Abstract
STUDY QUESTION
Does frozen-thawed or fresh embryo transfer (ET) influence utero-placental (vascular) development, when studied using three-dimensional (3D) ultrasound and virtual reality imaging techniques?
SUMMARY ANSWER
In the first trimester, placental developmental parameters, that is, placental volume (PV) and utero-placental vascular volume (uPVV), were comparable between pregnancies resulting from frozen-thawed ET, fresh ET and natural conception; and in the second and the third trimester, uterine artery Doppler indices were lower in pregnancies after frozen-thawed ET compared to pregnancies after fresh ET and natural conception.
WHAT IS KNOWN ALREADY
Pregnancies after frozen-thawed ET are at risk of developing placenta-related pregnancy complications. There is strong evidence that impaired first-trimester spiral artery remodelling is involved in the pathophysiology of these complications. Studies on longitudinal placental development in pregnancies with different modes of conception, that is, after frozen-thawed ET, fresh ET or natural conception, are lacking.
STUDY, DESIGN, SIZE, DURATION
Women with singleton pregnancies were included before 10 weeks of gestation, between January 2017 and July 2018, as a subcohort of the ongoing Rotterdam Periconception cohort. Results were partially validated in 722 women from the total cohort, which was conducted from November 2010 onwards.
PARTICIPANTS/MATERIALS, SETTING, METHODS
A total of 214 women, of whom 32 conceived after frozen-thawed ET, 56 conceived after fresh ET and 126 conceived naturally, were selected. PV and uPVV measurements were obtained at 7, 9 and 11 weeks of gestation by transvaginal 3D (power Doppler) ultrasound. The uterine artery pulsatility index (UtA-PI) and resistance index (UtA-RI) were measured transvaginally at 7, 9, 11 and 13 weeks and abdominally at 22 and 32 weeks of gestation by pulsed wave Doppler ultrasound. In the validation cohort, the PV was measured in 722 women. Associations between mode of conception and placental development were studied using linear mixed models.
MAIN RESULTS AND THE ROLE OF CHANCE
First-trimester parameters of placental development, that is, PV, uPVV, UtA-PI and UtA-RI, were comparable between pregnancies after frozen-thawed and fresh ET and naturally conceived pregnancies. In our validation cohort, comparable results were found for PV. However, the second- and third-trimester UtA-PI and UtA-RI in pregnancies after frozen-thawed ET were significantly lower than in pregnancies after fresh ET (βUtA-PI −0.158 (95% CI: −0.268, −0.048), P = 0.005; βUtA-RI −0.052 (95% CI: −0.089, −0.015), P = 0.006). The second- and third-trimester uterine artery indices in pregnancies after fresh ET were comparable to those in pregnancies after natural conception.
LIMITATIONS, REASONS FOR CAUTION
The main limitation of this study is the lack of power to optimally detect differences in placental development and placenta-related pregnancy outcomes between pregnancies after different modes of conception. Moreover, our population was selected from a tertiary hospital and included a relatively limited number of pregnancies. Therefore, external validity of the results should be confirmed in a larger sample size.
WIDER IMPLICATIONS OF THE FINDINGS
These findings indicate no significant impact of conception mode on early placental development and a beneficial impact for frozen-thawed ET on the second- and third-trimester Doppler indices. This suggests that frozen-thawed ET may not be as detrimental for placental perfusion as previous research has demonstrated. As the number of clinics applying the ‘freeze-all strategy’ increases, future research should focus on establishing the optimal uterine environment, with regards to hormonal preparation, prior to ET to reduce placental-related pregnancy complications after frozen-thawed ET.
STUDY FUNDING/COMPETING INTEREST(S)
This research was funded by the Erasmus MC Medical Research Advisor Committee’s ‘Health Care Efficiency Research’ program and the department of Obstetrics and Gynaecology of the Erasmus MC, University Medical Center, Rotterdam, The Netherlands. JSEL reports grants and personal fees from Ferring, personal fees from Titus Healthcare, grants and personal fees from Ansh Labs, grants from NIH, grants from Dutch Heart Association and grants from ZonMW outside the submitted work. None of the other authors have a conflict of interest.
TRIAL REGISTRATION NUMBER
Registered at the Dutch Trial Register (NTR6684).
Keywords: placenta, trophoblasts, in-vitro fertilisation, ultrasound, frozen-thawed embryo transfer
Introduction
In-vitro fertilisation (IVF) treatment is a relatively successful fertility treatment to achieve pregnancy. However, these pregnancies are associated with increased risks of placenta-related pregnancy complications, such as preeclampsia and foetal growth restriction (Pandey et al., 2012). These complications also have consequences for long-term maternal and offspring health (de Boo and Harding, 2006; Brown et al., 2013). Moreover, recent studies have demonstrated that some complications are more prevalent in pregnancies after frozen-thawed embryo transfer (ET) than after fresh ET (Maheshwari et al., 2018; Wei et al., 2019). However, a potential life-threatening complication of IVF treatment is the ovarian hyperstimulation syndrome (OHSS), which is caused by pharmacologic ovarian stimulation (Blumenfeld, 2018). To lower the risks of this complication, an increasing number of fertility clinics transfer only frozen-thawed embryos as the standard procedure (Wong et al., 2017). Although this strategy does not seem to reduce IVF success rates in terms of implantation, there may be serious consequences for the prevalence of preeclampsia after IVF-treatment (Roque et al., 2019).
A well-developed placenta is essential for successful pregnancy. It is crucial that the placenta is able to exchange gasses and supply nutrients to facilitate the embryo in reaching its full growth potential (Sandovici et al., 2012). Placental development starts 6–10 days after conception, when embryonic trophoblast cells invade the decidua (Boss et al., 2018). The maternal decidua is supplied with blood from the spiral arteries, which extensively remodel from the first trimester onwards after invasion of the extravillous trophoblast (Brosens, 2011). Deficiencies in remodelling have been associated with severe pregnancy complications, such as miscarriage, foetal growth restriction and preeclampsia (Steegers et al., 2010; Reijnders et al., 2018).
