Abstract
STUDY QUESTION
Are children of mothers with polycystic ovary syndrome (PCOS) or anovulatory infertility at increased risks of obesity or diabetes?
SUMMARY ANSWER
Maternal PCOS/anovulatory infertility is associated with an increased risk of offspring obesity from early age and diabetes in female offspring from late adolescence.
WHAT IS KNOWN ALREADY
Women with PCOS often have comorbid metabolic disorders such as obesity and diabetes, and children of mothers with PCOS have an increased risk of subtle signs of cardiometabolic alterations.
STUDY DESIGN, SIZE, DURATION
This was a nationwide cohort study of all live births (n = 1 105 997) during 1996–2014 in Finland, excluding those with maternal diagnoses sharing signs and symptoms with PCOS (n = 8244). A total of 1 097 753 births were included and followed up until 31 December 2018.
PARTICIPANTS/MATERIALS, SETTING, METHODS
National registries were linked to identify births with maternal PCOS or anovulatory infertility (n = 24 682). The primary outcomes were diagnoses of obesity (ICD-10: E65, E66) and diabetes (ICD-10: E10–E14) in offspring recorded in the Finnish Care Register for Health Care. Cox proportional hazards regression was modeled to analyze the risk of offspring obesity and diabetes in relation to prenatal exposure to maternal PCOS/anovulatory infertility. Differently adjusted models and stratified analyses were used to assess whether the risk was modified by maternal obesity or diabetes diagnoses, pre-pregnancy BMI, fertility treatment or perinatal problems.
MAIN RESULTS AND THE ROLE OF CHANCE
Exposure to maternal PCOS/anovulatory infertility was associated with a higher cumulative incidence of obesity in the children (exposed: 1.83%; 95% CI 1.66–2.00% vs unexposed: 1.24%; 95% CI 1.22–1.26%). Accounting for birth factors and maternal characteristics such as obesity and diabetes diagnoses, the hazard ratio (HR) for obesity was increased in offspring below 9 years of age (HR 1.58; 95% CI 1.30–1.81), and in those 10–16 years of age (HR 1.37; 95% CI 1.19–1.57), but not in those aged 17–22 years (HR 1.24; 95% CI 0.73–2.11). Sex-stratified analyses revealed similar risk estimates for boys (HR 1.48; 95% CI 1.31–1.68) and girls (HR 1.45; 95% CI 1.26–1.68). Notably, the joint effect of PCOS/anovulatory infertility and BMI-based pre-pregnancy obesity on offspring obesity (HR 8.89; 95% CI 7.06–11.20) was larger than that of either PCOS/anovulatory infertility or obesity alone. Furthermore, PCOS/anovulatory infertility was associated with offspring obesity in children without perinatal problems (HR 1.27; 95% CI 1.17–1.39), with larger effect size for maternal PCOS/anovulatory infertility and joint perinatal problems (HR 1.61; 95% CI 1.35–1.91). However, the risk estimates were comparable between maternal PCOS/anovulatory infertility with (HR 1.54; 95% CI 1.17–2.03) and without fertility treatment (HR 1.46; 95% CI 1.32–1.61). For offspring diabetes, the HR was increased only between 17 and 22 years of age (HR 2.06; 95% CI 1.23–3.46), and specifically for Type 1 diabetes in females (HR 3.23; 95% CI 1.41–7.40).
LIMITATIONS, REASONS FOR CAUTION
The prevalence of PCOS/anovulatory infertility in this study was 2.2%, lower than that reported in previous studies. In addition, the incidence of obesity in offspring was lower than that reported in studies based on measured or self-reported weight and height and may include mainly moderate and severe obesity cases who needed and/or actively sought medical care. Moreover, mothers with PCOS/anovulatory infertility were identified based on ICD codes, with no information on PCOS phenotypes. Furthermore, maternal pre-pregnancy BMI was available only from 2004. The PCOS/anovulatory infertility association with female offspring diabetes was based on only a few cases. Mothers’ weight gain during pregnancy, use of fertility treatment other than fresh or frozen IVF/ICSI, offspring lifestyle, as well as fathers’ age, medical disorders or medication prescriptions were not available for this study.
WIDER IMPLICATIONS OF THE FINDINGS
These findings support that prenatal PCOS/anovulatory infertility exposure influences metabolic health in the offspring from early age.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by Shandong Provincial Natural Science Foundation, China [ZR2020MH064 to X.C.], Shandong Province Medical and Health Technology Development Plan [2018WS338 to X.C.], the joint research funding of Shandong University and Karolinska Institute [SDU-KI-2019-08 to X.C. and C.L.], the Finnish Institute for Health and Welfare: Drug and Pregnancy Project [M.G.], the Swedish Research Council [2014-10171 to C.L.], the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institute Stockholm County Council [SLL20170292 and SLL20190589 to C.L.], the Swedish Brain Foundation [FO2018-0141 and FO2019-0201 to C.L.]. X.C. received grants from the China Scholarship Council at the beginning of the study. The authors have no competing interests to disclose.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: polycystic ovary syndrome, anovulatory infertility, offspring, obesity, diabetes, perinatal outcomes, fertility treatment
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine-metabolic disorder in reproductive-aged women, characterized by oligoovulation/anovulation, clinical or biochemical hyperandrogenism, and polycystic ovaries. Though women with PCOS have children as often as women without PCOS (Joham et al., 2014), the affected women are more likely to face fertility problems and to seek help from ART. In addition, mothers with PCOS more often have pregnancy complications and less favorable pregnancy outcomes (Palomba et al., 2015; Escobar-Morreale, 2018; Bahri Khomami et al., 2019). Furthermore, PCOS is featured by a range of metabolic dysfunctions, such as obesity and Type 2 diabetes (Teede et al., 2018).
PCOS status, associated comorbidities and pregnancy complications, provide a suboptimal intrauterine environment for the fetus. Together with genetic predisposition, and in consistence with the Barker hypothesis (Hales and Barker, 2001), PCOS may have implications for metabolic health in offspring. A meta-analysis observed subtle signs of altered cardiometabolic health such as increased insulin resistance and HDL-cholesterol concentrations in PCOS-exposed offspring aged 2–17 years old (Gunning et al., 2020). In addition, cohort studies have revealed higher post-stimulated insulin levels or hyperinsulinism in PCOS-exposed daughters, present around puberty and persisting during postmenarchal period (Sir-Petermann et al., 2007; Kent et al., 2008; Sir-Petermann et al., 2009; Crisosto et al., 2019). In male children with PCOS exposure, heavier weight from infancy to adulthood, and altered lipid metabolism during puberty have been reported (Recabarren et al., 2008; Crisosto et al., 2017). Recently, a cohort study found increased risks of PCOS diagnosis in daughters with maternal PCOS (Risal et al., 2019), but whether offspring with maternal PCOS are more likely to develop metabolic diseases such as obesity and diabetes remains unknown. In the general population, perinatal problems including preterm birth, large or small for gestational age (SGA) have been well established as risk factors for metabolic health (Mericq et al., 2017). Also, it was recently reported that children born after fertility treatment were at increased risk of metabolic dysfunctions (Cui et al., 2020). Whether a putative association of maternal PCOS with offspring obesity and/or diabetes would be modified by perinatal problems and fertility treatment, or not, is unknown.
Based on the nationwide birth cohort in Finland, this study aimed to examine the risks of offspring obesity and diabetes until 22 years of age in relation to maternal PCOS. The risks were assessed in both male and female children. Differently adjusted models and stratified analyses were performed to investigate the effects of maternal diagnosis of obesity and diabetes, pre-pregnancy BMI, fertility treatment, and perinatal problems.
