Abstract
Juniperus communis (JCo) is a well-known traditional Chinese medicinal plant that has been used to treat wounds, fever, swelling, and rheumatism. However, the mechanism underlying the anticancer effect of JCo extract on colorectal cancer (CRC) has not yet been elucidated. This study investigated the anticancer effects of JCo extract in vitro and in vivo as well as the precise molecular mechanisms. Cell viability was evaluated using the MTT assay. Cell cycle distribution was examined by flow cytometry analysis, and cell apoptosis was determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay. Protein expression was analyzed using western blotting. The in vivo activity of the JCo extract was evaluated using a xenograft BALB/c mouse model. The tumors and organs were examined through hematoxylin-eosin (HE) staining and immunohistochemistry. The results showed that JCo extract exhibited higher cytotoxicity against CRC cells than against normal cells and showed synergistic effects when combined with 5-fluorouracil. JCo extract induced cell cycle arrest at the G0/G1 phase via regulation of p53/p21 and CDK4/cyclin D1 and induced cell apoptosis via the extrinsic (FasL/Fas/caspase-8) and intrinsic (Bax/Bcl-2/caspase-9) apoptotic pathways. In vivo studies revealed that JCo extract suppressed tumor growth through the inhibition of proliferation and induction of apoptosis. In addition, there was no obvious change in body weight or histological morphology of normal organs after treatment. JCo extract suppressed CRC progression by inducing cell cycle arrest and apoptosis in vitro and in vivo, suggesting the potential application of JCo extract in the treatment of CRC.
Keywords: Colorectal cancer, Juniperus communis, Cell cycle, Apoptosis, Synergistic effect
Introduction
Colorectal cancer (CRC) is the third most common malignant cancer in humans, accounting for nearly 9.2% of all annual cancer-related deaths worldwide (1). The tumorigenesis of CRC is multifactorial and associated with progressive accumulation of epigenetic and genetic alterations that result in the transformation of normal rectal mucosa into malignant metastatic carcinoma (2). Diet is one of the main environmental factors involved in the etiology of CRC, with about 90% of CRC cases related to high intakes of red meat, saturated fats, and n-6 polyunsaturated fatty acids and low intakes of vitamins and fibers (3). The standard treatment for CRC is surgery combined with radiotherapy or chemotherapy, depending on tumor size, location, and disease stage (4). However, the standard chemotherapy regimens, including 5-fluorouracil (5-FU), doxorubicin, and mitomycin, exhibit side effects including mucositis, diarrhea, and dermatitis (5 –8). Therefore, there remains an unmet clinical requirement for novel anti-CRC agents or combination therapy for the treatment of CRC. Recently, natural materials have shown potential as preventive or therapeutic agents for various cancers (7,8).
Natural materials and natural health products have recently been used in the development of new drugs. The chemodrugs that are currently available are derived from natural materials such as plants, marine organisms, and microbes (9). Recent analyses have shown that at least 73 approved anticancer drugs in clinical use, including paclitaxel, vinblastine, topotecan, and etoposide, were derived from plants (10,11). Natural products have been widely used in the discovery of anticancer agents because of their diverse molecular structures and biological affinities (12). Natural products mainly include traditional and herbal medicines, and recent studies have focused on their biofunctions and applications in cancer therapy (13).
Juniperus communis (JCo) is an evergreen coniferous shrub belonging to the Cuppressaceae family (14). JCo extract has been traditionally used as an herbal medicine for the treatment of pain, wounds, fever, swelling, and rheumatism (15,16). Recent studies have demonstrated that JCo extract exhibits anti-oxidative, anti-microbial, anti-inflammatory, anti-diabetic, and anti-hyperlipidemic activities (17 –20). The anticancer activity of JCo extract has been demonstrated based on tumor growth inhibition with regard to melanoma, oral cancer, breast cancer, lung cancer, and carcinomas of the liver and colon (21–24). The compounds in JCo extract, including α-pinene, d-limonene, and terpinolene, have shown anti-carcinogenic activities (25–31). However, few studies have investigated the anticancer mechanism of JCo extract in vitro and in vivo.
This study aimed to examine the anticancer effects of JCo extract in vitro and in vivo and to determine the precise molecular mechanism underlying the tumor growth inhibition induced by JCo extract in CRC. In addition, the combination of JCo extract and the clinical drug 5-FU was analyzed for its anticancer effects in vitro as well as the tolerance of JCo extract in vivo. Our results indicated the potential of JCo extract as a clinical therapeutic agent or adjuvant therapeutic agent in CRC therapy.