IVF-treatment procedures can have several consequences for embryo implantation and placental development. For example, it has been suggested that the supraphysiological levels of hormones used for ovarian stimulation during IVF-treatment impact endometrial development (Kolibianakis et al., 2002; Bourgain and Devroey, 2003; Devroey et al., 2004). Also, studies show that the hormonal preparation of the endometrium prior to frozen-thawed ET may have a detrimental effect on maternal adaptation to pregnancy (Saito et al., 2019).
It is challenging to assess in vivo placental development during pregnancy. To measure features of placental development, two-dimensional (2D) and three-dimensional (3D) ultrasound techniques, as well as 3D power Doppler (PD) can be applied. Such features include first-trimester placental volume (PV), utero-placental vascular volume (uPVV) and uterine vascular parameters such as pulsatility and resistance indices of the uterine arteries (UtA-PI and UtA-RI). Some of these features have been associated with birth weight, placental weight and even placenta-related pregnancy complications (Schuchter et al., 2001; Effendi et al., 2014; Plasencia et al., 2015; Papastefanou et al., 2018; Soongsatitanon and Phupong, 2019).
Less is known about the impact of IVF treatment with fresh or frozen-thawed ET on these features of first-trimester placental development. The only two studies that distinguished between pregnancies after fresh and frozen-thawed ET have demonstrated a larger first-trimester PV in pregnancies after frozen-thawed ET than in pregnancies after fresh ET (Rizzo et al., 2016; Choux et al., 2019). However, both studies had a cross-sectional design and PV was obtained at the end of the first trimester. Although this may provide insight in the pathophysiology underlying the increased risk of placenta-related pregnancy complications in pregnancies after frozen-thawed ET, longitudinal data of several features of placental development are lacking. It is likely that variations in IVF-treatment have already had an impact on placental development earlier in gestation, as implantation initiates placental development by inducing alterations in the decidua and spiral arteries (Turco and Moffett, 2019). Therefore, our aim is to study differences in placental development, measured by longitudinal 2D and 3D (including virtual reality (VR)) ultrasound measurements in the first, second and third trimester, between pregnancies resulting from fresh ET, frozen-thawed ET and after natural conception.
Materials and methods
Study population
The data used for this study were collected as part of the VIRTUAL Placenta study (Dutch Trial Register number: 6684), a subcohort of the Rotterdam Periconception cohort (Predict Study), which focuses on ultrasound markers of (early) placentation (Steegers-Theunissen et al., 2016). This study was conducted from January 2017 to March 2018 at the outpatient clinic of the Department of Obstetrics and Gynaecology of the Erasmus MC, University Medical Center, Rotterdam, the Netherlands. Women 18 years and older, before 10 weeks of gestation with a viable singleton pregnancy were eligible for participation. To validate our results in a larger cohort, we studied the associations between mode of conception and PV in the total Rotterdam Periconception cohort. Other features of placental development were not measured in this study. The Predict study is an ongoing prospective cohort study, which has been conducted from November 2010 onwards after a pilot phase. Eligibility criteria were similar to those of the VIRTUAL Placenta study. Pregnancies after intrauterine insemination (IUI) or ovulation induction (OI) were included as natural conceptions, since fertilisation occurs in vivo and gonadotrophin levels are within physiological ranges. Pregnancies after oocyte donations, pregnancies resulting in a miscarriage and drop-outs were excluded.
Ethical approval
The studies were approved by the Medical Ethical and Institutional Review Board of the Erasmus University Medical Center, Rotterdam, The Netherlands (MEC-2004-227 and METC 2015-494). Prior to participation, written informed consent was obtained from women and their partners, as well as on behalf of their unborn child.
IVF, cryopreservation and culture procedures
Procedures for ovarian stimulation, oocyte retrieval, IVF, intracytoplasmic sperm injection (ICSI) and assessment of embryo morphology were performed as described extensively in two other publications (Hohmann et al. 2003; Heijnen et al., 2007). Until November 2014, embryos were cultured in Vitrolife G5 series (Vitrolife, Goteborg, Sweden); thenceforth embryos were cultured in SAGE 1-StepTM (CooperSurgical, Trumbull, CT, USA). Evaluation and selection of embryos for transfer was performed on Day 3 after oocyte retrieval, based on morphology. Supernumerary embryos of adequate quality were cultured until Day 4, when selection for cryopreservation was performed based on the degree of compaction and presence of fragmentation (Ebner et al., 2009). First, selected embryos were incubated for 10 min in culture medium containing 1.0 di-methyl sulfoxide (DMSO) and loaded into straws (CBS High Security embryo straw, CryoBioSystem, Saint-Ouen-Sur-Iton, France). Second, cryopreservation was performed by slow-freezing the straws in a controlled rate freezer (Kryo 360, Planer, Sunbury-on-Thames, UK) to −40°C at −0.3°C/min. Subsequently, the straws were cooled rapidly at −25°C/min to −140°C. Finally, after immersion in liquid nitrogen, the straws were stored in nitrogen vapour. Duration of storage varied between 1 month and 5 years.
If patients had a regular menstrual cycle, frozen-thawed ET was performed in a natural cycle. If patients had an irregular or absent menstrual cycle, frozen-thawed ET was performed in a hormonally-prepared endometrium. This was achieved by increasing dosages of intravaginal oestrogens and optionally daily injections of a GnRH-agonist. Embryos were thawed 4 days after spontaneous ovulation, or after approximately 19 days of endometrial preparation. Until March 2016, this was accomplished by consecutive washes in decreasing concentrations of DMSO in buffered culture medium at room temperature. Thereafter, the Quinn’s AdvantageTM Thaw Kit (CooperSurgical) was used for thawing. One hour after thawing, embryos were checked for survival and cultured overnight in an atmosphere of 5% CO2 and 7% O2 in 1 ml of SAGE 1-Step culture medium at 37°C. The following day, morphology was evaluated and only embryos demonstrating developmental progression were transferred.
