Abstract

An efficient, direct sulfinylation of organoborons catalyzed by palladium is disclosed. Treatment of organoborons and sulfinate esters in the presence of a palladium precatalyst provided a broad range of sulfoxides. Various organosulfur compounds having oxidizable functional groups were successfully prepared through the sulfoxide synthesis.
Sulfoxides are a fundamental class of compounds in a broad range of research fields such as synthetic organic chemistry, pharmaceutical sciences, agrochemistry, and materials chemistry.1,2 Particularly, recent remarkable progress on versatile transformations of sulfoxides have allowed us to synthesize a wide variety of molecules.2 These recent advances clearly enhanced the synthetic utility of not only chiral but also achiral sulfoxides.2 Despite their great significance, accessible sulfoxides by conventional methods through sulfanylation of Grignard reagents, organic bromides, or organoborons and following S-oxidation are limited since various functional groups can be damaged in the oxidation step (Figure 1A).2,3 Thus, an efficient method for direct sulfinylation is highly sought after. We herein describe a direct method for sulfinylation of aryl- and alkenylborons with sulfinate esters catalyzed by palladium, enabling the preparation of a wide variety of sulfoxides having easily oxidizable functional groups.
Figure 1.
(A) Conventional methods for sulfoxide synthesis. (B) Pioneering study. (C) This work.
Conventional direct sulfoxide synthesis has been achieved from nucleophilic carbanions with sulfinate esters as a sulfur surrogate.4−6 A pioneering study on the sulfinylation of organomagnesiums using sulfinate esters was reported by Andersen and co-workers in 1962 (Figure 1B).4 Considering that recent significant successes of modern organometallic chemistry have greatly improved the availability of diverse molecules including biaryls and amines, an efficient cross-coupling reaction using sulfinate esters is highly demanded for synthesizing diverse sulfoxides. With our recent achievements in organosulfur chemistry using thiosulfonates catalyzed by transition-metals in mind (Figure 1A, bottom),7 we envisioned that a wide range of sulfoxides can be prepared by direct sulfinylation of organoborons catalyzed by a transition-metal complex using sulfinate esters as electrophilic sulfur surrogates under mild conditions (Figure 1C).
A sulfoxide synthesis from 4-tolylboronic acid (1a) and methyl 4-methoxybenzenesulfinate (2a) was chosen as a model reaction (Table 1). After a number of examinations, we found that a catalytic amount of Pd(dba)2 with XPhos as a ligand promoted the sulfinylation in the presence of potassium carbonate in 1,4-dioxane and water (v/v = 10/1) at 80 °C (entry 1). In contrast, the yields of sulfoxide 3a were significantly decreased when the reaction was conducted using triphenylphosphine or an N-hetero cyclic carbene ligand (entries 2 and 3). Palladium precatalysts XPhos Pd G3 and XPhos Pd G4 also catalyzed the synthesis of sulfoxide 3a (entries 4 and 5).8 While the reaction performed at 100 °C lowered the efficiency (entry 6), the yield of 3a based on recovered starting material was improved by decreasing the reaction temperature to 40 °C (entry 7). Although the reaction without water decreased the yield of 3a (entry 8), we accomplished the synthesis of sulfoxide 3a in high yield by changing the ratio of solvents from 10/1 to 5/1 (entry 9). Sulfoxide 3a was obtained in low yield when further increasing the ratio of water (entry 10). We succeeded in decreasing the catalyst loading from 10 to 5 mol % (entry 11). Further improvement of the efficiency was achieved by increasing the amount of 1a and decreasing the concentration of substrates from 0.1 to 0.05 M, enabling us to prepare sulfoxide 3a in high yield (entry 12). The reaction with a catalytic amount of XPhos in the absence of palladium precatalysts did not afford sulfoxide 3a, in which 94% of sulfinate ester 2a was recovered. This result clearly showed that palladium catalyzed the sulfinylation of 4-tolylboronic acid (1a) with sulfinate ester 2a.
