. The inverse care law suggests it might be women who are most economically advantaged (O'Dea and Kilham, 2002; Marmot, 2018), since they are most likely to have the personal and financial resources to consult medical specialists.
In our community-based cohort study, half of women with PCOS were not diagnosed until they participated in the research (March et al., 2010). We therefore have a unique opportunity to examine characteristics of women who were diagnosed through their own encounters with the healthcare system and those who were diagnosed through study participation. The aim of this study was to compare these groups of women, and the remainder of the cohort, in order to identify the sociodemographic and health profiles of women who received an earlier diagnosis.
Materials and methods
Study recruitment/participants/establishment of the cohort
The establishment of the cohort and the study protocol have been published previously (March et al., 2010; Moran et al., 2015; March et al., 2018). Cohort participants were born in 1973–75 in a large maternity hospital in Adelaide, South Australia. Around 30 years later, the 2199 eligible female births were traced. There were 1984 (90.2%) women confirmed still to be living, without any severe impairment, and these were invited to participate by letter and telephone. After acceptance, core first wave data were obtained from 974 (49.1%) women at a median age of 30.2 years.
Participants were broadly representative of all eligible female births at the maternity hospital, although with a slightly greater proportion of mothers from the highest socio-economic category (8.8% vs. 5.5%, derived from postcode of residence (Trewin, 2003)). The sociodemographic profile of participants’ mothers was similar to that of all women giving birth in Adelaide at the time, reflecting the fact that the hospital was then one of only two major maternity hospitals in the city, with a geographically large catchment area.
Study protocol/diagnosis of PCOS
A core set of information was obtained through a structured interview with a trained nurse, usually in person. The interview covered many aspects of women’s lives and medical history, including symptoms of PCOS, whether a clinical diagnosis of PCOS had been obtained, and details of any pregnancies. For women seen in person, height and weight were measured following standard protocols, otherwise self-reports were obtained. All women were invited to provide a fasting blood sample following the interview, for biochemical analysis. Subsequently, women with at least one symptom of PCOS (menstrual dysfunction or hyperandrogenism) were referred to a reproductive medicine clinic for a transvaginal ultrasound. Women who did not report a clinical diagnosis of PCOS before participation in the study were classified as having PCOS if they met the Rotterdam criteria for diagnosis.
Menstrual irregularity was defined as chronic amenorrhoea, or cycle length of less than 21 days or more than 35 days, or variation of more than 4 days between cycles. Hyperandrogenism was based on measurement of free testosterone in the blood sample and/or the modified Ferriman-Gallwey (mF-G) score for hirsutism of >7 (Hatch et al., 1981). Polycystic ovaries (PCO) were identified by the presence of either 12 or more follicles measuring 2–9 mm in diameter, or increased ovarian volume (>10 cm3) in at least one of the ovaries (Balen et al., 2003).
For the present work, the cohort was divided into three comparison groups. The first consisted of women who reported a diagnosis of PCOS before participating in the study (prior diagnosis group). The second group comprised women who were diagnosed through participation in our research and undergoing testing in the first wave of follow-up of the cohort (study diagnosis group), and the third was women who did not receive a diagnosis of PCOS through either of these pathways (no PCOS group). This last group may contain some women with undiagnosed PCOS as not all women with either menstrual irregularity or hyperandrogenism accepted the invitation to have an ultrasound, including 15 of those who reported a prior diagnosis of PCOS. Of the 56 with a prior diagnosis, 33 (59%) fulfilled the criteria for PCOS at the time of the study, a further 15 (27%) may have, and 8 (14%) did not.
Description of explanatory variables
Age of diagnosis was obtained directly from women’s self-report or derived from the reported year of diagnosis. Eight women with a prior PCOS diagnosis did not provide either information, so age was estimated based on reports of when treatment commenced (n = 2) or diagnostic tests were performed (n = 1) or, in the absence of that information, when symptoms were experienced (n = 3). Two women indicated that they were diagnosed after the birth of their last child, so age of diagnosis was estimated as birth year of that child, plus 1 year.
Women reported their ethnicity and educational attainment. An area-level index of socioeconomic status was derived from current postcode of residence (Trewin, 2003). Women’s BMI at the time of interview was calculated as weight (kg) divided by the square of height (m); women were classified as overweight or obese using cut-offs of 25, 30 and 35 kg/m2, respectively. Current BMI was not available for 32 women who were missing height (n = 27) and/or weight (n = 32) measurements. For 19 women, BMI was imputed from self-reported heaviest or lowest weight (ascertained in a specific question) if the corresponding year was at most 2 years before study participation in combination with either measured height or the average height of the cohort (164 cm).
Daily intake of alcohol was classified as none, one, two or more standard drinks. Other health risk factors were classified as present or absent, including daily smoking and ever having a clinical diagnosis of high blood pressure, high cholesterol, high blood glucose levels, diabetes or depression. Current symptoms of depression were assessed using the Center for Epidemiological Studies Depression Scale (CES-D), with a score of 16 or more indicating the presence of clinical depression (Radloff, 1977; Weissman et al., 1977). Current medications were recorded, with use of metformin, antidepressants and asthma medications and thyroxine extracted for this analysis.