Study parameters
At enrolment, women completed a self-administered questionnaire regarding general characteristics and periconceptional lifestyle factors. All data were verified by a research nurse and anthropometric measurements were performed. Mean arterial pressure (MAP) was calculated using the following formula: (systolic blood pressure + 2*diastolic blood pressure)/3. In IVF/ICSI pregnancies, medical records were screened to assess conception mode (i.e. frozen-thawed ET or fresh ET) and fertilisation method (IVF or ICSI). Geographical origin was classified conform the definition of Statistics Netherlands, that is, Western or non-Western. Educational level was categorised into low, intermediate and high. The use of cigarettes or alcohol was defined as any use during the periconception period, defined as 14 weeks prior to conception to 10 weeks after (Steegers-Theunissen et al., 2013). Preconception daily use of folic acid supplements was defined as adequate, whereas no or post-conception initiation was defined as inadequate.
In pregnancies after IVF/ICSI, gestational age (GA) at ultrasound was determined based on the moment of transfer. For frozen-thawed ET, GA was calculated by adding 19 days to the date of transfer, since frozen-thawed embryos were transferred at Day 5 of embryo development. For fresh ET, GA was calculated by adding 14 days to the date of oocyte retrieval. In pregnancies after natural conception, GA at ultrasound was based on the last menstrual period (LMP). If participants had a regular menstrual cycle of <25 or >32 days, GA was adjusted for the duration of the menstrual cycle. If LMP was unknown or if the GA based on LMP and GA based on crown-rump length (CRL) differed >7 days, GA was based on the CRL at ultrasound performed at 9 weeks of gestation.
Ultrasound data
All ultrasound scans were performed according to the international guidelines for safe use of (Doppler) ultrasound imaging in pregnancy (Bhide et al., 2013). Ultrasound images were obtained by three trained researches who have their expertise in first-trimester imaging. All three also first conducted a learning curve and their reliability was tested, which was good for all three. Placental volume (PV, cm3) was measured by using 3D ultrasound volumes of the whole gestational sac including the placenta, obtained at Weeks 7, 9 and 11 of gestation, using a 6–12 MHz transvaginal probe compatible with the GE Voluson E8 and E10 Expert system in the total cohort. A minimum of two volumes were recorded in a perpendicular angle (90°) to increase the chances of obtaining a complete and high-quality volume. PV was measured offline by using Virtual Organ Computer-Aided AnaLyses (VOCAL) software. This technique has been previously described and is validated to measure PV in the first trimester of pregnancy, with intra-class correlation coefficients (ICC) >0.97 (Rifouna et al., 2014). uPVV was measured in 3D-PD ultrasound volumes in the subcohort, obtained conform PV, with standardised settings (pulse repetition frequency 0.6 kHz, wall motion filter ‘low 1’, quality ‘high’, gain −8.0) and expressed in cm3. The uPVV was measured offline on a VR desktop system, which projects the ultrasound datasets as holograms that can be enlarged and rotated for more precision (Reijnders et al., 2018). By erasing the volume of the embryonic and myometrial vessels, only the vessels up to the myometrial-placental border remained, thus, the uPVV. This technique has an ICC >0.94 (Reijnders et al., 2018).
The pulsatility index (PI) and resistance index (RI) of the uterine artery (UtA) was measured bilaterally in 3-fold at Weeks 7, 9, 11 and 13 of gestation by using transvaginal pulsed wave Doppler ultrasound in the subcohort. The mean of these indices at each measurement moment was used for analysis. At Weeks 22 and 32 of gestation, these indices were measured transabdominally by a single researcher with expertise in second- and third-trimester ultrasound imaging.
Statistical analyses
The study populations were stratified into three groups according to mode of conception: pregnancies after IVF/ICSI with (1) fresh ET or (2) frozen-thawed ET and (3) naturally conceived pregnancies. Baseline characteristics between these three groups were compared with either the Kruskall–Wallis test (continuous variables) or χ2 (categorical variables). The associations between conception mode and PV, uPVV, UtA-PI and UtA-RI were assessed by linear mixed models. Prior to analyses, PV and uPVV were cube-root transformed to obtain linearity. For the UtA indices, the linear mixed models were performed separately for the first-, second- and third-trimester measurements. All linear mixed model analyses were performed using both pregnancies after fresh ET as well as naturally conceived pregnancies as reference. This approach enables comparison between all modes of conception. Two linear mixed models were constructed; the first was adjusted for GA only (Model 1) and the second was additionally adjusted for confounders based on literature (Model 2). Similar models were applied in our validation cohort. P-values <0.05 were considered statistically significant. All analyses were performed in SPSS 24.0 (IBM SPSS Statistics, Armonk, NY, USA).
Results
Baseline characteristics
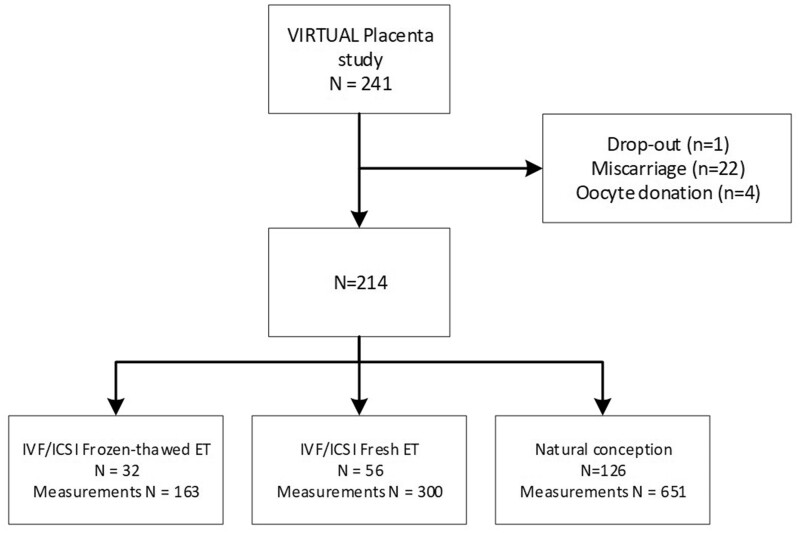
The VIRTUAL Placenta study population comprised 32 pregnancies after frozen-thawed ET, 56 after fresh ET and 126 that were naturally conceived, corresponding to a total of 1114 measurements (Fig. 1). Of the IVF/ICSI pregnancies, 49 (55.6%) were after the first cycle of ovarian stimulation. Six women in the frozen-thawed ET group did not have a fresh ET after fertilisation, four due to OHSS. In the frozen thawed-ET-group, 8 received hormonal preparation and 22 did not. Of the 126 naturally conceived pregnancies, five were achieved by a natural IUI cycle, eight were by OI and two were from a combination of IUI and OI.