Materials and methods
Ethical statement
Study approvals were obtained from the Finnish authorities providing the data, as well as the data protection authority (THL/1662/5.05.00/2015, THL/1853/5.05.00/2016 and THL/1496/5.05.00/2019). Based on regulations in Finland, informed consent was not required for analysis of anonymous data from registers.
Study population and data source
The index cases were all live births in Finland between 1996 and 2014, identified from the Drugs and Pregnancy Database (Artama et al., 2011), originally registered in the Medical Birth Register (MBR). Clinical diagnoses were retrieved from the Finnish Care Registers for Health Care (HILMO), which covers all hospitals and healthcare providers in Finland. Due to the availability of data on maternal BMI only in 2004–2014, analysis on offspring obesity risks in relation to maternal PCOS of different BMI strata was performed within the birth cohort 2004–2014. Purchases of prescribed drugs were obtained from the Finnish Register on Reimbursement Drugs (Supplementary materials and methods). Data from different datasets and registers were merged with the anonymized personal identification numbers issued to Finnish citizens and permanent residents. The data analysis was performed between 1 October 2019 and 10 March 2021.
Main exposures
The exposure was maternal PCOS, identified from the HILMO based on diagnosis of PCOS (International Statistical Classification of Diseases and Related Health Problems codes ninth version [ICD-9]: 256.4; tenth version [ICD-10]: E28.2) or anovulatory infertility (ICD-9: 628.0; ICD-10: N97.0) (Supplementary Table SI), as PCOS is the most common cause for anovulatory infertility. Because the hormonal and metabolic disturbances of PCOS persist throughout a woman’s lifespan, such a diagnosis was identified as an exposure regardless of the year. In this study, births to mothers with diagnoses sharing signs and symptoms with PCOS were excluded (n = 8244): pituitary gland disorders including hypo/hyper function (ICD-9: 253; ICD-10: E22, E23), pituitary adenoma (ICD-9: 227.3; ICD-10: D35.2), adrenal gland disorders including congenital adrenal hyperplasia and Cushing’s syndrome (ICD-9: 255; ICD-10: E24/E25/E27), suprarenal tumor (ICD-9: 194; ICD-10: C74), galactorrhea (ICD-9: 611.6; ICD-10: N64.3), and Turner syndrome (ICD-9: 758.6; ICD-10: Q96). In the final analysis, 24 682 children with maternal PCOS/anovulatory infertility and 1 073 071 without maternal PCOS/anovulatory infertility were included. All children were followed up until 31 December 2018.
Preterm birth was birth before gestational Week 37. SGA (birth weight and/or length 2 SDs below mean) and large for gestational age (LGA, birth weight and/or length 2 SDs above mean) are defined according to age- and sex-specific reference means of Finnish standards (Sankilampi et al., 2013), following the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society (Clayton et al., 2007). Appropriate for gestational age (AGA) is the interval between SGA and LGA.
Fertility treatment was fresh and frozen IVF/ICSI (check-box in the MBR).
Outcome measures
The primary outcomes were diagnoses of obesity (ICD-10: E65, E66) and diabetes (ICD-10: E10–E14) in offspring recorded in the HILMO. Diagnosis in the HILMO covered patients from all hospital inpatient care and outpatient clinics in Finland.
In Finland, regular health check-ups for young children are provided by municipal child health clinics free of charge, covering about 99% of families. During the first year, a child visits health clinic at least nine times, and a series of check-ups is followed before primary school. Children ≥7 years attend primary school, where they are provided with periodical health check-ups by school nurses. For each visit, height and body weight are registered. Therefore, child health care and school health care are essentially responsible for early detection of obesity and diabetes (Stockmarr et al., 2016). Cases are then referred to pediatricians in outpatient clinics. However, not all children identified as obese by school nurses attend clinical care. Therefore, the pediatric obesity cases from the HILMO based on ICD codes may include mainly moderate-to-severe obesity cases that needed and/or actively sought medical care for their symptoms.
The obesity status for offspring ≥18 years is assessed with body mass index (BMI, weight/height2). For children (below 18 years), the distribution of BMI changes with age and is dependent on sex (Cole et al., 2000). Obesity in children from Finland is assessed using weight-for-height or BMI-for-sex-and-age (ISO-BMI), with reference values based on 561 392 height and 75 810 weight measurements in Finnish children (Saari et al., 2011). When using weight-for-height, in children below 7 years, obesity is defined as >20% deviation above the reference population, whereas in children ≥7 years, >40% deviation above reference defines obesity. When using ISO-BMI, age- and sex-specific BMI cutoffs corresponding to adults' cutoff 30 kg/m2 is used for obesity (Library FH, 2020). From 2011 onwards, utilization of ISO-BMI has been recommended (Saari et al., 2011). There might be misclassification for offspring diabetes type when the children are young; hence, we recorded the first and second diagnosis codes (Supplementary Table SII). In this study, the child was classified based on the second diabetes diagnosis code (if there was any), which was regarded more trustable.
Covariates
Information on mothers’ age at delivery, smoking during pregnancy, marital status at delivery, maternal socioeconomic status (SES) (based on maternal occupation during pregnancy and categorized in upper white collar worker, lower white collar worker, blue collar worker, and other [e.g. entrepreneur, student or housewife]), mothers’ psychiatric disorders (inpatient and outpatient care due to mental health disorders before pregnancy according to ICD-9: 290–319, and ICD-10: F00–F99) (Supplementary Table SI), offspring birth year, mode of delivery, number of fetuses were obtained from the Drugs and Pregnancy Database and used as covariates when applicable.
Maternal obesity (ICD-10: E65, E66) and diabetes (ICD-10: E10–E14, O24, or prescription of A10A [insulin and analogues] or A10B [other blood glucose lowering drugs excluding insulin, also referred to as oral antidiabetic drugs] following the Anatomical Therapeutic Chemical Classification System [ATC]) were obtained from the Drugs and Pregnancy Database. Insulin-treated pre-gestational diabetes was identified using the Register on Reimbursement Drugs, from records on the special reimbursements of insulin medication for diabetes. Maternal insulin-treated pre-gestational diabetes, other diabetes (E11–E14, O24, A10B treated before pregnancy, A10A or A10B treated only during pregnancy), and maternal obesity (E65, E66) were used as three covariates in the main analysis. Mothers’ BMI was calculated based on pre-pregnancy weight and height (recorded at gestational Weeks 7–10 of the first prenatal visit), retrieved from the MBR and was available from 2004.
Statistical analyses
All statistical analyses were performed using SAS versions 9.3 and 9.4 (SAS Institute, Cary, USA). Descriptive statistics were reported as numbers of observations and prevalence (%). The Kaplan–Meier (KM) method was used to calculate cumulative incidence and 95% CI for offspring obesity and diabetes. Cox proportional hazards modeling was used to estimate the association of maternal PCOS/anovulatory infertility with obesity and diabetes in offspring, overall and in sex-stratified models, reporting hazard risk ratios (HRs) with 95% CI as measures of effect size. Missing data for caesarean section, marital status and SES was handled as a separate group. To account for potential effects of puberty on the development of obesity and diabetes (Hockett et al., 2019), the risk was examined for all offspring first, and then stratified by ages into three groups: ≤9 years, 10–16 years, and 17–22 years. Type 1 and Type 2 diabetes differ in pathophysiology; therefore, besides all types of offspring diabetes combined, the association of maternal PCOS/anovulatory infertility with Type 1 diabetes in offspring was also assessed. For offspring Type 2 diabetes, the number was too small for a subgroup analysis. Pre-pregnancy BMI (three strata: <25, 25–29, ≥30 kg/m2), fertility treatment (yes/no) and perinatal problems (yes/no) were tested for statistical interaction (on the multiplicative scale) with PCOS/anovulatory infertility on offspring obesity, followed by stratified analyses.