As part of the medical histories, women reported the age at which they first menstruated (age of menarche), and whether or not they had ‘ever had problems’ with weight, acne, excess body hair or periods, and whether they had ever used the contraceptive pill to manage period problems. Women were asked whether they had ever been pregnant and, if so, to provide details of all outcomes: number of live births, stillbirths, miscarriages, ectopic pregnancies and terminations. Women who had attempted or achieved pregnancy were asked whether they ever had any difficulty getting pregnant and, if so, to describe the difficulty and whether a healthcare practitioner was consulted.
After the clinical interview, women completed the Multidimensional Body-Self Relations Questionnaire—Appearance Scales (MBSQ-AS). This is a validated 34-item questionnaire assessing body image on five subscales; Appearance Evaluation (feelings about physical attractiveness), Appearance Orientation (extent of investment in one’s appearance), Body Areas Satisfaction Scale (BASS, satisfaction with discrete aspects of one’s appearance), Overweight Preoccupation (fat anxiety and weight vigilance) and Self-Classified Weight (how one perceives one’s weight) (Brown et al., 1990; Cash, 2000). Higher scores on the Appearance Evaluation and Body Areas Satisfaction subscales indicate greater satisfaction with one’s appearance and more positive body image construct. Higher scores on the Appearance Orientation, Overweight Preoccupation and Self-Classified Weight indicate lower satisfaction with one’s appearance or more negative body image construct.
Statistical analysis
The average age of diagnosis for women who reported being diagnosed with PCOS before participation in the study was computed. Age at diagnosis was also categorized in 5-year age bands.
Characteristics of women in three mutually exclusive groups were compared: those with a prior diagnosis of PCOS, those diagnosed through study participation, and those without a diagnosis. Distributions of sociodemographic, health-related and reproductive variables were tabulated. Group differences in categorical variables were assessed using chi-square tests. Continuous variables were presented as means and standard deviations, or medians and interquartile ranges and group differences assessed using Student’s t-test or Mann–Whitney U as appropriate.
For each question in the MBSRQ-AS, participants indicated a score on Likert scales. An average score was computed for each of the five subscales for each woman. Comparison of average scores on each of the subscales were compared across the three groups using one-way ANOVA.
Multivariable logistic regression was undertaken to identify independent predictors of prior diagnosis, presented as odds ratios and 95% CI. Potential predictive variables were included in the model if the P-value was ≤0.20 in univariate analyses comparing the prior and study diagnosis groups. All tests used the 5% significance level. Data analysis was performed using Stata version 14.2.
Ethical approval
The study was approved by the Queen Elizabeth Hospital and University of Adelaide ethics committees (H/36/99, March 2000) and all participants gave written informed consent.
Results
The average age of diagnosis for the 56 women who had received a clinical diagnosis of PCOS before participating in the cohort study was 24.4 years. A prior diagnosis was received at less than 20 years for 9 women (16.1%), at 20–24 years for 16 women (28.6%), 25–29 years for 28 women (50.0%) and 30–35 years for 3 women (5.4%). There were 64 women who received a diagnosis of PCOS through participation in the study (during 2003–2007) at an average age of 30.4 years, range 28.7–32.2 years.