Table 1. Optimization of the Reaction Conditions.
| entry | cat. Pd, (+ cat. ligand)a | temp | 3a/%b | 2a/%b |
|---|---|---|---|---|
| 1 | Pd(dba)2 (10), XPhos (10) | 80 | 59 | 22 |
| 2 | Pd(dba)2 (10), PPh3 (20) | 80 | n.d.c | <99 |
| 3 | PEPPSI-IPr (10) | 80 | 7 | 78 |
| 4 | XPhos Pd G3 (10) | 80 | 55 | 7 |
| 5 | XPhos Pd G4 (10) | 80 | 63 | trace |
| 6 | XPhos Pd G4 (10) | 100 | 48 | 14 |
| 7 | XPhos Pd G4 (10) | 40 | 61 | 28 |
| 8d | XPhos Pd G4 (10) | 40 | 48 | 40 |
| 9e | XPhos Pd G4 (10) | 40 | 78f | 4 |
| 10g | XPhos Pd G4 (10) | 40 | 18 | 0 |
| 11 | XPhos Pd G4 (5) | 40 | 58 | 39 |
| 12e,h | XPhos Pd G4 (5) | 40 | 78f | n.d. |
Catalyst amount is shown in the parentheses.
Yields based on 1H NMR analysis.
Not detected.
The reaction was performed without water.
The reaction was performed in dioxane and water (v/v = 5/1).
Isolated yields.
The reaction was performed in 1,4-dioxane and water (v/v = 1/1).
The reaction was performed using 1a (2.0 equiv) at 0.05 M.
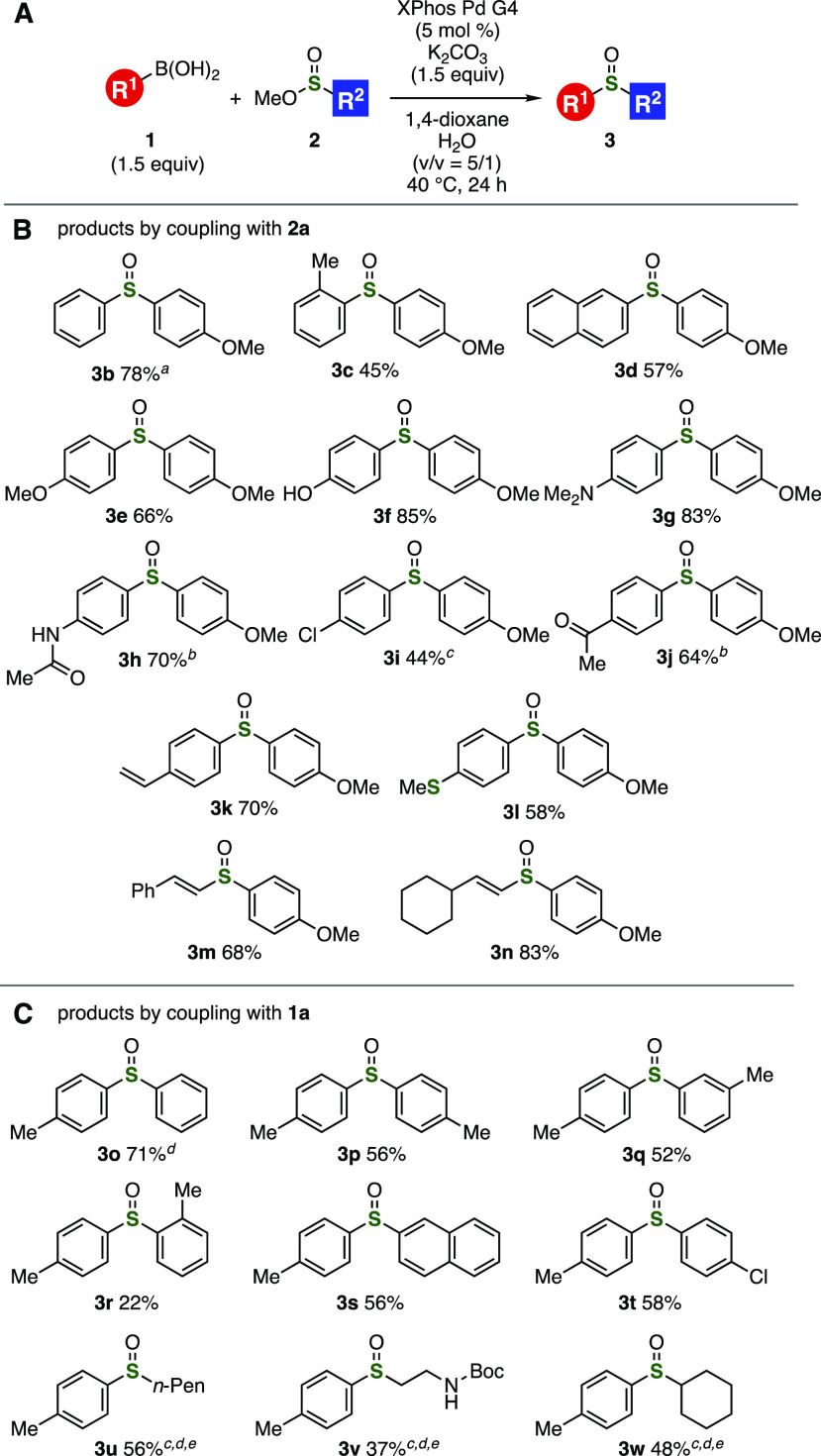
A wide range of aryl- and alkenylborons were successfully sulfinylated catalyzed by palladium under the optimized conditions (Figure 2A,B). The reaction using phenylboronic acid pinacol ester took place smoothly to afford sulfoxide 3b in high yield. Sulfoxides 3c and 3d were prepared in moderate yields by 4-methoxyphenylsulfinylation of 2-tolyl- and 2-naphthylboronic acids, respectively. Of note, the sulfinylation of electron-rich 4-methoxy-, 4-hydroxy-, 4-(dimethylamino)-, and 4-(acetylamino)phenylboronic acids proceeded efficiently to provide sulfoxides 3e–3h in good yields, leaving a broad range of electron-donating groups untouched.9 Sulfoxides 3i and 3j were also synthesized by the sulfinylation of electron-deficient 4-chloro- and 4-acetylphenylboronic acid in moderate to high yields. It is worth noting that we achieved the facile