Material and Methods
Cell culture and reagents
Human colorectal adenocarcinoma (HT-29, ATCC® HTB-38), mouse colon cancer (CT-26, BCRC 60447), canine kidney epithelial (MDCK, BCRC 60004), and mouse vascular endothelial (SVEC, BCRC 60220) cell lines were obtained from the American Type Culture Collection (ATCC, USA) or the Bioresource Collection and Research Center (BCRC, Taiwan). HT-29, SVEC, and MDCK cells were cultured in Dulbecco's modified Eagle’s medium (DMEM, Gibco BRL, USA); CT-26 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, HEPES (10 mM), pyruvate (1 mM), and penicillin/streptomycin solution (100 U/mL penicillin and 100 µg/mL streptomycin; all from Gibco BRL). The cells were routinely grown in culture dishes in a 5% CO2 humidified atmosphere at 37°C. The status of TP53 in HT-29 cells was determined by automated extraction of nucleic acids (AccuBioMed Co., Ltd., Taiwan) and sequencing using Femtopath Human Primer Sets (HongJing Biotech, Taiwan).
JCo fruits were freshly obtained from Nepal and subjected to extraction by steam distillation (22). The detailed extraction flowchart was tested on a small scale in our laboratory. Approximately 400 g of fruit was steam-distilled in a 2-L steam distillation unit for 100 min at 100-105°C at a flow rate of approximately 7.2 mL/min. Phoenix (USA) was then commissioned for the large-scale production of JCo extract. 5-FU and etoposide (VP-16) were purchased from Sigma-Aldrich (USA). Before each experiment, all extracts or chemicals were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and diluted in fresh medium.
Determination of cytotoxicity
Cytotoxicity was analyzed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, cells were cultured overnight in 96-well plates (5×103 cells/well) containing the corresponding culture media, followed by treatment with various concentrations of JCo extract (0-100 μg/mL) for 24, 48, or 72 h. Next, the medium in each well was replaced with MTT solution (400 μg/mL; Sigma), and the plates were incubated for 6-8 h. After incubation, DMSO was added to solubilize the formazan crystals, and absorbance at 550 nm was measured using a microplate reader (Molecular Devices, Spec384, USA). The 50% inhibitory concentration (IC50) was calculated based on the graph of relative viability vs JCo extract concentration. Cell viability (%) was calculated as absorbance (treated cells) / absorbance (control cells) × 100. After testing the concentration of JCo extract (0-100 μg/mL) in HT-29 cells, the dose of 65 μg/mL (IC70), which induced cell death, was used in further experiments to study the anticancer mechanism against CRC cells.
Synergistic effects of JCo extract and clinical drugs
HT-29 cells were treated with a combination of JCo extract (0, 20, 40, 60, and 80 μg/mL) and 0.25 μg/mL 5-FU, or a combination of 5-FU (0, 0.125, 0.25, 0.5, and 1 μg/mL) and 40 μg/mL JCo extract for 72 h. Cell viability was then determined by the MTT assay. The combination index (CI) was calculated as follows: [IC50 (drug A+B) / IC50 (drug A)] + [IC50 (drug A+B) / IC50 (drug B)]. The combination effects of drugs were evaluated based on the CIs for assessing synergism (CI<1), additivity (CI=1), and antagonism (CI>1) (32).
Cell cycle analysis
Cell cycle distribution was determined using propidium iodide (PI) staining and flow cytometry analysis. Briefly, HT-29 cells (2×106 cells/dish) were treated with 65 μg/mL JCo extract for 0, 6, 12, 24, and 48 h, followed by the addition of PI/RNase staining solution (40 μg/mL of PI and 100 μg/mL of RNase; Sigma) and incubation at 4°C overnight. The DNA content (FL2 intensity) of each cell was measured using FACScan (Beckton Dickinson, USA) and Kaluza Flow Cytometry Analysis software (version 1.2, Beckman Coulter, USA).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Apoptosis was detected using the In Situ Cell Death Detection kit, POD (Roche, Germany). Cells on silane-coated glass slides or deparaffinized tissue sections were rehydrated with phosphate-buffered saline (PBS), treated with 3% H2O2 in methanol to inactivate endogenous peroxidase, and incubated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate buffer) on ice. The samples were then incubated with the TUNEL reaction solution for 2 h at 37°C and counterstained with PI. TUNEL-positive cells were observed and photographed using a fluorescence microscope (ZEISS AXioskop2, Germany) at 400× magnification.