The full distribution of socio-economic status was represented in the cohort (Table I), roughly in proportion to the wider population (from quartile bands). Educational attainment was similar to that of all Australian women in this age group born in the same period (Tunny, 2006). The low number of Asian women is expected in a birth cohort from this era. Although there were no statistically significant differences between the PCOS groups, it is notable that the group with a prior diagnosis had the smallest proportion of women with university qualifications and the largest proportion of women living in the most disadvantaged areas.
Table I.
Demographic, lifestyle and health characteristics of women by polycystic ovary syndrome diagnosis group.
| PCOS diagnosis | |||||
|---|---|---|---|---|---|
| Prior diagnosis (N = 56) | Study diagnosis (N = 64) |
Prior vs Study
P-valuea |
No PCOS (N = 854) | Three-group comparison P-valuea | |
| Age at follow-up | |||||
| Median (IQR) | 30.6 (30.0–31.4) | 30.2 (29.9–30.8) | 0.20 | 30.6 (30.0–31.5) | 0.02 |
| Sociodemographics | n (%) | n (%) | n (%) | ||
| Educational attainment | 0.47 | 0.50 | |||
| Some high school | 19 (33.9) | 24 (37.5) | 253 (29.6) | ||
| Completed high school | 26 (46.4) | 23 (35.9) | 377 (44.2) | ||
| University | 11 (19.6) | 17 (26.6) | 224 (26.2) | ||
| Socio-economic index for area | 0.75 | 0.21 | |||
| Quartile 1 (most disadvantaged) | 19 (33.9) | 17 (26.6) | 188 (22.0) | ||
| Quartile 2 | 14 (25.0) | 20 (31.3) | 212 (24.8) | ||
| Quartile 3 | 11 (19.6) | 11 (17.2) | 236 (27.6) | ||
| Quartile 4 (least disadvantaged) | 12 (21.4) | 16 (25.0) | 218 (25.5) | ||
| Ethnicity | 0.63 | 0.23 | |||
| Asian | 0 (0.0) | 0 (0.0) | 9 (1.1) | ||
| European | 53 (94.6) | 59 (92.2) | 809 (94.7) | ||
| Pacific/aboriginal | 3 (5.4) | 4 (6.3) | 18 (2.1) | ||
| Other/unknown | 0 (0.0) | 1 (1.6) | 18 (2.1) | ||
| Health risk factors | n (%) | n (%) | n (%) | ||
| Current BMIb | 0.67 | <0.001 | |||
| Underweight/normal (<25) | 14 (25.5) | 15 (23.8) | 412 (48.9) | ||
| Overweight (25–<30) | 14 (25.5) | 13 (20.6) | 232 (27.5) | ||
| Obese 1 (30–<35) | 13 (23.6) | 11 (17.5) | 109 (12.9) | ||
| Obese 2/3 (35+) | 14 (25.5) | 24 (38.1) | 90 (10.7) | ||
| Current smoker | 21 (37.5) | 19 (29.7) | 0.37 | 230 (26.9) | 0.22 |
| Current alcoholic drinks per day | |||||
| 0 | 14 (25.0) | 11 (17.2) | 0.53 | 147 (17.4) | 0.09 |
| 1 | 16 (28.6) | 18 (28.1) | 344 (40.6) | ||
| ≥2 | 26 (46.4) | 35 (54.7) | 356 (42.0) | ||
| Medical conditions (ever diagnosed) | |||||
| High blood pressure | 10 (17.9) | 5 (7.8) | 0.10 | 43 (5.0) | <0.001 |
| High cholesterol | 9 (16.1) | 6 (9.4) | 0.27 | 44 (5.2) | 0.002 |
| High blood sugar | 6 (10.7) | 4 (6.3) | 0.38 | 14 (1.6) | <0.001 |
| Diabetes | 0 (0.0) | 1 (1.6) | 0.35 | 5 (0.6) | 0.52 |
| Depression | 18 (32.1) | 20 (31.3) | 0.92 | 175 (20.5) | 0.021 |
| Asthma | 21 (37.5) | 17 (26.6) | 0.20 | 209 (24.0) | 0.092 |
| Thyroid condition | 3 (5.4) | 5 (7.8) | 0.59 | 28 (3.3) | 0.14 |
| Clinical depression from symptom scorec | 26 (51.0) | 29 (50.0) | 0.92 | 235 (29.2) | <0.001 |
| Current medication use | |||||
| Any medication | 27 (48.2) | 20 (31.3) | 0.06 | 226 (26.5) | 0.002 |
| Metformin | 5 (8.9) | 0 (0.0) | 0.02 | 1 (0.1) | <0.001 |
| Antidepressant | 4 (7.4) | 3 (4.7) | 0.57 | 38 (4.5) | 0.65 |
| Asthma medications | 4 (7.1) | 2 (3.1) | 0.31 | 39 (4.6) | 0.57 |
| Thyroxine | 0 (0.0) | 3 (4.7) | 0.11 | 9 (1.1) | 0.03 |
Pearson’s Chi2 test.
BMI data was missing for 13 women (Prior PCOS = 1, Study PCOS = 1, No PCOS = 11).
Center for Epidemiological Studies Depression Scale (CES-D) > 16; score missing for 60 women (Prior PCOS = 5, Study PCOS = 6, No PCOS = 49).
IQR, interquartile range; PCOS, polycystic ovary syndrome.
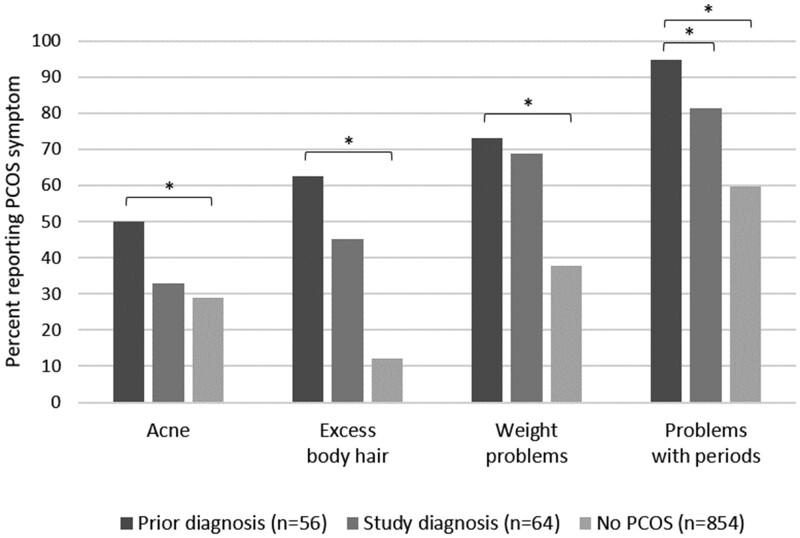
Compared with women diagnosed with PCOS during the study, among those with a prior diagnosis there were more reports of ‘problems with periods’ (P = 0.02) (Fig. 1). Differences between these two groups in reports of ever having problems with acne (P = 0.06) or excess body hair (P = 0.06) were of borderline statistical significance. All comparisons that included the remainder of the cohort as a third group were statistically significant.
Figure 1.
Frequency of self-reported polycystic ovary syndrome (PCOS) symptoms in women by PCOS diagnosis group. *P < 0.05.