preparation of sulfoxides 3k and 3l having a vinyl and a methylthio group, which can be damaged by oxidation in the conventional synthesis. Furthermore, efficient sulfinylation took place to furnish alkenyl sulfoxide 3m or 3n when using phenyl- or cyclohexyl-substituted alkenylboronic acid, respectively. This broad substrate scope obviously demonstrated a benefit of the palladium-catalyzed direct sulfinylation of organoborons.
Figure 2.
(A) General scheme. (B) Results using various organoborons 1. (C) Results using various sulfinate esters 2. See the Supporting Information for the structures of 1 and 2. aPhenylboronic acid pinacol ester was used. bXPhos Pd G4 (25 mol %) was used. cXPhos Pd G4 (10 mol %) was used. dTMEDA (20 mol %) was added. eThe reaction was performed using 1a (3.0 equiv) at 60 °C.
Diverse sulfinate esters participated in the catalytic sulfinylation of organoborons allowing us to synthesize a wide variety of sulfoxides 3o–3w (Figures 2A,C).10 Phenylation of 4-tolylboronic acid (1a) with methyl benzenesulfinate was facilitated by the palladium catalysis to furnish 3o in good yield, in which the addition of N,N,N′,N′-tetramethylethylenediamine (TMEDA) slightly improved the efficiency. Sulfoxides 3p and 3q were prepared in moderate yields by the reaction using 4- and 3-toluenesulfinic acid methyl esters. Additionally, S-tolylation of bulky methyl 2-toluenesulfinate took place albeit in low yield. The palladium-catalyzed sulfinylation with 2-naphthalene- and 4-chlorobenzenesulfinic acid methyl esters was achieved to provide sulfoxides 3s and 3t in moderate yields. It is worthy to note that a variety of alkyl aryl sulfoxides 3u–3w were successfully synthesized using primary and secondary sulfinate esters. In particular, we accomplished the catalytic synthesis of sulfoxide 3v without damaging a (tert-butoxycarbonyl)amino group.