Results
Study population
We followed up 1 097 753 individuals from birth to 22 years for the oldest, that is, 14 035 113 person-years in this study. Of them, 24 682 children with maternal PCOS/anovulatory infertility contributed to 277 097 person-years of observation. A total of 5052 (20.5%) children with maternal PCOS/anovulatory infertility were 17–22 years old at the end of follow-up, versus 327 533 (30.5%) in the unexposed group. Compared with PCOS/anovulatory infertility-unexposed children, children with maternal PCOS/anovulatory infertility had an age distribution skewed toward younger age, indicating more deliveries in recent years. They were also more likely to be born preterm and LGA, whereas mothers with PCOS/anovulatory infertility more often had obesity and diabetes. Pre-pregnancy BMI (available 2004 onwards) was 25.8 ± 5.7 for mothers with PCOS/anovulatory infertility and 24.3 ± 4.8 for mothers without PCOS/anovulatory infertility (Table I).
Table I.
Demographic and clinical characteristics of offspring stratified by maternal polycystic ovary syndrome (PCOS) or anovulatory infertility.
| Variable | Maternal PCOS/anovulatory infertility (n = 24 682) | No maternal PCOS/anovulatory infertility (n = 1 073 071) |
|---|---|---|
| Birth year | ||
| 1996–2000 | 4255 (17.2) | 283 112 (26.4) |
| 2001–2005 | 5540 (22.4) | 274 431 (25.6) |
| 2006–2010 | 7602 (30.8) | 288 828 (26.9) |
| 2011–2014 | 7285 (29.5) | 226 700 (21.1) |
| Offspring sex | ||
| Female | 11 949 (48.4) | 524 538 (48.9) |
| Male | 12 733 (51.6) | 548 533 (51.1) |
| Offspring age in 2018, years | ||
| 0–9 | 10 929 (44.3) | 352 940 (32.9) |
| 10–16 | 8701 (35.3) | 392 598 (36.6) |
| 17–22 | 5052 (20.5) | 327 533 (30.5) |
| Caesarean section | ||
| Yes | 5698 (23.1) | 154 953 (14.4) |
| No | 18 967 (76.8) | 916 721 (85.4) |
| Missing | 17 (0.1) | 139 (0.1) |
| Number of fetuses | ||
| 1 | 23 539 (95.4) | 1 041 732 (97.1) |
| ≥2 | 1143 (4.6) | 31 339 (2.9) |
| Birth weight | ||
| SGA | 824 (3.3) | 33 971 (3.2) |
| AGA | 22 913 (92.8) | 1 007 377 (93.9) |
| LGA | 945 (3.8) | 31 723 (3.0) |
| Preterm birth | 2168 (8.8) | 58 377 (5.4) |
| Mother born in Finland | ||
| Yes | 22 670 (91.8) | 987 139 (92.0) |
| No | 2012 (8.2) | 85 932 (8.0) |
| Mothers age at delivery, years | ||
| <25 | 3335 (13.5) | 199 630 (18.6) |
| 25–29 | 7622 (30.9) | 340 611 (31.7) |
| 30–34 | 8541 (34.6) | 334 348 (31.2) |
| ≥35 | 5184 (21.0) | 198 484 (18.5) |
| Mother smoking during pregnancy | ||
| Yes | 3817 (15.5) | 187 942 (17.5) |
| No | 20 865 (84.5) | 885 129 (82.5) |
| Marital status at birth | ||
| Married | 16 554 (67.1) | 636 044 (59.3) |
| Cohabiting | 6028 (24.4) | 313 006 (29.2) |
| Single | 1769 (7.2) | 103 245 (9.6) |
| Missing | 331 (1.3) | 20 776 (1.9) |
| Mother SES | ||
| Upper white collar worker | 4127 (16.7) | 178 991 (16.7) |
| Lower white collar worker | 9564 (38.7) | 382 070 (35.6) |
| Blue collar worker | 3604 (14.6) | 155 596 (14.5) |
| Other status | 3591 (14.5) | 187 070 (17.4) |
| Missing | 3796 (15.4) | 169 344 (15.8) |
| Maternal psychiatric disorders | ||
| Yes | 3092 (12.5) | 83 793 (7.8) |
| No | 21 590 (87.5) | 989 278 (92.2) |
| Maternal obesity | ||
| Yes | 1592 (6.5) | 28 119 (2.6) |
| No | 23 090 (93.5) | 1 044 952 (97.4) |
| Maternal diabetes | ||
| PGDM | 311 (1.3) | 5616 (0.5) |
| Other diabetes | 5755 (23.3) | 154 107 (14.4) |
| No diabetes | 18 616 (75.4) | 913 348 (85.1) |
| Maternal BMI (mean/SD)*, kg/m2 | 25.8 (5.7) | 24.3 (4.8) |
| Offspring obesity | ||
| Yes | 451 (1.8) | 13 342 (1.2) |
| No | 24 231 (98.2) | 1 059 729 (98.8) |
| Offspring diabetes | ||
| Yes | 214 (0.9) | 9391 (0.9) |
| No | 24 468 (99.1) | 1 063 680 (99.1) |
| Offspring Type 1 diabetes | ||
| Yes | 202 (0.8) | 9075 (0.8) |
| No | 24 480 (99.2) | 1 063 996 (99.2) |
| Offspring Type 2 diabetes | ||
| Yes | 13 (0.0) | 400 (0.0) |
| No | 24 669 (100.0) | 1 072 671 (100.0) |
| Offspring other diabetes | ||
| Yes | 17 (0.1) | 472 (0.0) |
| No | 24 665 (99.9) | 1 072 599 (100.0) |
| Median age (IQR) at obesity diagnosis, years | 9 (6–13) | 11(7–13) |
| Median age (IQR) at diabetes diagnosis, years | 7 (4–11) | 7 (4–11) |
Values are expressed as n (%) unless otherwise indicated.
IQR, interquartile range; SD, standard deviation; SES, socioeconomic status.
Maternal pre-pregnancy BMI was available for birth years 2004–2014. PCOS was identified by polycystic ovary syndrome (ICD-9 256.4 or ICD-10 E28.2) and/or anovulatory infertility (ICD-9 628.0 or ICD-10 N97.0). Small for gestational age (SGA, birth weight and/or length 2 SDs below mean), and large for gestational age (LGA, birth weight and/or length 2 SDs above mean) are defined according to age- and sex-specific reference mean of Finnish standards, following the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. Appropriate for gestational age (AGA) is the interval between SGA and LGA. Preterm birth was defined as birth before gestational week 37. Maternal psychiatric disorders included inpatient and outpatient care due to mental health disorders before pregnancy according to ICD-9: 290–319, and ICD-10: F00–F99. Obesity was identified by ICD-10 codes E65, E66. Maternal PGDM (insulin treated pregestational diabetes) was identified based on special reimbursement of insulin for diabetes. Maternal other diabetes was identified based on ICD-10 E11–E14, O24, A10B treatment before pregnancy and A10A or A10B treated only during pregnancy. Offspring Type 1 diabetes, and Type 2 diabetes was identified based on ICD-10 E10, and E11, respectively. Offspring other diabetes including nutrition-related diabetes, other specified diabetes, and other unspecified diabetes was identified based on ICD-10 E12, E13, and E14.