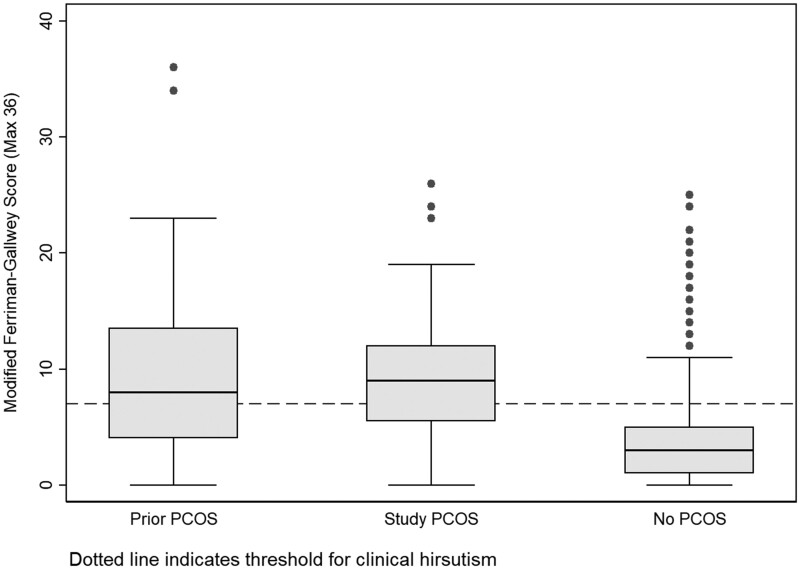
Over half of both groups of women with PCOS had clinical hirsutism (prior diagnosis n = 31, 55.4%, and study diagnosis n = 45, 70.3%). The difference in proportions was not statistically significant (P = 0.09), but box and whisker plots (Fig. 2) suggested that women with the most extreme hirsutism scores may be in the prior diagnosis group. Among women without PCOS, the median hirsutism score was low, although a proportion of this group had clinical hirsutism (n = 127, 14.9%).
Figure 2.
Distribution of the modified Ferriman-Gallwey Score by polycystic ovary syndrome (PCOS) diagnosis group. Dotted line indicates threshold for clinical hirsutism. Upper and lower bounds of the boxes represent the upper (75%) and lower (25%) quartiles. Median values are indicated by the horizontal bar across the boxes. Whiskers indicate the spread of the data, and are calculated as 1.5 × interquartile range above the upper quartile (upper whisker) and below the lower quartile (lower whisker). Points above the box and whisker plots indicate outliers.
Lifestyle variables did not differ substantially between the prior diagnosis and study diagnosis groups at age 27–34 years (Table I) although the proportion of women in the former group that smoked (37.5%) and of the latter group with BMI of at least 35 kg/m2 (38.1%) are noteworthy. While the proportions of the prior diagnosis group with medical conditions and taking medications were consistently higher than those for the study diagnosis group, only the increase for metformin was statistically significant. Half of women in the prior diagnosis group and the study diagnosis group had current symptoms of depression consistent with clinical depression (CES-D > 16). Thus, both groups had relatively poor health profiles compared with those with no PCOS.
By age 35 years, over two-thirds of women in all three groups had attempted or achieved pregnancy (Table II). Among these women, 33 in each of the two PCOS groups (72–73%) had a live birth and 503 (83%) of those without PCOS (P = 0.1). Women diagnosed with PCOS prior to the study were significantly more likely to have experienced difficulties becoming pregnant compared to women diagnosed as part of the study. Of note, 57.8% of the prior diagnosis group consulted a medical practitioner for infertility compared with 19.6% of the study diagnosis group and 9.1% of the no PCOS group; treatment frequently ensued. Aside from use of the oral contraceptive pill to manage period problems, there were no other statistically significant differences in reproductive history and pregnancy outcomes between these groups, although data were sparse for uncommon outcomes; patterns observed in the data suggested that the adverse outcome of pregnancy loss may be elevated among women with PCOS.
Table II.
Reproductive history of women by polycystic ovary syndrome diagnosis group.
| PCOS diagnosis | |||||
|---|---|---|---|---|---|
| Prior diagnosis (N = 56) | Study diagnosis (N = 64) |
Prior vs study
P-valuea |
No PCOS (N = 854) | Three-group comparison P-valuea | |
| n (%) | n (%) | n (%) | |||
| Age of menarche | 0.68 | 0.37 | |||
| <11 years | 3 (5.4) | 4 (6.3) | 32 (3.8) | ||
| 11 to <12 years | 7 (12.5) | 14 (21.9) | 110 (12.9) | ||
| 12 to <13 years | 18 (32.1) | 18 (28.1) | 241 (28.2) | ||
| 13 to <14 years | 15 (26.8) | 13 (20.3) | 231 (27.1) | ||
| ≥14 years | 13 (23.2) | 14 (21.8) | 238 (27.9) | ||
| Ever used oral contraceptive pill to manage period problems | 35 (62.5) | 32 (50.0) | 0.17 | 305 (35.7) | <0.001 |
| Attempted or achieved pregnancy | 45 (80.4) | 46 (71.9) | 0.28 | 614 (71.9) | 0.39 |
| Among those who attempted or achieved pregnancy | N = 45 | N = 46 | N = 614 | ||
| Difficulties becoming pregnant | 32 (71.1) | 17 (37.0) | 0.001 | 114 (18.6) | <0.001 |
| Consulted health practitioner for infertility | 26 (57.8) | 9 (19.6) | <0.001 | 56 (9.1) | <0.001 |
| Pregnancy outcomes, among those ever pregnant | N = 41 | N = 44 | N = 586 | ||
| One or more terminations | 7 (17.1) | 11 (25.0) | 0.37 | 165 (28.2) | 0.29 |
| One or more ectopic pregnancies, miscarriages or stillbirths | 15 (36.6) | 15 (34.1) | 0.81 | 139 (23.7) | 0.07 |
| One or more live births | 33 (80.5) | 33 (75.0) | 0.54 | 503 (85.8) | 0.11 |
Pearson’s Chi2 test.