To obtain insights into the reaction mechanism, we conducted a number of control experiments (Figure 3). For example, a mixture of 1a and 2a was treated with XPhos Pd G4 as a precatalyst in the presence of a catalytic amount of potassium carbonate (Figure 3A). As a result, sulfoxide 3a was obtained in moderate yield even when using only 5 mol % of base. Treatment of sulfinate ester 2a with an equimolar amount of XPhos Pd G4 and potassium carbonate followed by the addition of 1a and potassium carbonate resulted in affording a complex mixture of products, in which sulfoxide 3a was not detected (Figure 3B, upper). In contrast, sulfoxide 3a was synthesized in high yield when the palladium precatalyst loading was reduced to 10 mol % (Figure 3B, lower). A plausible reaction mechanism on the basis of these results is illustrated in Figure 3C-a. First, the oxidative addition of sulfinate esters to XPhos-ligated Pd(0) I generated in situ would proceed leading to Pd(II) complex II.11,12 Then, transmetalation between II and borates III and subsequent reductive elimination will provide sulfoxides, where liberating methoxide from borate V can facilitate the reaction. Another mechanism through transmetalation between Pd(II) complex VI and borates III followed by σ-bond metathesis of Pd(II) intermediate VII with sulfinate esters through transition state VIII is also possible (Figure 3C-b).11 Although further mechanistic studies should be performed to reveal the reaction pathway, it is worth noting that sulfinate esters successfully served as sulfur building blocks without C–S cleavage.12,13
Figure 3.
Control experiments and plausible reaction mechanisms. (A) Reaction using a catalytic amount of base. (B) Control experiments from 2a. (C) Plausible reaction mechanisms. (a) Catalytic cycle via Pd(0) and Pd(II). (b) Catalytic cycle via Pd(II).
An advantage of the palladium-catalyzed sulfoxide synthesis was showcased by consecutive cross-coupling reactions using bromo-substituted sulfinate ester 4 (Figure 4A,B). Bromide-selective Suzuki–Miyaura cross-coupling of 4 catalyzed by palladium with a variety of arylboronic acids proceeded efficiently keeping the sulfinate moiety unreacted (Figure 4A). Then, following S-arylation with arylboronic acids realized the synthesis of diverse sulfoxides 6a–6d without damaging hydroxy, formyl, dimethylamino, acetylamino, methylthio, and vinyl groups. Furthermore, we succeeded in the synthesis of sulfoxide 6b by the consecutive coupling of 4 with arylboronic acids in a one-pot manner (Figure 4B). Since sequential coupling reactions were realized even in the presence of reactive functional groups including formyl and dimethylamino groups owing to the good functional group tolerance, this one-pot procedure will contribute to the modular synthesis of diverse sulfoxides from bromo-substituted sulfinate esters and easily available organoboron derivatives.
Figure 4.
Application of the palladium-catalyzed sulfoxide synthesis. (A) Sequential cross-couplings. (B) One-pot synthesis of sulfoxide 6b. (C) Aryne reaction of sulfoxide 3j or 3m. See the Supporting Information for the details.
The palladium-catalyzed sulfoxide synthesis significantly improved the accessibility of diaryl sulfides by oxythiolation of aryne intermediates IX (Figure 4C).14 Treatment of o-silylaryl triflates 7 and sulfoxide 3j or 3m with potassium fluoride and 18-crown-6 in hot dioxane provided a range of diaryl sulfides 8a–8c via selective oxythiolation of arynes IX and subsequent O-arylation, where an electron-deficient aryl or alkenyl group was selectively migrated. Of note, the synthesis of highly functionalized diaryl sulfides was achieved by virtue of the enhancing the accessibility of sulfoxides developed in this study. Since various functional groups were tolerated in the palladium-catalyzed sulfinylation and this aryne reaction, a modular synthesis of a wide range of diaryl sulfides will be realized from easily available sulfinate esters, organoborons, and o-silylaryl triflates.
In summary, we have developed an efficient catalytic method for sulfinylation of organoborons. A wide variety of sulfoxides were synthesized from sulfinate esters and organoborons, keeping easily oxidizable functional groups unreacted. Further studies including detailed mechanistic studies and applications to the synthesis of bioactive organosulfurs are ongoing.
Acknowledgments
The authors thank Dr. Yuki Sakata at Tokyo Medical and Dental University for HRMS analyses. This work was supported by JSPS KAKENHI Grant Nos. JP19K05451 (C; S.Y.); the Naito Foundation (S.Y.); the Japan Agency for Medical Research and Development (AMED) under Grant Nos. JP20am0101098 (Platform Project for Supporting Drug Discovery and Life Science Research, BINDS); and the Cooperative Research Project of Research Center for Biomedical Engineering.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c01292.