Western blotting
JCo extract-treated cells were lysed with RIPA lysis buffer containing a protease inhibitor (Bio Basic Inc., Canada) and a phosphatase inhibitor (Bionovas, Canada) incubated on ice for 30 min. After centrifugation at 12,000 g for 30 min at 4°C, the protein content of the supernatant was measured using a bicinchoninic acid (BCA) protein assay kit (Pierce, USA). Equal amounts (20 μg) of protein samples were resolved using 8-12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to 0.22-μm polyvinylidene difluoride (PVDF) membranes (PALL Corp., USA). The membranes were blocked with 5% nonfat dry milk and incubated with the primary antibodies anti-p53, anti-p-p53, anti-Rb, anti-pRb, anti-CDK2, anti-CDK4, anti-cyclin A, anti-cyclin B1, anti-cyclin D1, anti-FasL, anti-Fas, anti-Bax, anti-Bcl-2, anti-caspase-8, anti-caspase-9, anti-caspase-3, anti-proliferating cell nuclear antigen (PCNA), anti-vascular endothelial growth factor (VEGF), anti-VEGF receptor 1 (VEGFR1), anti-VEGF receptor 2 (VEGFR2), anti-matrix metalloproteinase (MMP)-2, anti-MMP-9 (Santa Cruz, USA), anti-p21, and anti-β-actin (iReal Biotechnology Co., Ltd., Taiwan) overnight at 4°C. The membranes were washed three times with 0.5% Tween-20 in tris-buffered saline and incubated with biotin-conjugated secondary antibodies (Santa Cruz) for 2 h, followed by incubation with peroxidase-conjugated streptavidin (Jackson ImmunoResearch Inc., USA) for 1 h. Antibody-reactive proteins were treated with an enhanced chemiluminescence reagent (ECL, T-Pro Biotechnology, Taiwan) and detected using a fluorescence/chemiluminescence imaging analyzer (GE LAS-4000, GE Healthcare Life Sciences, USA). Protein expression was quantified using ImageJ 1.47t software (NIH, USA) and calculated as follows: (sample intensity / β-actin intensity of the sample) / (control intensity / β-actin intensity of the control).
Detection of caspase-3 activation
HT-29 cells (5×105 cells/well in a 6-well culture plate) were pretreated with caspase-3 inhibitor (1 μM; Z-DEVD-FMK, BIOSCIENCES, USA) for 2 h, followed by treatment with 65 μg/mL JCo extract for 24 h. The expression levels of pro-caspase-3 and cleaved caspase-3 in treated cells were determined by western blotting.
In vivo tumor growth and immunohistochemistry
Female BALB/c mice (10-12 weeks; 19-23 g) were obtained from the National Laboratory Animal Center (Taiwan), housed (6 per cage) in a laminar airflow room, maintained with a 12-h light/dark cycle (relative humidity: 55-60%; temperature: 25±1°C), and allowed free access to a balanced diet and water. Before experimentation, the mice were acclimated to laboratory conditions for 1 week. The experiment was performed at Chung Shan Medical University (CSMU) according to the Guide for the Care and Use of Laboratory Animals. The CRC model was established by injecting 1×106 CT-26 cells sc into the flanks of BALB/c mice. The mice were randomized into the vehicle control (n=4) and JCo extract treatment (n=6) groups. When the tumor volumes exceeded 15 mm3 (cell injection for 7 days), the tumor-bearing mice were treated with 200 mg/kg JCo extract once every 2 days for 40 days and sacrificed by carbon dioxide asphyxiation when the tumor volume exceeded 1500 mm3 (L×H×W×π/6 mm3). This procedure was approved by the Institutional Animal Care and Use Committee (IACUC) of CSMU (allowance number: CSMU-IACUC-1543). The tumor masses and organs were collected, fixed with 4% neutral formalin, embedded in paraffin, and cut into sections for immunohistochemical (IHC) and hematoxylin-eosin (HE) staining.