PCOS, polycystic ovary syndrome.
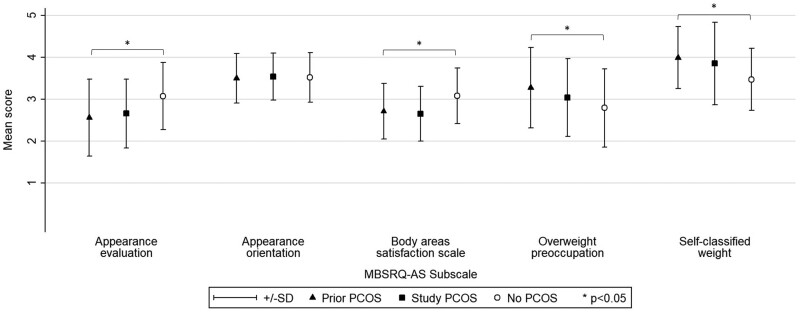
One-way ANOVA analysis of the MBSRQ-AS subscales across the three groups indicated that the mean scores differed significantly (P < 0.001) for all but the Appearance Orientation subscale (Fig. 3). However, the prior and study diagnosis groups did not differ in their mean scores for any of the five MBSRQ-AS subscales.
Figure 3.
Mean and SD of scores for the Multidimensional Body-Self Relational Questionnaire—Appearance Scale components by polycystic ovary syndrome (PCOS) diagnosis group. *P < 0.05.
In the logistic regression analyses, experiencing difficulties becoming pregnant was the only variable that was statistically significant and independently associated with diagnosis of PCOS prior to study entry in comparison with diagnosis through study participation (Table III). Other characteristics that approached statistical significance were self-reported acne and self-reported period problems. Several variables that were potentially associated with prior PCOS diagnosis in univariate analysis (i.e. P ≤ 0.2) could not be included in regression models due to collinearity (consulted health practitioner for infertility) or sparse data (specific medications). Ever using the oral contraceptive pill to manage period problems was entered into the model, but not retained (P = 0.56). Likewise, year of birth was not retained (P = 0.44).
Table III.
Logistic regression analysis of factors associated with gaining a prior diagnosis of polycystic ovary syndrome compared with diagnosed through study participation (n = 120).
| Factor | Odds ratio [95% CI] a |
|---|---|
| Self-reported acne | 2.11 [0.90–4.93] |
| Self-reported excess body hair | 1.67 [0.72–3.87] |
| Self-report weight problems | 0.84 [0.34–2.10] |
| Self-reported period problems | 3.27 [0.81–13.16] |
| Difficulties becoming pregnant | |
| No difficulties/never attempted | Reference |
| Difficulties | 4.05 [1.74–9.45] |
| Ever diagnosed with high blood pressure | 2.98 [0.80–11.10] |
| Any medication use | 1.76 [0.76–4.10] |
Multiple logistic regression analysis, including all variables presented in the table.
Discussion
In this community-based cohort, around 6% of women reported a prior diagnosis of PCOS and a similar proportion were diagnosed through study participation when aged around 30 years. With regard to the pathways to a diagnosis, the great majority of women with a prior diagnosis of PCOS reported having had ‘problems with periods’ including irregular cycles and amenorrhoea, and at least half reported problems with acne and excess body hair. That these proportions were somewhat higher than for women diagnosed through study participation suggests symptom severity and/or concerns about symptoms contributed to gaining a diagnosis. Some support for more severe objective symptoms is provided by the finding that the group with a prior diagnosis of PCOS contained women with the most extreme clinical hirsutism scores.
Commencement of the oral contraceptive pill for irregular menstrual cycles or acne may delay diagnosis of PCOS (Prelevic et al., 1993). Consistent with women’s reports of pill use for period problems, it seems that this occurred quite widely when these women were teenagers and young adults in the late 1980s and through the 1990s. Recent recommendations from the International Evidence-based Guideline for the Assessment and Treatment of PCOS promote the identification of ‘at risk’ girls who should receive follow-up and re-assessment for PCOS at reproductive maturity (3 years post-menarche), whilst also being provided with immediate support and treatment for presenting symptoms (Peña et al., 2020).