Experimental procedures and characterization of new compounds including NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Legros J.; Dehli J. R.; Bolm C. Applications of Catalytic Asymmetric Sulfide Oxidations to the Syntheses of Biologically Active Sulfoxides. Adv. Synth. Catal. 2005, 347, 19. 10.1002/adsc.200404206. [DOI] [Google Scholar]; b Bentley R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609. 10.1039/b418284g. [DOI] [PubMed] [Google Scholar]; c Işık D.; Quaas E.; Klinger D. Thermo- and oxidation-sensitive poly(meth)acrylates based on alkyl sulfoxides: dual-responsive homopolymers from one functional group. Polym. Chem. 2020, 11, 7662. 10.1039/D0PY01321H. [DOI] [Google Scholar]
- a Bur S. K.; Padwa A. The Pummerer Reaction: Methodology and Strategy for the Synthesis of Heterocyclic Compounds. Chem. Rev. 2004, 104, 2401. 10.1021/cr020090l. [DOI] [PubMed] [Google Scholar]; b Feldman K. S. Modern Pummerer-type reactions. Tetrahedron 2006, 62, 5003. 10.1016/j.tet.2006.03.004. [DOI] [Google Scholar]; c Akai S.; Kita Y. Recent Advances in Pummerer Reactions. Top. Curr. Chem. 2007, 274, 35. 10.1007/128_073. [DOI] [Google Scholar]; d Smith L. H. S.; Coote S. C.; Sneddon H. F.; Procter D. J. Beyond the Pummerer Reaction: Recent Developments in Thionium Ion Chemistry. Angew. Chem., Int. Ed. 2010, 49, 5832. 10.1002/anie.201000517. [DOI] [PubMed] [Google Scholar]; e Shafir A. The emergence of sulfoxide and iodonio-based redox arylation as a synthetic tool. Tetrahedron Lett. 2016, 57, 2673. 10.1016/j.tetlet.2016.05.013. [DOI] [Google Scholar]; f Pulis A. P.; Procter D. J. C–H Coupling Reactions Directed by Sulfoxides: Teaching an Old Functional Group New Tricks. Angew. Chem., Int. Ed. 2016, 55, 9842. 10.1002/anie.201601540. [DOI] [PubMed] [Google Scholar]; g Yorimitsu H. Cascades of Interrupted Pummerer Reaction-Sigmatropic Rearrangement. Chem. Rec. 2017, 17, 1156. 10.1002/tcr.201700017. [DOI] [PubMed] [Google Scholar]; h Yanagi T.; Nogi K.; Yorimitsu H. Recent development of ortho-C–H functionalization of aryl sulfoxides through [3,3] sigmatropic rearrangement. Tetrahedron Lett. 2018, 59, 2951. 10.1016/j.tetlet.2018.06.055. [DOI] [Google Scholar]; i Kaiser D.; Klose I.; Oost R.; Neuhaus J.; Maulide N. Bond-Forming and -Breaking Reactions at Sulfur(IV): Sulfoxides, Sulfonium Salts, Sulfur Ylides, and Sulfinate Salts. Chem. Rev. 2019, 119, 8701. 10.1021/acs.chemrev.9b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhang L.; Hu M.; Peng B. [3,3]- and [5,5]-Sigmatropic Rearrangements of Aryl Sulfoxides Using An ‘Assembly/Deprotonation’ Technology. Synlett 2019, 30, 2203. 10.1055/s-0039-1690212. [DOI] [Google Scholar]
- a Kondo T.; Mitsudo T. Metal-Catalyzed Carbon–Sulfur Bond Formation. Chem. Rev. 2000, 100, 3205. 10.1021/cr9902749. [DOI] [PubMed] [Google Scholar]; b Hartwig J. F. Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides. Acc. Chem. Res. 2008, 41, 1534. 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Bichler P.; Love J. A.. Organometallic Approaches to Carbon–Sulfur Bond Formation. In C–X Bond Formation: Topics in Organometallic Chemistry; Vigalok A., Eds.; Springer, Heidelberg, 2010, Vol. 31, pp 39–64. [Google Scholar]; d Liu C.; Zhang H.; Shi W.; Lei A. Bond Formations between Two Nucleophiles: Transition Metal Catalyzed Oxidative Cross-Coupling Reactions. Chem. Rev. 2011, 111, 1780. 