Cumulative incidences of obesity and diabetes
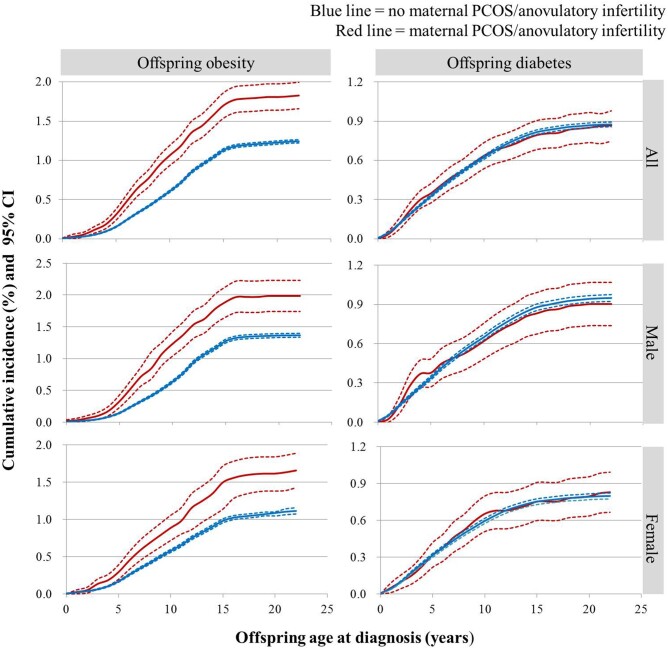
During follow-up, a total of 13 793 offspring were diagnosed with obesity and 9605 with diabetes, of which 451 (1.83%) and 214 (0.87%) were to mothers with PCOS/anovulatory infertility (Table I). As shown in Fig. 1, the cumulative incidence of obesity in PCOS/anovulatory infertility -exposed offspring was substantially higher than in unexposed offspring (1.83% [451], 95% CI 1.66–2.00% vs 1.24% [13 342], 95% CI 1.22–1.26%). The incidence for obesity in offspring with maternal PCOS/anovulatory infertility was increased until around 16 years old. However, there was no significant difference for diabetes between PCOS/anovulatory infertility-exposed and unexposed offspring (0.87% [214], 95% CI 0.75–0.98% vs 0.88% [9391], 95% CI 0.86–0.89%). Sex-stratified analysis revealed higher cumulative incidence of obesity in PCOS/anovulatory infertility-exposed male (1.99% [253], 95% CI 1.74–2.23% vs 1.37% [7502], 95% CI 1.34–1.40%) and female children (1.66% [198], 95% CI 1.43–1.89% vs 1.11% [5840], 95% CI 1.07–1.13%), whereas for diabetes there was no difference between PCOS/anovulatory infertility-exposed and unexposed offspring for any sex (male: 0.90% [115], 95% CI 0.74–1.07% vs 0.95% [5207], 95% CI 0.92–0.98%; female: 0.83% [99], 95% CI 0.67–0.99% vs 0.80% [4184], 95% CI 0.77–0.82%) (Fig. 1). In subgroup analysis of mothers with only PCOS, the cumulative incidence of offspring obesity remained increased (Supplementary Fig. S1).
Figure 1.
Cumulative incidences of obesity and diabetes in the offspring, overall and sex-stratified, in relation to maternal polycystic ovary syndrome (PCOS)/anovulatory infertility. The cumulative incidence of obesity was significantly higher for offspring with maternal PCOS/anovulatory infertility than those without, with similar patterns in males and females. There was no difference in cumulative incidence of diabetes between offspring with and without maternal PCOS/anovulatory infertility. PCOS/anovulatory infertility was identified by ICD-9 256.4, 628.0, ICD-10 E28.2, N97.0. Obesity was identified by ICD-10 E65 and E66. Diabetes was identified by ICD-10 E10–E14. Dotted lines represent the 95% CI.
HRs for offspring obesity and diabetes adjusted for birth and maternal factors
To account for offspring and maternal factors on the association of PCOS/anovulatory infertility exposure with offspring obesity and diabetes, adjusted HRs were calculated. Adjusting for offspring factors and maternal characteristics such as an obesity or diabetes diagnosis, the HR for obesity in PCOS/anovulatory infertility-exposed children was increased compared with unexposed offspring (incidence rate per 1000 person-years 1.63 [451] vs 0.97 [13 342]; HR 1.47, 95% CI 1.34–1.61). Age-stratified analysis revealed increased HR for obesity in offspring aged ≤9 years (1.18 [231] vs 0.62 [5482]; HR 1.58, 95% CI 1.30–1.81) and 10–16 years (3.18 [206] vs 1.92 [7164]; HR 1.37, 95% CI 1.19–1.57), but not in those 17–22 years old (0.86 [14] vs 0.59 [696]; HR 1.24, 95% CI 0.73–2.11). Sex-stratified analysis found comparable effect sizes between males (1.79 [253] vs 1.08 [7502]; HR 1.48, 95% CI 1.31–1.68) and females (1.46 [198] vs 0.86 [5840]; HR 1.45, 95% CI 1.26–1.68) (Table II). Subgroup analysis within births to mothers with only PCOS implied consistent results for offspring obesity (Supplementary Table SIII).
Table II.
Adjusted hazard ratios (HRs) for offspring obesity in relation to maternal polycystic ovary syndrome (PCOS)/anovulatory infertility (AI) in birth cohort 1996–2014.
| Variable | Overalla |
Male |
Female |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Person-years | IRb | HR (95% CI) |
No. of cases | IRb | HR (95% CI) |
No. of cases | IRb | HR (95% CI) |
||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | ||||||||
| All | |||||||||||||
| No maternal PCOS/AI | 13 342 | 13 758 016 | 0.97 | 1.00 | 1.00 | 7502 | 1.08 | 1.00 | 1.00 | 5840 | 0.86 | 1.00 | 1.00 |
| Maternal PCOS/AI | 451 | 277 097 | 1.63 | 1.77 (1.61–1.94) | 1.47 (1.34–1.61) | 253 | 1.79 | 1.75 (1.54–1.98) | 1.48 (1.31–1.68) | 198 | 1.46 | 1.79 (1.55–2.06) | 1.45 (1.26–1.68) |
| ≤9 years | |||||||||||||
| No maternal PCOS/AI | 5482 | 8 852 347 | 0.62 | 1.00 | 1.00 | 2856 | 0.63 | 1.00 | 1.00 | 2626 | 0.61 | 1.00 | 1.00 |
| Maternal PCOS/AI | 231 | 196 156 | 1.18 | 1.89 (1.65–2.15) | 1.58 (1.30–1.81) | 137 | 1.36 | 2.12 (1.79–2.52) | 1.80 (1.51–2.14) | 94 | 0.99 | 1.62 (1.32–1.99) | 1.35 (1.10–1.66) |
| 10–16 years | |||||||||||||
| No maternal PCOS/AI | 7164 | 3 731 103 | 1.92 | 1.00 | 1.00 | 4424 | 2.38 | 1.00 | 1.00 | 2740 | 1.46 | 1.00 | 1.00 |
| Maternal PCOS/AI | 206 | 64 719 | 3.18 | 1.66 (1.45–1.91) | 1.37 (1.19–1.57) | 114 | 3.52 | 1.48 (1.23–1.78) | 1.25 (1.04–1.51) | 92 | 2.85 | 1.97 (1.60–2.42) | 1.55 (1.25–1.91) |
| 17–22 years | |||||||||||||
| No maternal PCOS/AI | 696 | 1 174 566 | 0.59 | 1.00 | 1.00 | 222 | 0.38 | 1.00 | 1.00 | 474 | 0.80 | 1.00 | 1.00 |
| Maternal PCOS/AI | 14 | 16 222 | 0.86 | 1.50 (0.88–2.55) | 1.24 (0.73–2.11) | 2 | 0.25 | 0.63 (0.16–2.54) | 0.55 (0.14–2.23) | 12 | 1.45 | 1.96 (1.11–3.48) | 1.58 (0.89–2.80) |
PCOS/AI was identified by ICD-9 256.4, 628.0, ICD-10 E28.2 and N97.0. Obesity was identified by ICD-10 codes E65, E66. Model 1 was adjusted for offspring birth year, caesarean section (yes/no) and number of fetuses. Model 2 was further adjusted for maternal age, smoking during pregnancy (yes/no), married at delivery (yes/no), maternal SES (upper white collar worker, lower white collar worker, blue collar worker and other status), maternal psychiatric disorders (yes/no), maternal obesity (yes/no), maternal insulin-treated pregestational diabetes (yes/no) and maternal other diabetes (yes/no).