The great majority of the group of women with a prior diagnosis of PCOS had attempted or achieved pregnancy. The socio-economic background of this group is consistent with somewhat earlier plans for family formation. Difficulties becoming pregnant were commonly experienced in those desiring parenthood in this group, with over half having sought assistance. Thus it is highly plausible that fertility concerns contributed to clinical contact and a diagnosis of PCOS. Gaining a diagnosis in this way may partly reflect greater knowledge of PCOS among gynaecology and fertility specialists, whereas women with PCOS symptoms other than fertility problems may have consulted healthcare practitioners who were less alert to the possibility. It is noteworthy that around a third of women attempting pregnancy in both PCOS groups had experienced ectopic pregnancy, miscarriage or stillbirth; while not reaching statistical significance, this finding is consistent with other reports for women with PCOS (Bahri Khomami et al., 2019).
Socio-economic advantage did not appear to explain why women did or did not gain a diagnosis of PCOS through their own encounters with the healthcare system, ruling out the inverse care law—at least in Australia which has universal health coverage. The two groups of women with PCOS were in many ways more similar to each other than to the remainder of the cohort, suggesting an arbitrary aspect to gaining a diagnosis. The association between socio-economic status and PCOS is likely to be complicated, if poor psychological health is a feature of the condition as our data suggest, since this affects engagement with schooling, further study, employment and careers (Lagerveld et al., 2010; Finning et al., 2019). A Danish study of socio-economic factors and PCOS considers the further complexity of migration and ethnic background in relation to socio-economic status (Rubin et al., 2019).
The current health profiles of women not diagnosed with PCOS until they participated in the study raise some concerns. Over one third had severe (class 2/3) obesity. That they had fewer diagnosed metabolic conditions compared with those with a prior PCOS diagnosis (among who a quarter had severe obesity) points to lack of appropriate health care, rather than better health.
These findings do not support the argument that remaining undiagnosed avoids harms that may come with a disease label (Copp et al., 2017). To the contrary, irrespective of when the PCOS diagnosis occurred, almost a third of women with PCOS had experienced clinically diagnosed depression, a prevalence 50% higher than for women without PCOS. In addition, half of women with PCOS reported depressive symptoms consistent with current clinical depression (assessed using CES-D, a validated scale), compared with 30% of women without PCOS. This provides evidence that poor psychological health is a feature of the condition, rather than produced by the label, consistent with the findings of Karjula et al. (2017). Furthermore, it suggests that this aspect of health had not received appropriate attention and care in women known to have PCOS. These findings reinforce new recommendations in the international evidence-based PCOS guideline to screen all women with PCOS for depression and anxiety (Jacob and Balen, 2019).
Regardless of when they were diagnosed, women with PCOS scored similarly on the MBSQ-AS subscales for assessing body image. Specifically, feelings about physical attractiveness, extent of investment in one’s appearance, satisfaction with aspects of appearance, anxiety about fatness, vigilance around weight and perception of weight were similar. On all but one subscale (Appearance Orientation), scores for women with PCOS indicated more negative body image compared to women without PCOS. Negative perceptions of body image have been found previously among women with PCOS and are likely to contribute to their poorer psychological health (Deeks et al., 2011; Alur-Gupta et al., 2019) although we emphasise that this does not adequately account for or diminish the seriousness of mental health problems experienced by women with PCOS (Veltman-Verhulst et al., 2012).
While it is hoped that early symptoms of PCOS receive more attention now, inconsistent knowledge and practices regarding PCOS diagnosis by care providers continues to be a concern (Dokras et al., 2017; Gibson-Helm et al., 2018; Piltonen et al., 2019). A recent qualitative study also found that a negative response to diagnosis of PCOS, particularly among women with milder phenotypes, was influenced by diagnostic experience, doctor communications and lack of evidence-based information about the condition (Copp et al., 2019). We welcome the substantial clinician and patient education strategies accompanying the implementation of the international evidence-based PCOS guideline to improve the timely diagnosis of PCOS (Teede et al., 2018). This is a critical step in improving the support women receive to manage the distressing and often debilitating consequences of this complex condition. Furthermore, our study shows that despite the high likelihood of difficulties in becoming pregnant among the women with a diagnosis of PCOS who desired motherhood, the proportion achieving a live birth was similar to that of their peers by the time of the study. This could provide women with PCOS reassurance about their prospects of having children (with medical assistance if required) and suggests healthcare attention should be directed to improved management of other aspects of the condition.
A strength of this study is that it involved the use of data from an unselected community-based cohort of women. This overcomes the limitations of clinic-based cohorts, which tend to comprise women with the most severe manifestations of PCOS who have concerns about fertility. Other strengths are that women from across the spectrum of socio-economic status were represented in the cohort and the demographic profile of participants was broadly similar to that of the wider population of women the same age. Also, women reported on their history of clinically diagnosed depression and current depression symptoms before they knew they met criteria for PCOS. We note that the international guideline published in 2018 recommends new diagnostic criteria for PCOS (mF-G score greater than 3, 20 follicles but same ovarian volume threshold for PCO) (Teede et al., 2018). However, the relevant comparison group in this study was identified with the criteria that then applied, comprising women who did not receive the clinical attention that they merited and could have gained at the time.