10.1021/cr100379j. [DOI] [PubMed] [Google Scholar]; e Eichman C. C.; Stambuli J. P. Transition Metal Catalyzed Synthesis of Aryl Sulfides. Molecules 2011, 16, 590. 10.3390/molecules16010590. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Beletskaya I. P.; Ananikov V. P. Transition-Metal-Catalyzed C–S, C–Se, and C–Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 111, 1596. 10.1021/cr100347k. [DOI] [PubMed] [Google Scholar]; g Takise R.; Isshiki R.; Muto K.; Itami K.; Yamaguchi J. Decarbonylative Diaryl Ether Synthesis by Pd and Ni Catalysis. J. Am. Chem. Soc. 2017, 139, 3340. 10.1021/jacs.7b00049. [DOI] [PubMed] [Google Scholar]; h Lee S.-C.; Liao H.-H.; Chatupheeraphat A.; Rueping M. Nickel-Catalyzed C–S Bond Formation via Decarbonylative Thioetherification of Esters, Amides and Intramolecular Recombination Fragment Coupling of Thioesters. Chem. - Eur. J. 2018, 24, 3608. 10.1002/chem.201705842. [DOI] [PubMed] [Google Scholar]; i Liu C.; Szostak M. Decarbonylative thioetherification by nickel catalysis using air- and moisture-stable nickel precatalysts. Chem. Commun. 2018, 54, 2130. 10.1039/C8CC00271A. [DOI] [PubMed] [Google Scholar]; j Ichiishi N.; Malapit C. A.; Woźniak Ł.; Sanford M. S. Palladium- and Nickel-Catalyzed Decarbonylative C–S Coupling to Convert Thioesters to Thioethers. Org. Lett. 2018, 20, 44. 10.1021/acs.orglett.7b03305. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Zhang R.; Ding H.; Pu X.; Qian Z.; Xiao Y. Recent Advances in the Synthesis of Sulfides, Sulfoxides and Sulfones via C-S Bond Construction from Non-Halide Substrates. Catalysts 2020, 10, 1339. 10.3390/catal10111339. [DOI] [Google Scholar]
- a Andersen K. K. Synthesis of (+)-ethyl p-tolyl sulfoxide from (−)-menthyl (−)-p-toluenrsulfinate. Tetrahedron Lett. 1962, 3, 93. 10.1016/S0040-4039(00)71106-3. [DOI] [Google Scholar]; b Andersen K. K.; Gaffield W.; Papanikolaou N. E.; Foley J. W.; Perkins R. I. Optically Active Sulfoxides. The Synthesis and Rotatory Dispersion of Some Diaryl Sulfoxides. J. Am. Chem. Soc. 1964, 86, 5637. 10.1021/ja01078a047. [DOI] [Google Scholar]; c Andersen K. K.; Bujnicki B.; Drabowicz J.; Mikolajczyk M.; O’Brien J. B. Synthesis of enantiomerically pure alkyl and aryl methyl sulfoxides from cholesteryl methanesulfinates. J. Org. Chem. 1984, 49, 4070. 10.1021/jo00195a042. [DOI] [Google Scholar]; d Solladie G.; Hutt J.; Girardin A. Improved preparation of optically active methyl p-tolyl sulfoxide. Synthesis 1987, 173. 10.1055/s-1987-27877. [DOI] [Google Scholar]
- a Lu B. Z.; Jin F.; Zhang Y.; Wu X.; Wald S. A.; Senanayake C. H. New General Sulfinylating Process for Asymmetric Synthesis of Enantiopure Sulfinates and Sulfoxides. Org. Lett. 2005, 7, 1465. 10.1021/ol0501020. [DOI] [PubMed] [Google Scholar]; b Maitro G.; Vogel S.; Prestat G.; Madec D.; Poli G. Aryl Sulfoxides via Palladium-Catalyzed Arylation of Sulfenate Anions. Org. Lett. 2006, 8, 5951. 10.1021/ol062315a. [DOI] [PubMed] [Google Scholar]; c Maitro G.; Prestat G.; Madec D.; Poli G. Preparation of Allyl Sulfoxides by Palladium-Catalyzed Allylic Alkylation of Sulfenate Anions. J. Org. Chem. 2006, 71, 7449. 10.1021/jo061359u. [DOI] [PubMed] [Google Scholar]; d Signore G.; Samaritani S.; Malanga C.; Menicagli R. Reinheckel Protocol Revisited: Synthesis of (E)-α,β-Unsaturated Sulfoxides. Synthesis 2006, 762. 