The analyses were also adjusted for offspring sex.
IR, incidence rate per 1000 person-years. Reference group was total, male or female births to non-PCOS/anovulatory infertility mothers.
The HR for diabetes was not increased in offspring ≤16 years old but was substantially increased in those 17–22 years (HR 2.06; 95% CI 1.23–3.46) (Supplementary Table SIV). Sex- and diabetes type-specific analysis revealed that the association was significant only in female offspring (HR 3.06, 95% CI 1.56–6.03), and specifically for Type 1 diabetes (HR 3.23, 95% CI 1.41–7.40), although it was based on a very small number of cases (Supplementary Tables SIV and SV).
Offspring obesity relative to maternal/anovulatory infertility, pre-pregnancy BMI, and fertility treatment
We then examined to what extent the association between maternal PCOS/anovulatory infertility and offspring obesity could be explained by maternal BMI and fertility treatment. Maternal BMI data was available for the 2004–2014 birth cohort. A statistically significant interaction existed between maternal PCOS/anovulatory infertility and maternal pre-pregnancy BMI-strata (categorized into normal-weight, overweight, and obesity) on offspring obesity (F = 15.96, P < 0.001). The interaction existed also after excluding mothers with an obesity or diabetes diagnosis (F = 11.19, P < 0.001). As expected, stratified analysis revealed that among non-PCOS/anovulatory infertility mothers the HR of obesity in offspring was increased in overweight and obese mothers compared with normal-weight mothers. Of note, the effect size for offspring obesity was higher among obese mothers with PCOS/anovulatory infertility (HR 8.89; 95% CI 7.06–11.20) than among obese mothers without PCOS/anovulatory infertility (HR 6.33; 95% CI 5.83–6.88) (Table III). Fertility treatment, however, did not modify the association between PCOS/anovulatory infertility and offspring obesity (interaction between maternal PCOS/anovulatory infertility and fertility treatment [yes/no] on offspring obesity: P > 0.05) (Supplementary Table SVI).
Table III.
Adjusted hazard ratios (HRs) for offspring obesity in relation to maternal polycystic ovary syndrome (PCOS)/anovulatory infertility (AI) stratified by pre-pregnancy BMI in birth cohort 2004–2014.
| Groups | All births (n = 610 821) |
Excluding maternal obesity and diabetes (n = 588 344) |
||
|---|---|---|---|---|
| n (%) | HR (95% CI) | n (%) | HR (95% CI) | |
| No maternal PCOS/AI | ||||
| <25 | 524 942 (88.3) | 1.00 | 51 496 (89.8) | 1.00 |
| 25–29 | 47 180 (7.9) | 2.66 (2.45–2.88) | 41 298 (7.2) | 2.64 (2.43–2.87) |
| ≥30 | 22 210 (3.7) | 6.39 (5.89–6.92) | 17 034 (3.0) | 6.33 (5.83–6.88) |
| Maternal PCOS/AI | ||||
| <25 | 12 972 (78.7) | 1.19 (0.83–1.70) | 12 430 (82.2) | 1.23 (0.85–1.76) |
| 25–29 | 2216 (13.4) | 3.45 (2.54–4.70) | 1788 (11.8) | 3.61 (2.61–4.98) |
| ≥30 | 1301 (7.9) | 8.12 (6.61–9.96) | 898 (5.9) | 8.89 (7.06–11.20) |
PCOS/AI was identified by ICD-9 256.4, 628.0, ICD-10 E28.2, and N97.0. Obesity was identified by ICD-10 codes E65, E66. The analysis was adjusted for offspring birth year, sex, caesarean section (yes/no), number of fetuses, maternal age, smoking during pregnancy (yes/no), married at delivery (yes/no), maternal socio-economic status (SES) (upper white collar worker, lower white collar worker, blue collar worker, and other status), maternal psychiatric disorders (yes/no), maternal insulin-treated pregestational diabetes (yes/no), and maternal other diabetes (yes/no). Birth cohort 2004–2014 was used due to BMI data availability for only these years. Reference group was births to non-PCOS mothers with normal BMI (<25).
HRs for obesity relative to maternal PCOS/anovulatory infertility and perinatal problems
We also examined if perinatal problems including SGA, LGA, and preterm birth modified the association between PCOS/anovulatory infertility and offspring obesity. There was a statistically significant interaction between maternal PCOS/anovulatory infertility and perinatal problems (yes/no) on offspring obesity (F = 5.29, P < 0.001). In PCOS/anovulatory infertility-unexposed offspring, the HR of obesity was increased if they were born SGA, LGA or preterm (fully adjusted HR 1.16; 95% CI 1.11–1.21) compared with those born AGA and full term. Notably, in PCOS/anovulatory infertility-exposed children, the effect size was significantly larger even for offspring born AGA and full term (HR 1.27; 95% CI 1.17–1.39). In offspring exposed to both PCOS/anovulatory infertility and perinatal problems, the HR for obesity was further increased (HR 1.61; 95% CI 1.35–1.91) (Table IV).
Table IV.
Adjusted hazard ratios (HRs) for offspring obesity in relation to maternal polycystic ovary syndrome (PCOS)/anovulatory infertility (AI) and perinatal problems in birth cohort 1996–2014.
| Groups | No. of cases | Person-years | Incidence ratea | HR (95% CI) |
|
|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||
| No maternal PCOS/AI | |||||
| AGA & full term | 11 444 | 12 338 693 | 0.93 | 1.00 | 1.00 |
| SGA, LGA or preterm | 1898 | 1 453 005 | 1.31 | 1.42 (1.35–1.49) | 1.16 (1.11–1.21) |
| Maternal PCOS/AI | |||||
| AGA & full term | 360 | 237 462 | 1.52 | 1.72 (1.54–1.91) | 1.27 (1.17–1.39) |
| SGA, LGA or preterm | 91 | 39 525 | 2.30 | 2.70 (2.19–3.32) | 1.61 (1.35–1.91) |
PCOS/AI were identified by ICD-9 256.4, 628.0, ICD-10 E28.2, and N97.0. Obesity was identified by ICD-10 codes E65, E66. Small gestational age (SGA, birth weight and/or length 2 SDs below mean), and large (LGA, birth weight and/or length 2 SDs above mean) for gestational age are defined according to age- and sex-specific reference means of Finnish standards, following the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. Appropriate for gestational age (AGA) is the interval between SGA and LGA. Preterm birth was defined as birth before gestational week 37. Model 1 was adjusted for offspring birth year, sex, caesarean section (yes/no) and number of fetuses. Model 2 was further adjusted for maternal age, smoking during pregnancy (yes/no), married at delivery (yes/no), maternal socio-economic status (SES) (upper white collar worker, lower white collar worker, blue collar worker, and other status), maternal psychiatric disorders (yes/no), maternal obesity (yes/no), maternal insulin-treated pregestational diabetes (yes/no), and maternal other diabetes (yes/no).