Figure 1.
Flowchart of the Rotterdam Periconception subcohort: the VIRTUAL Placenta study. ET, embryo transfer.
The women who were pregnant after natural conception were less often nulliparous than women pregnant after IVF/ICSI with either frozen-thawed ET or fresh ET (39.7%, 71.9%, and 81.8%, respectively, P < 0.001) and had used folic acid supplements adequately less often (73.6%, 100% and 93.8%, respectively, P < 0.001). Women pregnant after fresh ET were more often from a Western background compared to women pregnant after frozen-thawed ET and after natural conception (90.9%, 71.9% and 77.8%, respectively, P = 0.048) (Table I). Maternal age, BMI, MAP, level of education, use of alcohol and cigarettes and fertilisation method were comparable between the three groups.
Table I.
Baseline characteristics of the VIRTUAL placenta subcohort, stratified for the mode of conception.
| IVF/ICSI Frozen-thawed ET n = 32 |
IVF/ICSI Fresh ET n = 56 |
Natural conception n = 126 |
||||||
|---|---|---|---|---|---|---|---|---|
| Median/N | IQR/% | Median/N | IQR/% | Median/N | IQR/% | P-value | Missing | |
| Maternal characteristics | ||||||||
| Age, years | 33.3 | 29.4–36.4 | 33.1 | 29.3–36.2 | 31.4 | 28.8–34.5 | 0.098 | 0 |
| Body mass index, kg/m2 | 24.2 | 21.9–28.4 | 23.9 | 21.8–27.3 | 25.3 | 22.5–29.7 | 0.118 | 0 |
| MAP, mmHg | 82.5 | 76.7–88.2 | 80 | 76.7–83.3 | 80 | 75.3–85.1 | 0.202 | 0 |
| Nulliparous | 23 | 71.9 | 45 | 81.8 | 50 | 39.7 | <0.001d,e | 1 |
| Geographical origin, Western | 23 | 71.9 | 50 | 90.9 | 98 | 77.8 | 0.048c,e | 0 |
| Education | 0.698 | 6 | ||||||
| Low | 4 | 12.5 | 4 | 7.3 | 10 | 8.3 | ||
| Middle | 8 | 28.1 | 22 | 40.0 | 38 | 31.4 | ||
| High | 19 | 59.4 | 29 | 52.7 | 73 | 60.3 | ||
| Alcohol, yesa | 13 | 40.6 | 10 | 17.9 | 34 | 27.0 | 0.067 | 0 |
| Smoking, yesb | 7 | 21.9 | 5 | 8.9 | 16 | 12.7 | 0.219 | 0 |
| Folic acid, yesb | 30 | 93.8 | 56 | 100 | 92 | 73.6 | <0.001d,e | 1 |
| Fertilisation method, ICSI | 15 | 46.9 | 38 | 67.9 | NA | 0.053 | 0 | |
IQR, interquartile range. MAP, mean arterial pressure.
aAny use during the 14 weeks prior to up to 10 weeks of gestation.
bDaily use during the 14 weeks prior to up to 10 weeks of gestation.
cSignificantly different between pregnancies after frozen-thawed ET and after fresh ET.
dSignificantly different between pregnancies after frozen-thawed ET and naturally conceived pregnancies.
eSignificantly different between pregnancies after fresh ET and naturally conceived pregnancies.
The validation cohort of the Predict study comprised a total of 722 pregnancies; 96 after frozen-thawed ET, 184 after fresh ET and 442 were naturally conceived (Supplementary Fig. S1). In the frozen-thawed ET group, 18 received hormonal preparation and 78 did not. Maternal age, BMI, parity, periconceptional use of alcohol and folic acid and fertilisation method were significantly different between the three groups (Supplementary Table SI).
PV and uPVV
The first-trimester PV growth trajectories in pregnancies after frozen-thawed ET were comparable to growth trajectories in pregnancies after fresh ET (Model 1: β −0.007 (95% CI: −0.184, 0.170), P = 0.936; Model 2: β −0.026 (95% CI: −0.206, 0.155), P = 0.778) and after natural conception (Model 1: β 0.002 (95% CI: −0.160, 0.163), P = 0.983; Model 2: β 0.006 (95% CI: −0.162, 0.174), P = 0.940) (Table II). A β greater than 0 indicates increased growth, whereas a β below 0 indicates reduced growth compared to the reference category. The first-trimester PV in pregnancies after fresh ET was also comparable to PV in pregnancies after natural conception (Table II). Similar results were observed in the validation cohort for both models (Table III).
Table II.
First-trimester trajectories of placental development and uterine artery indices in VIRTUAL placenta subcohort for pregnancies after frozen-thawed ET (n = 32), fresh ET (n = 56), and after natural conception (n = 126).
| Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|
| Beta (95% CI) | P-value | Beta (95% CI) | P-value | ||
| PV () | Frozen-thawed ET | −0.007 (−0.184, 0.170) | 0.936 | −0.026 (−0.206, 0.155) | 0.778 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | 0.002 (−0.160, 0.163) | 0.983 | 0.006 (−0.162, 0.174) | 0.940 | |
| Fresh ET | 0.003 (−0.127, 0.134) | 0.962 | 0.013 (−0.132, 0.158) | 0.857 | |
| Natural conception | Reference | Reference | |||
|
| |||||
| uPVV () | Frozen-thawed ET | 0.121(−0.098, 0.340) | 0.276 | 0.075 (−0.147, 0.297) | 0.506 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | 0.071 (−0.121, 0.263) | 0.465 | 0.112 (−0.085, 0.311) | 0.262 | |
| Fresh ET | −0.052 (−0.207, 0.103) | 0.505 | −0.009 (−0.180, 0.162) | 0.916 | |
| Natural conception | Reference | Reference | |||
|
| |||||
| UtA-PI | Frozen-thawed ET | 0.028 (−0.159 0.215) | 0.767 | 0.023 (−0.178, 0.223) | 0.833 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | −0.112 (−0.305, 0.080) | 0.251 | −0.130 (−0.330, 0.070) | 0.200 | |
| Fresh ET | −0.148 (−0.304, 0.009) | 0.064 | −0.125 (−0.298, 0.048) | 0.155 | |
| Natural conception | Reference | Reference | |||
|
| |||||
| UtA-RI | Frozen-thawed ET | −0.012 (−0.039, 0.015) | 0.383 | −0.012 (−0.041, 0.017) | 0.408 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | −0.022 (−0.050, 0.005) | 0.112 | −0.021 (−0.050, 0.007) | 0.114 | |
| Fresh ET | −0.011 (−0.034, 0.011) | 0.322 | −0.003 (−0.028, 0.021) | 0.783 | |
| Natural conception | Reference | Reference | |||
ET, embryo transfer. Model 1: Adjusted for GA.