10.1055/s-2006-926322. [DOI] [Google Scholar]; e Foucoin F.; Caupène C.; Lohier F.-F.; de Oliveira Santos J. S.; Perrio S.; Metzner P. 2-(Trimethylsilyl)ethyl Sulfoxides as a Convenient Source of Sulfenate Anions. Synthesis 2007, 1315. 10.1055/s-2007-966017. [DOI] [Google Scholar]; f Wei J.; Sun Z. tert-Butyl Sulfoxide as a Starting Point for the Synthesis of Sulfinyl Containing Compounds. Org. Lett. 2015, 17, 5396. 10.1021/acs.orglett.5b02743. [DOI] [PubMed] [Google Scholar]; g Lenstra D. C.; Vedovato V.; Flegeau E. F.; Maydom J.; Willis M. C. One-Pot Sulfoxide Synthesis Exploiting a Sulfinyl-Dication Equivalent Generated from a DABSO/Trimethylsilyl Chloride Sequence. Org. Lett. 2016, 18, 2086. 10.1021/acs.orglett.6b00712. [DOI] [PubMed] [Google Scholar]; h Jia T.; Zhang M.; McCollom S. P.; Bellomo A.; Montel S.; Mao J.; Dreher S. D.; Welch C. J.; Regalado E. L.; Williamson R. T.; Manor B. C.; Tomson N. C.; Walsh P. J. Palladium-Catalyzed Enantioselective Arylation of Aryl Sulfenate Anions: A Combined Experimental and Computational Study. J. Am. Chem. Soc. 2017, 139, 8337. 10.1021/jacs.7b03623. [DOI] [PubMed] [Google Scholar]; i Wang L.; Chen M.; Zhang P.; Li W.; Zhang J. Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General Sulfenate Anions: Scope and Synthetic Applications. J. Am. Chem. Soc. 2018, 140, 3467. 10.1021/jacs.8b00178. [DOI] [PubMed] [Google Scholar]; j Fu D.; Dong J.; Du H.; Xu J. Methanesulfinylation of Benzyl Halides with Dimethyl Sulfoxide. J. Org. Chem. 2020, 85, 2752. 10.1021/acs.joc.9b03041. [DOI] [PubMed] [Google Scholar]
- For recent transformations of sulfinate esters, see:; a Yuste F.; Linares H. L.; Mastranzo V. M.; Ortiz B.; Sanchez-Obregon R.; Fraile A.; Ruano J. L. G. Methyl Sulfinates as Electrophiles in Friedel–Crafts Reactions. Synthesis of Aryl Sulfoxides. J. Org. Chem. 2011, 76, 4635. 10.1021/jo2006335. [DOI] [PubMed] [Google Scholar]; b Lujan-Montelongo J. A.; Estevez A. O.; Fleming F. F. Alkyl Sulfinates: Formal Nucleophiles for Synthesizing TosMIC Analogs. Eur. J. Org. Chem. 2015, 1602. 10.1002/ejoc.201403615. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mohd A.; Anitha T.; Reddy K. R.; Wencel-Delord J.; Colobert F. P-Stereogenic Phosphonates via Dynamic Kinetic Resolution: A Route towards Enantiopure Tertiary Phosphine Oxides. Eur. J. Org. Chem. 2019, 7836. 10.1002/ejoc.201901475. [DOI] [Google Scholar]; d Li G.-J.; Pan Y.-L.; Liu Y.-L.; Xu H.-F.; Chen J.-Z. Ni/NHC-catalyzed cross-coupling of methyl sulfinates and amines for direct access to sulfinamides. Tetrahedron Lett. 2019, 60, 151260. 10.1016/j.tetlet.2019.151260. [DOI] [Google Scholar]; e Chen L.; Zhang J.; Wei Y.; Yang Z.; Liu P.; Zhang J.; Dai B. NH4I/1,10-phenanthroline catalyzed direct sulfenylation of N-heteroarenes with ethyl arylsulfinates. Tetrahedron 2019, 75, 130664. 10.1016/j.tet.2019.130664. [DOI] [Google Scholar]; f Kobayashi A.; Matsuzawa T.; Hosoya T.; Yoshida S. Sulfoxide synthesis from sulfinate esters under Pummerer-like conditions. Chem. Commun. 2020, 56, 5429. 10.1039/D0CC02253E. [DOI] [PubMed] [Google Scholar]