IR, incidence rate per 1000 person-years. Reference group was offspring of non-PCOS/anovulatory infertility mothers born AGA and full term.
Discussion
This large nationwide population-based study found that maternal PCOS/anovulatory infertility was associated with increased risk for the offspring to be diagnosed with obesity, irrespective of sex and fertility treatment. Maternal obesity explained part of the effect size. Moreover, the association remained significant even in AGA and full term offspring, with a larger effect size if the child was exposed to both maternal PCOS/anovulatory infertility and perinatal problems. However, maternal PCOS/anovulatory infertility was associated with diabetes diagnosis only in female offspring from late adolescence, an association based on a very small number of cases.
This study found that obesity was more often identified in offspring with maternal PCOS/anovulatory infertility, from early age until around age 16 years. To our knowledge, this is the largest cohort study with the longest follow-up durations to examine obesity risks in PCOS-exposed offspring. Our findings are in accordance with the study by Risal et al. (2019), which followed up 21 daughters with maternal PCOS until age 21 years, revealing higher BMI compared with daughters without maternal PCOS. Similarly, Doherty et al. (2015) reported more hospitalizations due to endocrine, nutritional and metabolic diseases (ICD-10 E00–E90, e.g. obesity and diabetes) in PCOS-exposed offspring up to 31 years of age. Moreover, case–control studies revealed higher glucose-stimulated insulin levels around 15 years of age, higher triglycerides and LDL-cholesterol at age 2.5–4 years old, as well as heavier body weight across from infancy (2 months after birth) to 29 years of age in offspring with maternal PCOS (Recabarren et al., 2008, Wilde et al., 2018; Crisosto et al., 2019). By contrast, Bell et al. (2018) found no association between self-reported PCOS and offspring obesity before 3 years of age, whereas another clinical study revealed similar BMI at all Tanner stages compared to daughters without maternal PCOS (Sir-Petermann et al., 2009), though short follow-up or small sample size may limit the ability to detect differences.
Importantly, this study found increased risk of obesity in not only female but also male offspring with maternal PCOS/anovulatory infertility, in line with studies reporting increased body weight and disturbed lipid metabolism in PCOS-exposed male offspring (Recabarren et al., 2008; Crisosto et al., 2017). Likewise, a study in sheep with PCOS-like phenotype also indicated lifelong altered metabolic health in male offspring (Siemienowicz et al., 2019). However, a meta-analysis found signs of compromised cardiometabolic health only in female PCOS-exposed offspring (Gunning et al., 2020), which could be due to a lower number of male children in that study. Obesity has been an epidemic worldwide and associated with both reproductive and non-reproductive health in later life (Bluher, 2019; Wong et al., 2020). Our findings suggest weight management from early age for all offspring born to mothers with PCOS/anovulatory infertility.
Obesity is closely related to PCOS. Women with PCOS are more often overweight or obese, and later, obesity amplifies the severity of PCOS symptoms. Notably, evidence suggests post-obesity development of PCOS (Anderson et al., 2014). A Mendelian randomization and genetic association study indicated that increased BMI appeared causal for PCOS (Brower et al., 2019). He et al. (2020) demonstrated from two longitudinal population-based studies that greater BMI in childhood was associated with PCOS in later life. Koivuaho et al. (2019) also found that early timing of adiposity rebound in childhood was associated with PCOS diagnosis in adulthood. Moreover, reports showed that PCOS development may be attributable to early life obesity with insulin resistance (Littlejohn et al., 2007; Ibanez et al., 2011). Given that daughters of women with PCOS have increased risk of PCOS diagnosis (Risal et al., 2019), the bulk of evidence, including ours, suggest a developmental process towards PCOS through obesity.
Notably, in our study, the PCOS/anovulatory infertility association with offspring obesity remained significant after adjusting for maternal obesity and diabetes diagnoses. However, in the 2004–2014 birth cohort, we could not detect any association between maternal PCOS/anovulatory infertility and offspring obesity in normal-weight mothers according to pre-pregnancy BMI. Our stratified analysis suggested a joint effect of PCOS/anovulatory infertility and pre-pregnancy BMI-based obesity on offspring obesity, larger than that of either exposure alone. Furthermore, our study revealed that the increased risk of offspring obesity in mothers with PCOS/anovulatory infertility was independent of fertility treatment, though it was recently reported that children born after fertility treatment were more likely to develop metabolic dysfunctions (Cui et al., 2020). A recent review, summarizing epidemiological human studies and causal evidence from animal studies, indicated that long-term metabolic health in offspring was affected by intrauterine environment (Fernandez-Twinn et al., 2019), which in PCOS/anovulatory infertility was characterized by excess androgen (Rosenfield and Ehrmann, 2016). In consistence, a recent study revealed metabolic and reproductive disturbances in mice prenatally exposed to elevated androgen levels (Risal et al., 2019). Additionally, it has been reported that women with PCOS are associated with an altered gut microbiota and its metabolites (Qi et al., 2019), which might reflect a maternal–fetal gut microbiota pathway in offspring metabolic health. However, besides intrauterine exposure, postnatal factors should also be considered. For example, lifestyle is an important contributing factor to obesity. Future studies are warranted to assess the association between maternal PCOS/anovulatory infertility and obesity in offspring taking into account familial and individual factors such as diet and exercise.
Offspring to mothers with PCOS are often delivered preterm or with extremes of birth weight, which are risk factors for metabolic health (de Zegher et al., 2017; Mericq et al., 2017). This study confirmed the risk of obesity in PCOS-exposed offspring with perinatal problems, and demonstrated increased risk also in children with appropriate birth weight and delivered at term. As such, metabolic disorders in PCOS offspring are not always attributed to excess catch-up growth (de Zegher et al., 2017). Our findings suggest screening and preventative measures for children with maternal PCOS/anovulatory infertility exposure, irrespective of birth outcomes.
In addition, this study found increased risks of diabetes in offspring with maternal PCOS/anovulatory infertility from age 17 years compared to unexposed offspring. Subgroup analysis revealed a significant association specifically for Type 1 diabetes in female offspring. Likewise, PCOS has been reported to be associated with Type 1 diabetes (Escobar-Morreale and Roldan-Martin, 2016; Thong et al., 2020). In addition, the increased incidence of obesity in this study, present from childhood, may predispose PCOS-exposed offspring at higher risk of subsequent Type 2 diabetes (Lee et al., 2018). PCOS has been associated with Type 2 diabetes (Thong et al., 2020). In children with maternal PCOS, increased fasting insulin levels and/or insulin resistance were identified in most (Kent et al., 2008; Sir-Petermann et al., 2009; Crisosto et al., 2019), though not all studies (Legro et al., 2017; Wilde et al., 2018). Also, children of mothers with pregestational hyperandrogenism were more likely to develop prediabetes before age 5 years (Tian et al., 2017). Offspring in our study were 22 years for the oldest, which may be too young to receive a diagnosis of Type 2 diabetes, as it was reported that the mean age of Type 2 diabetes onset was 55 ± 11 years old in the general population in Finland (Kurkela et al., 2021). Sir-Petermann et al. (2009) reported no increased Type 2 diabetes among 99 pubertal PCOS-exposed daughters, which may be restricted by small sample size and young age of the participants. Future long-term studies are warranted to examine the risk of diabetes in PCOS/anovulatory infertility-exposed offspring.