Model 2: Adjusted for GA, parity, periconceptional folic acid supplement use and smoking.
Table III.
First-trimester trajectories of placental volume in the total cohort for pregnancies after frozen-thawed ET (n = 93), fresh ET (n = 172), and after natural conception (n = 435).
| Model 1 |
Model 2 |
|||
|---|---|---|---|---|
| Beta (95% CI) 3√cm3 | P-value | Beta (95% CI) 3√cm3 | P-value | |
| Frozen-thawed ET |
0.016 (−0.076, 0.108) |
0.735 |
0.016 (−0.080, 0.111) |
0.742 |
| Fresh ET | Reference | Reference | ||
|
| ||||
| Frozen-thawed ET |
0.030 (−0.057, 0.117) |
0.493 |
0.021 (−0.071, 0.112) |
0.657 |
| Fresh ET |
0.007 (−0.061, 0.075) |
0.849 |
−0.018 (−0.092, 0.056) |
0.632 |
| Natural conception | Reference | Reference | ||
Model 1: Adjusted for GA.
Model 2: Adjusted for GA, parity, periconceptional use of folic acid supplements and smoking.
95% CI: 95% confidence interval.
The first-trimester uPVV in pregnancies after frozen-thawed ET were comparable to uPVV in pregnancies after fresh ET and naturally conceived pregnancies in both models (Table II). First-trimester uPVV in pregnancies after fresh ET was also similar to uPVV in pregnancies after natural conception in both models (Table II).
Uterine artery indices
First-trimester UtA-PI and UtA-RI trajectories were not significantly different between pregnancies after frozen-thawed ET, fresh ET and after natural conception in both models (Table II).
In the second and the third trimester, the decline of UtA-PI in pregnancies after frozen-thawed ET was stronger compared to the UtA-PI in pregnancies after fresh ET (Model 1: β −0.122 (95% CI: −0.238, −0.029), P = 0.013; Model 2: β −0.158 (95% CI: −0.268, −0.048), P = 0.005) indicating a lower distal vascular resistance (Table IV). This was also observed when compared to pregnancies after natural conception in the crude model (β −0.115 (95% CI: −0.218, −0.012), P = 0.029).
Table IV.
Second- and third-trimester trajectories of uterine indices in the VIRTUAL Placenta subcohort for pregnancies after frozen-thawed ET (n = 32), fresh ET (n = 56), and after natural conception (n = 126).
| Model 1 |
Model 2 |
||||
|---|---|---|---|---|---|
| Beta (95% CI) | P-value | Beta (95% CI) | P-value | ||
| UtA-PI | Frozen-thawed ET | −0.122 (−0.238, −0.029) | 0.013 | −0.158 (−0.268, −0.048) | 0.005 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | −0.115 (−0.218, −0.012) | 0.029 | −0.104 (−0.211, 0.002) | 0.055 | |
| Fresh ET | 0.019 (−0.063, 0.101) | 0.647 | 0.048 (−0.042, 0.138) | 0.291 | |
| Natural conception | Reference | Reference | |||
|
| |||||
| UtA-RI | Frozen-thawed ET | −0.043 (−0.078, −0.008) | 0.018 | −0.052 (−0.089, −0.015) | 0.006 |
| Fresh ET | Reference | Reference | |||
|
|
|||||
| Frozen-thawed ET | −0.033 (−0.066, −0.001) | 0.043 | −0.028 (−0.062, 0.005) | 0.093 | |
| Fresh ET | 0.010 (−0.016, 0.035) | 0.454 | 0.020 (−0.008, 0.048) | 0.159 | |
| Natural conception | Reference | Reference | |||
Statistically significant values are in bold. Model 1: Adjusted for GA.
Model 2: Adjusted for GA, parity, periconceptional folic acid supplement use and smoking.
The second- and third- trimester UtA-RI decline in pregnancies after frozen-thawed ET was stronger than after fresh ET (Model 1: β −0.043 (95% CI: −0.078, −0.008), P = 0.018; Model 2: β −0.052 (95% CI: −0.089, −0.015), P = 0.006) (Table IV). Similar associations were observed when compared to naturally conceived pregnancies (Model 1: β −0.033 (95% CI: −0.066, −0.001), P = 0.043). The second- and third-trimester UtA-PI and UtA-RI in pregnancies after fresh ET were similar to the indices in pregnancies after natural conception pregnancies, suggesting no difference in placental perfusion.
Discussion
Summary of results
The current study is the first to investigate longitudinal differences in non-invasive parameters of placental development between pregnancies of different conception modes, that is, after frozen-thawed ET, after fresh ET and after natural conception. In the first trimester, placental developmental parameters (PV, uPVV, UtA-PI and UtA-RI) were comparable between pregnancies after frozen-thawed ET, after fresh ET and after natural conception. In the second and third trimester, however, pregnancies after frozen-thawed ET showed a lower UtA-PI and UtA-RI compared to pregnancies after fresh ET, which suggests a lower distal vascular resistance. Interestingly, these indices were comparable between pregnancies after fresh ET and after natural conception. Validation analyses of the association between first-trimester PV and conception mode in a larger cohort demonstrated similar results.