- a Yoshida S.; Sugimura Y.; Hazama Y.; Nishiyama Y.; Yano T.; Shimizu S.; Hosoya T. A mild and facile synthesis of aryl and alkenyl sulfides via copper-catalyzed deborylthiolation of organoborons with thiosulfonates. Chem. Commun. 2015, 51, 16613. 10.1039/C5CC07463K. [DOI] [PubMed] [Google Scholar]; b Kanemoto K.; Sugimura Y.; Shimizu S.; Yoshida S.; Hosoya T. Rhodium-catalyzed odorless synthesis of diaryl sulfides from borylarenes and S-aryl thiosulfonates. Chem. Commun. 2017, 53, 10640. 10.1039/C7CC05868C. [DOI] [PubMed] [Google Scholar]; c Kanemoto K.; Yoshida S.; Hosoya T. Modified Conditions for Copper-catalyzed ipso-Thiolation of Arylboronic Acid Esters with Thiosulfonates. Chem. Lett. 2018, 47, 85. 10.1246/cl.170907. [DOI] [Google Scholar]; d Kanemoto K.; Yoshida S.; Hosoya T. Synthesis of Alkynyl Sulfides by Copper-Catalyzed Thiolation of Terminal Alkynes Using Thiosulfonates. Org. Lett. 2019, 21, 3172. 10.1021/acs.orglett.9b00875. [DOI] [PubMed] [Google Scholar]
- a Bruno N. C.; Niljianskul N.; Buchwald S. L. N-Substituted 2-Aminobiphenylpalladium Methanesulfonate Precatalysts and Their Use in C–C and C–N Cross-Couplings. J. Org. Chem. 2014, 79, 4161. 10.1021/jo500355k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bruneau A.; Roche M.; Alami M.; Messaoudi S. 2-Aminobiphenyl Palladacycles: The “Most Powerful” Precatalysts in C–C and C–Heteroatom Cross-Couplings. ACS Catal. 2015, 5, 1386. 10.1021/cs502011x. [DOI] [Google Scholar]
- Since sulfoxide 3e was not obtained and sulfinate ester 2a was recovered in the reaction using anisole instead of 4-methoxyphenylboronic acid (1e), the sulfoxide formation mechanism via protodeborylation and following sulfinylation would be excluded.
- Sulfoxides were not obtained from heteroaromatic sulfinate esters such as 2- and 4-pyridylsulfinic acid methyl esters.
- To our knowledge, oxidative addition of sulfinate esters to Pd(0) and σ-bond metathesis between arylpalladium species and sulfinate esters have not been reported so far.
- Desulfinative coupling reactions of sodium sulfinates through transmetalation between RPd(II)X and sodium sulfinates and following removal of SO2 were reported, see:; Qin A.; Zhu G.; Chen Q.; Qian H.; Ma S. Palladium-Catalyzed Desulfitative Cross-Coupling Reaction of Sodium Sulfinates with Propargylic Carbonates. Adv. Synth. Catal. 2019, 361, 4656. 10.1002/adsc.201900698. [DOI] [Google Scholar]; de Gombert A.; McKay A. I.; Davis C. J.; Wheelhouse K. M.; Willis M. C. Mechanistic Studies of the Palladium-Catalyzed Desulfinative Cross-Coupling of Aryl Bromides and (Hetero)Aryl Sulfinate Salts. J. Am. Chem. Soc. 2020, 142, 3564. 10.1021/jacs.9b13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A number of transformations via C–S cleavage by oxidative addition of organosulfur(IV) compounds to Pd(0) were reported, see:; Yoshida Y.; Otsuka S.; Nogi K.; Yorimitsu H. Palladium-Catalyzed Amination of Aryl Sulfoxides. Org. Lett. 2018, 20, 1134. 10.1021/acs.orglett.8b00060. [DOI] [PubMed] [Google Scholar]; and references cited therein. For a review of C–S cleavage, see:; Lou J.; Wang Q.; Wu P.; Wang H.; Zhou Y.-G.; Yu Z. Transition-metal mediated carbon–sulfur bond activation and transformations: an update. Chem. Soc. Rev. 2020, 49, 4307. 10.1039/C9CS00837C. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T.; Uchida K.; Yoshida S.; Hosoya T. Synthesis of Diverse o-Arylthio-Substituted Diaryl Ethers by Direct Oxythiolation of Arynes with Diaryl Sulfoxides Involving Migratory O-Arylation. Org. Lett. 2017, 19, 5521. 10.1021/acs.orglett.7b02599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.