The strength of our study lies in the comprehensive nationwide registries, which enable long follow-up for large cohort members to identify cases with obesity and diabetes diagnosis at early age. Adjustment for maternal obesity and diabetes, as well as stratification by perinatal problems, allowed us to examine potentially unique contributions of PCOS/anovulatory infertility. Furthermore, all data was prospectively collected, avoiding recall bias.
The limitations of this study should be noted. First, the prevalence of PCOS/anovulatory infertility was 2.2%, lower than that reported in previous studies (Karjula et al., 2020; Skiba et al., 2018). This was mainly attributable to the register-based design, identifying only women who sought medical care for their symptoms. This may drive our estimates towards null because some PCOS/anovulatory infertility-exposed children are misclassified as controls. However, it is also possible that we identified only severe PCOS/anovulatory infertility cases, thus, overestimating the effect sizes.
Second, the incidence of obesity in offspring was lower than that reported in studies based on measured or self-reported weight and height (Figueiredo et al., 2019). Our data were based on ICD codes to identify children and adolescents diagnosed in clinical care. It is likely that mainly children with moderate to severe obesity who needed and/or actively sought medical care were included, consequently limiting the generalizability of our results. The Finnish Primary Health Care Register was initiated in 2011 with a possibility to provide information on height and weight, if measured. For our study period, however, this register was not complete enough to be included as a data source. Children identified as obese by school nurses may not always be referred to diagnosis, thus not be included in this study. In addition, we noted in this study that much fewer obesity cases were identified after 16 years of age in both PCOS/anovulatory infertility exposed and unexposed offspring, in contrast to the upward trend before that age. That is less likely to be caused by biological reasons than by changes in health related behaviors. In Finland, children have annual or every-2-years health examinations at school between 6 and 16 years. After that, they themselves choose whether and when to have health examination. Overweight children might refuse health visits because they would not like to receive an obesity diagnosis. Moreover, mothers with PCOS, who were often obese or diabetic, might be more likely to take their children to health check; hence, more cases would be identified in PCOS/anovulatory infertility-exposed than the unexposed offspring. Also, lifestyle of offspring contributes to obesity and Type 2 diabetes; however, such information is not available in the national registers this study is based on.
Third, mothers with PCOS/anovulatory infertility were identified based on ICD codes, with no information on PCOS phenotypes. For this highly heterogeneous disorder, it is warranted for future studies to examine offspring metabolic disturbances in different PCOS phenotypes, especially hyperandrogenism.
Fourth, maternal pre-pregnancy BMI, an important factor influencing offspring metabolic health, was available only from 2004 and onwards, limiting maternal BMI strata-stratified analysis to the birth cohort 2004–2014. In addition, there was no data on gestational weight gain for mothers. Moreover, fertility treatment included IVF/ICSI only, without detailed information on other types of treatment, such as ovulation induction. Also, information on fathers, including age, medical disorders and medication prescriptions, was not available in this study.
In conclusion, maternal PCOS/anovulatory infertility was associated with an increased risk of obesity in male and female offspring from early age, and there was an increased risk for diabetes in female offspring from late adolescence, the latter based on only a few cases. The effect size of the association with offspring obesity was larger for those with perinatal problems and maternal pre-pregnancy obesity. This is by far the largest cohort study with the longest follow-up duration reporting risk estimates of obesity and diabetes in offspring exposed to PCOS/anovulatory infertility prenatally. It supports that prenatal PCOS/anovulatory infertility exposure influences metabolic health in the offspring. Information on the associations is fundamental for pediatric professionals to take preventative measures for PCOS/anovulatory infertility-exposed offspring.
Data availability
The data underlying this article were provided by the Finnish Institute for Health and Welfare Finland by permission. The data cannot be shared, since it has been given for this specific study, but similar data will be accessible with permission of the Finnish data providing authorities and the data protection authority.
Authors’ roles
All authors met conditions for authorship. X.C. designed the study, was the main interpreter of the data, prepared, reviewed and approved the manuscript. E.K. interpreted the data, reviewed/edited and approved the manuscript. T.T.P. designed the study, interpreted the data, reviewed/edited and approved the manuscript. M.G. designed the study, performed the statistical analyses, reviewed/edited and approved the manuscript. C.L. designed and conducted the study, interpreted the data, prepared, reviewed and approved the manuscript, and is guarantor of this study.
Funding
This study was supported by Shandong Provincial Natural Science Foundation, China [ZR2020MH064 to X.C.], Shandong Province Medical and Health Technology Development Plan [2018WS338 to X.C.], the joint research funding of Shandong University and Karolinska Institute [SDU-KI-2019-08 to X.C. and C.L.], the Finnish Institute for Health and Welfare: Drug and pregnancy project [M.G.], the Swedish Research Council [2014-10171 to C.L.], the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institute Stockholm County Council [SLL20170292 and SLL20190589 to C.L.], the Swedish Brain Foundation [FO2018-0141 and FO2019-0201 to C.L.]. X.C. received grants from the China Scholarship Council at the beginning of the study. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Supplementary Material
References
- Anderson AD, Solorzano CM, McCartney CR.. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med 2014;32:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artama M, Gissler M, Malm H, Ritvanen A; The Drugs and Pregnancy Study Group. Nationwide register-based surveillance system on drugs and pregnancy in Finland 1996–2006. Pharmacoepidemiol Drug Saf 2011;20:729–738. [DOI] [PubMed] [Google Scholar]
- Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, Misso ML, Teede HJ, Moran LJ.. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta-analysis, and meta-regression. Obes Rev 2019;20:659–674. [DOI] [PubMed] [Google Scholar]
- Bell GA, Sundaram R, Mumford SL, Park H, Broadney M, Mills JL, Bell EM, Yeung EH.. Maternal polycystic ovarian syndrome and offspring growth: the Upstate KIDS Study. J Epidemiol Community Health 2018;72:852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288–298. [DOI] [PubMed] [Google Scholar]
- Brower MA, Hai Y, Jones MR, Guo X, Chen YI, Rotter JI, Krauss RM, Legro RS, Azziz R, Goodarzi MO.. Bidirectional Mendelian randomization to explore the causal relationships between body mass index and polycystic ovary syndrome. Hum Reprod 2019;34:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PE, Cianfarani S, Czernichow P, Johannsson G, Rapaport R, Rogol A.. Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 2007;92:804–810. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH.. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisosto N, Echiburú B, Maliqueo M, Luchsinger M, Rojas P, Recabarren S, Sir-Petermann T.. Reproductive and metabolic features during puberty in sons of women with polycystic ovary syndrome. Endocr Connect 2017;6:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisosto N, Ladron de Guevara A, Echiburu B, Maliqueo M, Cavada G, Codner E, Paez F, Sir-Petermann T.. Higher luteinizing hormone levels associated with antimullerian hormone in postmenarchal daughters of women with polycystic ovary syndrome. Fertil Steril 2019;111:381–388. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhou W, Xi B, Ma J, Hu J, Fang M, Hu K, Qin Y, You L, Cao Y. et al. Increased risk of metabolic dysfunction in children conceived by assisted reproductive technology. Diabetologia 2020;63:2150–2157. [DOI] [PubMed] [Google Scholar]