A limitation is that the number of women who were diagnosed with PCOS both prior to and during the study was small relative to effect sizes, resulting in limited statistical power to detect modest but potentially clinically meaningful differences between the PCOS groups. The protocol for the present study may have misclassified some cases as not having PCOS, but any bias would be in a conservative direction, as this would render the group without PCOS more similar to the other two. Study participants were predominantly white, reflecting the ‘white Australia’ migration policy that was in place until 1973 and created an ethnically narrow population (Windschuttle, 2004). Sparse data for rarer outcomes, such as ectopic pregnancy and stillbirth, meant that several types of pregnancy loss were reported in a combined variable, despite different biological mechanisms. Another limitation is that anxiety was not assessed. In the Northern Finland Birth Cohort 1966, women who were aware that they had PCOS had moderate anxiety symptoms to a greater extent than women who met criteria for PCOS but were not aware of this; however, the two groups were similar in terms of severe anxiety and depression (Karjula et al., 2017).
Conclusion
In this community-based cohort, half of women who met the Rotterdam criteria for PCOS were undiagnosed by age 35 years. Women with fertility problems were most likely to gain a diagnosis. Women who were undiagnosed experienced an excess of period problems, and hirsutism, perhaps to a slightly lesser degree than women with a prior diagnosis. Importantly, both groups had relatively poor psychological health, evidence that anxiety and depression in women with PCOS is not a consequence of the disease label and clinical contact. Together, findings reinforce the need for monitoring symptomatic women from adolescence, so that timely diagnosis and appropriate health care may be provided.
Data availability
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. Any requests will be considered by the corresponding author.
Acknowledgements
We gratefully acknowledge the contribution from the women who participated in the study. We are also grateful to Kendall Smith for the study co-ordination and management of the interviews and Nanette Kretschmer for her involvement in tracing the women, and Fred Amato and staff at Repromed for their assistance with blood assays. Also, thank you to the many other staff members involved in the interviews, database construction and data entry.
Authors’ roles
Authors M.J.D. and V.M.M. were the principal investigators of the Lucina cohort. Together with R.C.F. and A.R.R. they formulated the research question reported in the manuscript and directed its implementation. R.C.F. planned and conducted the statistical analysis, and with V.M.M. drafted the manuscript. All authors contributed to the interpretation of analyses, the critical revision of important intellectual content and final approval of the manuscript.
Funding
This work was supported by a project grant (2017) from the National Health and Medical Research Council of Australia (NHMRC) Centre for Research Excellence in Polycystic Ovary Syndrome (Grant ID APP1078444). R.C.F. and J.C.A. were supported by Robinson Research Institute Lloyd Cox Career Development Fellowships (2018). Establishment of the cohort was funded by an NHMRC Strategic Award No. 465455, a Career Development Award in Population Health (No. 349548) and the Australian Research Council (Future Fellowship FT100101018) awarded to M.J.D.
The funding sources had no involvement in the conduct of the research.
Conflict of interest
The authors report no conflict of interest.
References
- Alur-Gupta S, Chemerinski A, Liu C, Lipson J, Allison K, Sammel MD, Dokras A.. Body-image distress is increased in women with polycystic ovary syndrome and mediates depression and anxiety. Fertil Steril 2019;112:930–938.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, Misso ML, Teede HJ, Moran LJ.. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta-analysis, and meta-regression. Obes Rev 2019;20:659–674. [DOI] [PubMed] [Google Scholar]
- Balen AH, Laven JSE, Tan SL, Dewailly D.. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 2003;9:505–514. [DOI] [PubMed] [Google Scholar]
- Barry JA, Kuczmierczyk AR, Hardiman PJ.. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2011;26:2442–2451. [DOI] [PubMed] [Google Scholar]
- Brown TA, Cash TF, Mikulka PJ.. Attitudinal body-image assessment: factor analysis of the body-self relations questionnaire. J Pers Assess 1990;55:135. [DOI] [PubMed] [Google Scholar]
- Cash TF. The Multidimensional Body-Self Relations Questionnaire (MBSRQ) users' manual, 3rd Revision. 2000, Norfolk VA. Available from: http://www.body-images.com/assessments/mbsrq.html.