Comparison with literature and interpretation
Two previous studies have investigated differences in first-trimester PV between pregnancies after fresh ET and after frozen-thawed ET (Rizzo et al., 2016; Choux et al., 2019). The study populations of these studies consisted of 532 (Rizzo et al.) and 252 (Choux. et al.) pregnancies. Contrary to our findings, both studies show that PV is smaller in pregnancies after frozen-thawed ET than in pregnancies after fresh ET. The method of cryopreservation may be of importance when investigating placental development. Research shows higher pregnancy rates for vitrification when compared to slow-freezing, but perinatal outcomes are comparable between the two methods (Rienzi et al., 2017; Gu et al., 2019). As Choux et al. applied cryopreservation methods similar to ours, and Rizzo et al. did not report the method of cryopreservation, our results may not be generalisable to vitrification. There are also considerable differences between these studies and ours. The first difference is that the results of these studies were based on a single measurement from an abdominally acquired ultrasound, whereas we performed multiple vaginal ultrasound examinations in the first trimester. Second, in both studies, the ultrasound examination was performed at a later GA than in our study, that is, between 11 + 0 and 13 + 6 weeks of gestation. To explain these differences further, we performed additional linear regression analyses of PV at 7, 9 and 11 weeks of gestation independently, showing no significant differences between pregnancies after fresh ET, after frozen-thawed ET and after natural conception (data not shown). Finally, the study by Rizzo et al. provided no information on endometrial preparation prior to frozen-thawed ET, whereas Choux et al. investigated PV in pregnancies after frozen-thawed ET in hormonally induced cycles. Hence, these study populations are not comparable to ours, in which approximately 75% of all frozen-thawed ETs were performed in a natural cycle. A sensitivity analysis excluding frozen-thawed ETs in a hormonally induced cycle also demonstrated no significant association between PV and conception mode (data not shown). However, another explanation might be that the current study was insufficiently powered to detect associations between PV and conception mode.
Nevertheless, there is emerging evidence that the endocrine uterine environment at the moment of ET plays a critical role in maternal vascular adaptation to pregnancy and thus might affect placental development (Wang and Dey, 2006; Weinerman and Mainigi, 2014; von Versen-Hoynck et al., 2019). During hormonally induced cycles, the hypothalamic-pituitary axis is suppressed by consecutive administration of oestrogen and progesterone to prepare the endometrium for transfer. As a result, the corpus luteum is absent, whereas it is present in natural cycles as a major source of reproductive and vasoactive hormones, such as prorenin. Conrad et al. suggested that the presence of a corpus luteum is essential for an optimal maternal hormonal environment during implantation and hemodynamic adaption to pregnancy, and its absence may impact the maternal physiology and pregnancy outcome (Conrad and Baker, 2013). This might explain why we did not find any differences in the first-trimester placental developmental parameters between naturally conceived pregnancies and pregnancies after frozen-thawed ET, as the corpus luteum is present in the majority of pregnancies after frozen-thawed natural cycle ET.
Since there are multiple corpora lutea after ovarian stimulation followed by fresh ET, one may also expect differences in first-trimester placental developmental parameters between pregnancies after fresh ET and naturally conceived pregnancies. However, we did not observe such differences. Most studies that have investigated first-trimester placental development after IVF do not distinguish between pregnancies after fresh ET and after frozen-thawed ET, which complicates comparison to previous results. Rifouna et al. investigated uPVV in relation to conception mode and showed no differences in uPVV between naturally conceived pregnancies and pregnancies after IVF (Rifouna et al., 2014). Their study population comprised 84 naturally conceived pregnancies, 59 pregnancies after fresh ET and 11 after frozen-thawed ET, but no distinction was made between pregnancies after fresh ET and after frozen-ET due to the small sample size. The lack of distinction between pregnancies after fresh ET and after frozen-thawed ET is also observed in studies regarding first-trimester uterine artery indices and conception mode. Only Cavoretto et al. demonstrated a lower first-trimester UtA-PI in pregnancies after frozen-thawed ET than in pregnancies after fresh ET, whereas Rizzo et al. found no differences (Rizzo et al., 2016; Cavoretto et al., 2020).
In the second and the third trimester, we found a stronger decline in UtA-PI and UtA-RI in pregnancies after frozen-thawed ET than in pregnancies after fresh ET. This observation is in contrast with the reported increased risk for placenta-related pregnancy complications in pregnancies after frozen-thawed ET, which are associated with increased uterine artery indices (Cnossen et al., 2008; Maheshwari et al., 2018; Wei et al., 2019). Although beyond the scope of this study, we found no associations with the incidence of placenta-related pregnancy complications in our cohort (data not shown). Moreover, we found decreased indices in pregnancies after frozen-thawed ET, which was also observed by Cavoretto et al. (2020).
Recently, a major randomised controlled trial in 1508 women with polycystic ovarian syndrome (PCOS) showed that the risk of preeclampsia is higher after elective frozen-thawed cleavage ET than after fresh cleavage ET, which was not observed in 2157 ovulatory women (Chen et al., 2016; Shi et al., 2018). This might be explained by the overt difference in study populations, as pregnant women with PCOS are at increased risk for preeclampsia (Palomba et al., 2015). However, another considerable difference is the endometrial preparation preceding the elective frozen-thawed ET. For ovulatory women, the frozen-thawed ET was primarily performed in a natural cycle, whereas for women with PCOS, this was in a hormonally induced cycle. Together with our results, this supports the hypothesis that placental development after frozen-thawed natural cycle ET might be superior to placental development after frozen-thawed hormonal treatment cycle ET.
Strengths and limitations
The main strength of this study is the collection of longitudinal non-invasive measurements of early placental development using 3D ultrasound in combination with VR. Placental parameters were measured by experienced researchers and the applied innovative techniques have an excellent reproducibility (Reijnders et al., 2018) Furthermore, our results regarding the associations between conception mode and PV, a thoroughly studied non-invasive parameter of placental development, were validated in a larger cohort in the same setting and study design with over 700 participants. Finally, the evaluation of both naturally conceived pregnancies as well as pregnancies after fresh ET as a reference category enabled interpretation of our results in a wider perspective.