- de Zegher F, Reinehr T, Malpique R, Darendeliler F, López-Bermejo A, Ibáñez L.. Reduced prenatal weight gain and/or augmented postnatal weight gain precedes polycystic ovary syndrome in adolescent girls. Obesity (Silver Spring) 2017;25:1486–1489. [DOI] [PubMed] [Google Scholar]
- Doherty DA, Newnham JP, Bower C, Hart R.. Implications of polycystic ovary syndrome for pregnancy and for the health of offspring. Obstet Gynecol 2015;125:1397–1406. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 2018;14:270–284. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Roldan-Martin MB.. Type 1 diabetes and polycystic ovary syndrome: systematic review and meta-analysis. Diabetes Care 2016;39:639–648. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R.. Intrauterine programming of obesity and type 2 diabetes. Diabetologia 2019;62:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo RAO, Simola-Strom S, Rounge TB, Viljakainen H, Eriksson JG, Roos E, Weiderpass E.. Cohort Profile: the Finnish Health in Teens (Fin-HIT) study: a population-based study. Int J Epidemiol 2019;48:23–24h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning MN, Sir Petermann T, Crisosto N, van Rijn BB, de Wilde MA, Christ JP, Uiterwaal C, de Jager W, Eijkemans MJC, Kunselman AR. et al. Cardiometabolic health in offspring of women with PCOS compared to healthy controls: a systematic review and individual participant data meta-analysis. Hum Reprod Update 2020;26:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ.. The thrifty phenotype hypothesis. Br Med Bull 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- He Y, Tian J, Blizzard L, Oddy WH, Dwyer T, Bazzano LA, Hickey M, Harville EW, Venn AJ.. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: the Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum Reprod 2020;35:1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockett CW, Harrall KK, Moore BF, Starling AP, Bellatorre A, Sauder KA, Perng W, Scherzinger A, Garg K, Ringham BM. et al. Persistent effects of in utero overnutrition on offspring adiposity: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia 2019;62:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F.. Early metformin therapy (age 8–12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab 2011;96:E1262–E1267. [DOI] [PubMed] [Google Scholar]
- Joham AE, Boyle JA, Ranasinha S, Zoungas S, Teede HJ.. Contraception use and pregnancy outcomes in women with polycystic ovary syndrome: data from the Australian Longitudinal Study on Women's Health. Hum Reprod 2014;29:802–808. [DOI] [PubMed] [Google Scholar]
- Karjula S, Morin-Papunen L, Franks S, Auvinen J, Järvelin M-R, Tapanainen JS, Jokelainen J, Miettunen J, Piltonen TT.. Population-based Data at Ages 31 and 46 Show Decreased HRQoL and Life Satisfaction in Women with PCOS Symptoms. The Journal of Clinical Endocrinology & Metabolism 2020;105:1814–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS.. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab 2008;93:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivuaho E, Laru J, Ojaniemi M, Puukka K, Kettunen J, Tapanainen JS, Franks S, Järvelin MR, Morin-Papunen L, Sebert S. et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int J Obes 2019;43:1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela O, Forma L, Ilanne-Parikka P, Nevalainen J, Rissanen P.. Association of diabetes type and chronic diabetes complications with early exit from the labour force: register-based study of people with diabetes in Finland. Diabetologia 2021;64:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, Giovannucci EL.. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: two large prospective studies in US men and women. Eur J Epidemiol 2018;33:1113–1123. [DOI] [PubMed] [Google Scholar]
- Legro RS, Kunselman AR, Stetter CM, Gnatuk CL, Estes SJ, Brindle E, Vesper HW, Botelho JC, Lee PA, Dodson WC.. Normal pubertal development in daughters of women with PCOS: a controlled study. J Clin Endocrinol Metab 2017;102:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Library FH. ISO-BMI in Finland. 2020.
- Littlejohn EE, Weiss RE, Deplewski D, Edidin DV, Rosenfield R.. Intractable early childhood obesity as the initial sign of insulin resistant hyperinsulinism and precursor of polycystic ovary syndrome. J Pediatr Endocrinol Metab 2007;20:41–51. [DOI] [PubMed] [Google Scholar]
- Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A.. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol 2017;13:50–62. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC.. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, Wang L, Zhang Y, Liang X, Wang L. et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med 2019;25:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Smith R, Rios R, Maliqueo M, Echiburú B, Codner E, Cassorla F, Rojas P, Sir-Petermann T.. Metabolic profile in sons of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2008;93:1820–1826. [DOI] [PubMed] [Google Scholar]
- Risal S, Pei Y, Lu H, Manti M, Fornes R, Pui H-P, Zhao Z, Massart J, Ohlsson C, Lindgren E. et al. Prenatal androgen exposure and transgenerational susceptibility to polycystic ovary syndrome. Nat Med 2019;25:1894–1904. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL, Ehrmann DA.. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev 2016;37:467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari A, Sankilampi U, Hannila ML, Kiviniemi V, Kesseli K, Dunkel L.. New Finnish growth references for children and adolescents aged 0 to 20 years: length/height-for-age, weight-for-length/height, and body mass index-for-age. Ann Med 2011;43:235–248. [DOI] [PubMed] [Google Scholar]
- Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L.. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med 2013;45:446–454. [DOI] [PubMed] [Google Scholar]
- Siemienowicz KJ, Filis P, Shaw S, Douglas A, Thomas J, Mulroy S, Howie F, Fowler PA, Duncan WC, Rae MT.. Fetal androgen exposure is a determinant of adult male metabolic health. Sci Rep 2019;9:20195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Codner E, Perez V, Echiburu B, Maliqueo M, Ladron de Guevara A, Preisler J, Crisosto N, Sanchez F, Cassorla F. et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2009;94:1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, Perez-Bravo F, Cassorla F.. Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 2007;92:4637–4642. [DOI] [PubMed] [Google Scholar]
- Skiba MA, Islam RM, Bell RJ, Davis SR. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2018;24:694–709. [DOI] [PubMed] [Google Scholar]
- Stockmarr A, Hejgaard T, Matthiessen J.. Obesity prevention in the Nordic countries. Curr Obes Rep 2016;5:156–165. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 2018;33:1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thong EP, Codner E, Laven JSE, Teede H.. Diabetes: a metabolic and reproductive disorder in women. Lancet Diabetes Endocrinol 2020;8:134–149. [DOI] [PubMed] [Google Scholar]
- Thong EP, Milat F, Joham AE, Mishra GD, Teede H.. Obesity, menstrual irregularity and polycystic ovary syndrome in young women with type 1 diabetes: a population-based study. Clin Endocrinol 2020;93:564–571. [DOI] [PubMed] [Google Scholar]
- Tian S, Lin XH, Xiong YM, Liu ME, Yu TT, Lv M, Zhao W, Xu GF, Ding GL, Xu CM. et al. Prevalence of prediabetes risk in offspring born to mothers with hyperandrogenism. EBioMedicine 2017;16:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde MA, Eising JB, Gunning MN, Koster MPH, Evelein AMV, Dalmeijer GW, Uiterwaal C, Eijkemans MJC, Ent CKV, Meijboom FJ. et al. Cardiovascular and metabolic health of 74 children from women previously diagnosed with polycystic ovary syndrome in comparison with a population-based reference cohort. Reprod Sci 2018;25:1492–1500. [DOI] [PubMed] [Google Scholar]
- Wong MCS, Huang J, Wang J, Chan PSF, Lok V, Chen X, Leung C, Wang HHX, Lao XQ, Zheng Z-J.. Global, regional and time-trend prevalence of central obesity: a systematic review and meta-analysis of 13.2 million subjects. Eur J Epidemiol 2020;35:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the Finnish Institute for Health and Welfare Finland by permission. The data cannot be shared, since it has been given for this specific study, but similar data will be accessible with permission of the Finnish data providing authorities and the data protection authority.