- Cooney LG, Dokras A.. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril 2018;110:794–809. [DOI] [PubMed] [Google Scholar]
- Copp T, Hersch J, Muscat DM, McCaffery KJ, Doust J, Dokras A, Mol BW, Jansen J.. The benefits and harms of receiving a polycystic ovary syndrome diagnosis: a qualitative study of women's experiences. Hum Reprod Open 2019;2019:hoz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp T, Jansen J, Doust J, Mol BW, Dokras A, McCaffery K.. Are expanding disease definitions unnecessarily labelling women with polycystic ovary syndrome? BMJ 2017;358:j3694. [DOI] [PubMed] [Google Scholar]
- Crosignani PG, Colombo M, Vegetti W, Somigliana E, Gessati A, Ragni G.. Overweight and obese anovulatory patients with polycystic ovaries: parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum Reprod 2003;18:1928–1932. [DOI] [PubMed] [Google Scholar]
- Deeks AA, Gibson-Helm ME, Paul E, Teede HJ.. Is having polycystic ovary syndrome a predictor of poor psychological function including anxiety and depression? Hum Reprod 2011;26:1399–1407. [DOI] [PubMed] [Google Scholar]
- Dokras A, Saini S, Gibson-Helm M, Schulkin J, Cooney L, Teede H.. Gaps in knowledge among physicians regarding diagnostic criteria and management of polycystic ovary syndrome. Fertil Steril 2017;107:1380–1386.e1. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270. [DOI] [PubMed] [Google Scholar]
- Fernandez RC, Moore VM, Van Ryswyk EM, Varcoe TJ, Rodgers RJ, March WA, Moran LJ, Avery JC, McEvoy RD, MD.. Sleep disturbances in women with polycystic ovary syndrome: prevalence, pathophysiology, impact and management strategies. Nat Sci Sleep 2018;2018:45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finning K, Ukoumunne OC, Ford T, Danielsson-Waters E, Shaw L, Romero De Jager I, Stentiford L, Moore DA.. The association between child and adolescent depression and poor attendance at school: a systematic review and meta-analysis. J Affect Disord 2019;245:928–938. [DOI] [PubMed] [Google Scholar]
- Gibson-Helm M, Dokras A, Karro H, Piltonen T, Teede HJ.. Knowledge and practices regarding polycystic ovary syndrome among physicians in Europe, North America, and Internationally: an online questionnaire-based study. Semin Reprod Med 2018;36:19–27. [DOI] [PubMed] [Google Scholar]
- Gibson-Helm M, Teede H, Dunaif A, Dokras A.. Delayed diagnosis and a lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2017;102:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch R, Rosenfield RL, Kim MH, Tredway D.. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 1981;140:815–830. [DOI] [PubMed] [Google Scholar]
- Homburg R. Polycystic ovary syndrome—from gynaecological curiosity to multisystem endocrinopathy. Hum Reprod 1996;11:29–39. [DOI] [PubMed] [Google Scholar]
- Huber-Buchholz M-M, Carey DGP, Norman RJ.. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab 1999;84:1470–1474. [DOI] [PubMed] [Google Scholar]
- Jacob S, Balen AH.. How will the new global polycystic ovary syndrome guideline change our clinical practice? Clin Med Insights Reprod Health 2019;13:1179558119849605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karjula S, Morin-Papunen L, Auvinen J, Ruokonen A, Puukka K, Franks S, Järvelin M-R, Tapanainen JS, Jokelainen J, Miettunen J. et al. Psychological distress is more prevalent in fertile age and premenopausal women with PCOS symptoms: 15-year follow-up. J Clin Endocrinol Metab 2017;102:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerveld SE, Bültmann U, Franche RL, van Dijk FJH, Vlasveld MC, van der Feltz-Cornelis CM, Bruinvels DJ, Huijs JJJM, Blonk RWB, van der Klink JJL. et al. Factors associated with work participation and work functioning in depressed workers: a systematic review. J Occup Rehabil 2010;20:275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan ME, Chizen DR, Pierson RA.. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol 2008;30:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ.. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544–551. [DOI] [PubMed] [Google Scholar]
- March WA, Whitrow MJ, Davies MJ, Fernandez RC, Moore VM.. Postnatal depression in a community-based study of women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2018;97:838–844. [DOI] [PubMed] [Google Scholar]
- Marmot M. An inverse care law for our time. BMJ 2018;362:k3216. [DOI] [PubMed] [Google Scholar]
- Moran LJ, March WA, Whitrow MJ, Giles LC, Davies MJ, Moore VM.. Sleep disturbances in a community-based sample of women with polycystic ovary syndrome. Hum Reprod 2015;30:466–472. [DOI] [PubMed] [Google Scholar]
- O'Dea JF, Kilham RJ.. The inverse care law is alive and well in general practice. Med J Aust 2002;177:78–79. [DOI] [PubMed] [Google Scholar]
- Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, Garad R, Dabadghao P, Teede H.. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piltonen TT, Ruokojärvi M, Karro H, Kujanpää L, Morin-Papunen L, Tapanainen JS, Stener-Victorin E, Sundrström-Poromaa I, Hirschberg AL, Ravn P. et al. Awareness of polycystic ovary syndrome among obstetrician-gynecologists and endocrinologists in Northern Europe. PLoS One 2019;14:e0226074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelevic GM, Wurzburger MI, Balint-Peric L.. 24-hour serum cortisol profiles in women with polycystic ovary syndrome. Gynecol Endocrinol 1993;7:179–184. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- Rubin KH, Andersen MS, Abrahamsen B, Glintborg D.. Socioeconomic status in Danish women with polycystic ovary syndrome: a register-based cohort study. Acta Obstet Gynecol Scand 2019;98:440–450. [DOI] [PubMed] [Google Scholar]
- Teede H, Misso ML, Costello M, Dokras A, Laven JS, Moran LJ, Piltonen T, Norman RJ.. International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Melbourne, Australia: Monash University, 2018. [Google Scholar]
- Trewin D. Information Paper: Census of Population and Housing Socio-Economic Indexes for Areas, Australia, 2001. Canberra, Australia: Australian Bureau of Statistics, 2003. [Google Scholar]
- Tunny G. Educational attainment in Australia. Econ Roundup 2006;2:1–10. [Google Scholar]
- Veltman-Verhulst SM, Boivin J, Eijkemans MJ, Fauser BJ.. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum Reprod Update 2012;18:638–651. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ.. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203–214. [DOI] [PubMed] [Google Scholar]
- Windschuttle K. The White Australia Policy. Sydney: Macleay Press, 2004. [Google Scholar]
- Witchel SF, Teede HJ, Peña AS.. Curtailing PCOS. Pediatr Res 2020;87:353–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. Any requests will be considered by the corresponding author.