The main limitation of this study is the relatively small size of the VIRTUAL Placenta study to detect differences in placental development and placenta-related pregnancy outcomes between pregnancies of different modes of conception. Also, our frozen-thawed ET group consisted of both freeze-all cycles as well as cycles with an initial fresh ET. This may have implications, as it is not a homogeneous study population. Moreover, we performed cryopreservation on Day 4 using a slow-freeze method and frozen-thawed ET was usually performed in a natural cycle. As this is not common practice for all clinics, this may have affected our results and their generalisability. Although we adjusted for multiple confounders to minimise the effect of differences between groups, for example, previous unsuccessful ET, we cannot eliminate residual confounding, due to the observational character of this study. For example, the endometrium might have been a confounding factor in our analyses, but the small sample size and inconsistent data collection prevented adequate adjustment. Finally, as this study was conducted at a tertiary hospital, the naturally conceived pregnancies were at risk of developing pregnancy complications due to the presence of maternal comorbidities. This may have consequences for the extrapolation of our results to the general population.
Conclusion
In this study, we investigated differences in placental development between pregnancies resulting from fresh ET, frozen-thawed ET and natural conception, but did not find differences in first-trimester placental development. Furthermore, the second- and third-trimester uterine artery indices in pregnancies after frozen-thawed ET were lower than indices in pregnancies after fresh ET, and this is associated with higher placental perfusion. This is contrast with findings of other studies demonstrating a higher risk of placenta-related pregnancy complications after frozen-thawed ET (Maheshwari et al., 2018; Wei et al., 2019). These complications have serious implications for short- and long-term maternal and offspring health (Barker, 2007). In conclusion, this study can be considered hypothesis generating. For example, the maternal hormonal environment at the moment of frozen-thawed ET may have an essential role in placental development. Larger longitudinal studies are necessary to study this hypothesis and should also include measurements of this maternal environment, for example, endometrial thickness or quality. These studies are crucial, since approximately 1% of all newborns are conceived after frozen-thawed ET and the number of frozen-thawed ETs only continues to rise (De Geyter et al., 2018).
Data availability
The data underlying this article can be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
The authors thank the participating couples, gynaecologists and midwifery practices in Rotterdam for their contributions and the Rotterdam Periconception cohort team for data acquisition. We also thank Dr. Annemarie Mulders for her support in data acquisition and Dr. Anton Koning for his assistance with the acquisition and accessibility of VR data.
Authors’ roles
RST initiated the research question and supervised all aspects of the study. LvD and IR contributed to data-acquisition. EB and JL were responsible for IVF/ICSI patients. SW, MR and RST initiated and supervised statistical procedures of the manuscript. LvD and MR wrote the first version of the manuscript. All authors contributed to the writing and critical revisions of the manuscript and all authors approved the final version of the manuscript.
Funding
This research was funded by the Erasmus MC Medical Research Advisor Committee’s ‘Health Care Efficiency Research’ program and the Department of Obstetrics and Gynecology of the Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Conflict of interest
JSEL reports grants and personal fees from Ferring, personal fees from Titus Healthcare, grants and personal fees from Ansh Labs, grants from NIH, grants from Dutch Heart Association and grants from ZonMW outside the submitted work. None of the other authors have a conflict of interest.
References
- 2016 Assisted Reproductive Technology National Summary Report. Centers for Disease Control and Prevention. American Society for Reproductive Medicine. Atlanta (GA): Society for Assisted Reproductive Technology, 2018. [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–417. [DOI] [PubMed] [Google Scholar]
- Bhide A, Acharya G, Bilardo CM, Brezinka C, Cafici D, Hernandez-Andrade E, Kalache K, Kingdom J, Kiserud T, Lee W.. et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41:233–239. [DOI] [PubMed] [Google Scholar]
- Blumenfeld Z. The ovarian hyperstimulation syndrome. Vitam Horm 2018;107:423–451. [DOI] [PubMed] [Google Scholar]
- Boss AL, Chamley LW, James JL.. Placental formation in early pregnancy: how is the centre of the placenta made? Hum Reprod Update 2018;24:750–760. [DOI] [PubMed] [Google Scholar]
- Bourgain C, Devroey P.. The endometrium in stimulated cycles for IVF. Hum Reprod Update 2003;9:515–522. [DOI] [PubMed] [Google Scholar]
- Brosens I. Placental bed & maternal – fetal disorders. Preface. Best Pract Res Clin Obstet Gynaecol 2011;25:247–248. [DOI] [PubMed] [Google Scholar]
- Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R.. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- Cavoretto P, Farina A, Gaeta G, Sigismondi C, Spinillo S, Casiero D, Pozzoni M, Vigano P, Papaleo E, Candiani M.. Longitudinal cohort study of uterine artery Doppler in singleton pregnancies obtained by IVF/ICSI with fresh or frozen blastocyst transfers in relation to pregnancy outcomes. Ultrasound Obstet Gynecol 2020;56:603–610. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, Yang J, Liu J, Wei D, Weng N.. et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med 2016;375:523–533. [DOI] [PubMed] [Google Scholar]
- Choux C, Ginod P, Barberet J, Rousseau T, Bruno C, Sagot P, Astruc K, Fauque P.. Placental volume and other first-trimester outcomes: are there differences between fresh embryo transfer, frozen-thawed embryo transfer and natural conception? Reprod Biomed Online 2019;38:538–548. [DOI] [PubMed] [Google Scholar]
- Cnossen JS, Morris RK, ter Riet G, Mol BW, van der Post JA, Coomarasamy A, Zwinderman AH, Robson SC, Bindels PJ, Kleijnen J.. et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. Cmaj 2008;178:701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Baker VL.. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 2013;304:R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boo HA, Harding JE.. The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol 2006;46:4–14. [DOI] [PubMed] [Google Scholar]
- De Geyter C, Calhaz-Jorge C, Kupka MS, Wyns C, Mocanu E, Motrenko T, Scaravelli G, Smeenk J, Vidakovic S, Goossens V, European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2014: results generated from European registries by ESHRE: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum Reprod 2018;33:1586–1601.30032255 [Google Scholar]
- Devroey P, Bourgain C, Macklon NS, Fauser BC.. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab 2004;15:84–90. [DOI] [PubMed] [Google Scholar]
- Ebner T, Moser M, Shebl O, Sommergruber M, Gaiswinkler U, Tews G.. Morphological analysis at compacting stage is a valuable prognostic tool for ICSI patients. Reprod Biomed Online 2009;18:61–66. [DOI] [PubMed] [Google Scholar]
- Effendi M, Demers S, Giguere Y, Forest JC, Brassard N, Girard M, Gouin K, Bujold E.. Association between first-trimester placental volume and birth weight. Placenta 2014;35:99–102. [DOI] [PubMed] [Google Scholar]
- Gu F, Li S, Zheng L, Gu J, Li T, Du H, Gao C, Ding C, Quan S, Zhou C.. et al. Perinatal outcomes of singletons following vitrification versus slow-freezing of embryos: a multicenter cohort study using propensity score analysis. Hum Reprod 2019;34:1788–1798. [DOI] [PubMed] [Google Scholar]
- Heijnen EM, Eijkemans MJ, De Klerk C, Polinder S, Beckers NG, Klinkert ER, Broekmans FJ, Passchier J, Te Velde ER, Macklon NS.. et al. A mild treatment strategy for in-vitro fertilisation: a randomised non-inferiority trial. Lancet 2007;369:743–749. [DOI] [PubMed] [Google Scholar]
- Hohmann FP, Macklon NS, Fauser BC.. A randomized comparison of two ovarian stimulation protocols with gonadotropin-releasing hormone (GnRH) antagonist cotreatment for in vitro fertilization commencing recombinant follicle-stimulating hormone on cycle day 2 or 5 with the standard long GnRH agonist protocol. J Clin Endocrinol Metab 2003;88:166–173. [DOI] [PubMed] [Google Scholar]
- Kolibianakis E, Bourgain C, Albano C, Osmanagaoglu K, Smitz J, Van Steirteghem A, Devroey P.. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil Steril 2002;78:1025–1029. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S.. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update 2018;24:35–58. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC.. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A.. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update 2012;18:485–503. [DOI] [PubMed] [Google Scholar]
- Papastefanou I, Chrelias C, Siristatidis C, Kappou D, Eleftheriades M, Kassanos D.. Placental volume at 11 to 14 gestational weeks in pregnancies complicated with fetal growth restriction and preeclampsia. Prenat Diagn 2018;38:928–935. [DOI] [PubMed] [Google Scholar]
- Plasencia W, Gonzalez-Davila E, Gonzalez LA, Armas-Gonzalez M, Padron E, Gonzalez-Gonzalez NL.. First trimester placental volume and vascular indices in pregnancies complicated by preeclampsia. Prenat Diagn 2015;35:1247–1254. [DOI] [PubMed] [Google Scholar]
- Reijnders IF, Mulders A, Koster MPH.. Placental development and function in women with a history of placenta-related complications: a systematic review. Acta Obstet Gynecol Scand 2018;97:248–257. [DOI] [PubMed] [Google Scholar]
- Reijnders IF, Mulders A, Koster MPH, Koning AHJ, Frudiger A, Willemsen SP, Jauniaux E, Burton GJ, Steegers-Theunissen RPM, Steegers EAP.. New imaging markers for preconceptional and first-trimester utero-placental vascularization. Placenta 2018;61:96–102. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C.. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifouna MS, Reus AD, Koning AH, van der Spek PJ, Exalto N, Steegers EA, Laven JS.. First trimester trophoblast and placental bed vascular volume measurements in IVF or IVF/ICSI pregnancies. Hum Reprod 2014;29:2644–2649. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Aiello E, Pietrolucci ME, Arduini D.. Are there differences in placental volume and uterine artery Doppler in pregnancies resulting from the transfer of fresh versus frozen-thawed embryos through in vitro fertilization. Reprod Sci 2016;23:1381–1386. [DOI] [PubMed] [Google Scholar]
- Roque M, Haahr T, Geber S, Esteves SC, Humaidan P.. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update 2019;25:2–14. [DOI] [PubMed] [Google Scholar]
- Saito K, Kuwahara A, Ishikawa T, Morisaki N, Miyado M, Miyado K, Fukami M, Miyasaka N, Ishihara O, Irahara M.. et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum Reprod 2019;34:1567–1575. [DOI] [PubMed] [Google Scholar]
- Sandovici I, Hoelle K, Angiolini E, Constancia M.. Placental adaptations to the maternal-fetal environment: implications for fetal growth and developmental programming. Reprod Biomed Online 2012;25:68–89. [DOI] [PubMed] [Google Scholar]
- Schuchter K, Metzenbauer M, Hafner E, Philipp K.. Uterine artery Doppler and placental volume in the first trimester in the prediction of pregnancy complications. Ultrasound Obstet Gynecol 2001;18:590–592. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H.. et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med 2018;378:126–136. [DOI] [PubMed] [Google Scholar]
- Soongsatitanon A, Phupong V.. First trimester 3D ultrasound placental volume for predicting preeclampsia and/or intrauterine growth restriction. J Obstet Gynaecol 2019;39:474–479. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD.. The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update 2013;19:640–655. [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen RP, Verheijden-Paulissen JJ, van Uitert EM, Wildhagen MF, Exalto N, Koning AH, Eggink AJ, Duvekot JJ, Laven JS, Tibboel D.. et al. Cohort profile: the Rotterdam Periconceptional Cohort (Predict Study). Int J Epidemiol 2016;45:374–381. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R.. Pre-eclampsia. Lancet 2010;376:631–644. [DOI] [PubMed] [Google Scholar]
- Turco MY, Moffett A.. Development of the human placenta. Development 2019;146:dev163428. [DOI] [PubMed] [Google Scholar]
- von Versen-Hoynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR.. et al. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension 2019;73:640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dey SK.. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 2006;7:185–199. [DOI] [PubMed] [Google Scholar]
- Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, Tan J, Liang X, Cao Y, Wang Z.. et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet 2019;393:1310–1318. [DOI] [PubMed] [Google Scholar]
- Weinerman R, Mainigi M.. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril 2014;102:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S.. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2017;3:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article can be shared on reasonable request to the corresponding author.