The sections were deparaffinized and rehydrated, and the endogenous peroxidase was inactivated. The sections were blocked with 10% bovine serum albumin (BSA) in PBS and incubated at 4°C overnight with the primary antibodies anti-PCNA, anti-VEGF, anti-VEGFR1, anti-VEGFR2, anti-MMP-2, anti-MMP-9, and anti-cleaved caspase-3 (Santa Cruz). After washing, the slides were incubated with a biotinylated secondary antibody (Super Sensitive Polymer HRP IHC Detection System kit, BioGenex, USA) for 2 h. Finally, the slides were incubated with an avidin-biotin complex, reacted with 3,3′-diaminobenzidine (DAB), and counterstained with hematoxylin. All samples were observed and photographed using a microscope and scored using the Quickscore method (33).
Statistical analyses
The data are reported as means±SD or standard error. The IC50 values were determined by linear regression analysis using Microsoft Excel 2016 (USA). Statistical significance was determined using the Student's t-test and one-way analysis of variance. Survival analyses were performed using the Kaplan-Meier method. P-values <0.05 were considered statistically significant.
Results
JCo extract decreased the viability of CRC cells
CRC cells were treated with various concentrations of JCo extract, and cell viability was detected by the MTT assay. The results showed that JCo extract reduced the viability of HT-29 and CT-26 cells in a dose-dependent manner (Figure 1). As shown in Table 1, the mean IC50 of JCo extract in tumor cells (HT-29 and CT-26 cells) was significantly lower than that in normal cells (MDCK and SVEC cells), indicating higher selectivity of JCo extract for tumor cells than for normal cells.
Figure 1. Effect of Juniperus communis (JCo) extract on growth inhibition in colorectal cancer cell lines. HT-29 (A), CT-26 (B), MDCK (C), and SVEC (D) cells were treated with serial dilutions of JCo extract for 24, 48, and 72 h, and cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data are reported as means±SD. *P<0.05 vs control (ANOVA).
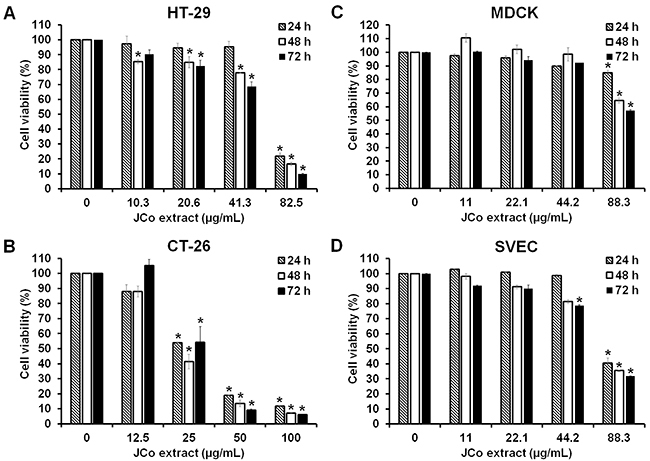
Table 1. Cytotoxicity (IC50) of Juniperus communis (JCo) extract in colorectal cancer cells and normal cells.
| Drugs | Colorectal cancer cells | Normal cells | ||
|---|---|---|---|---|
| HT-29 | CT-26 | MDCK | SVEC | |
| JCo extract | ||||
| 24 h | 66.71±0.48.0a,c | 27.8±0.2a,b,c | >88.3c | 81.1±2.2b,c |
| 48 h | 60.02±0.21a,b,c | 22.7±0.9a,b,c | >88.3c | 74.8±0.3b,c |
| 72 h | 54.32±1.58a,b,c | 27.3±4.5a,b,c | >88.3b,c | 71.2±0.6b,c |
| 5-FU | ||||
| 24 h | >10 | 2.7±0.03a | >10 | 6.8±1.3 |
| 48 h | 1.3±0.4 | 0.3±0.02a | >10 | 1.4±0.05 |
| 72 h | 0.7±0.03 | 0.4±0.01a | 1.1±0.01 | 0.8±0.03 |
| VP-16 | ||||
| 24 h | >100a | 44.1±0.4 | 66.0±1.0 | 46.2±3.2 |
| 48 h | 3.3±2.2 | 9.4±0.03 | 25.7±2.7 | 2.2±0.6 |
| 72 h | 2.3±0.02 | 4.1±0.1a | 2.7±0.2 | 1.0±0.001 |
IC50: half maximal inhibitory concentration. Data are reported as means±SD in µg/mL. aP<0.05 between tumor cells and normal cells; bP<0.05 between JCo extract group and 5-FU treatment group; cP<0.05 between JCo extract group and VP-16 treatment group (ANOVA).
Synergistic effects of JCo extract and 5-FU
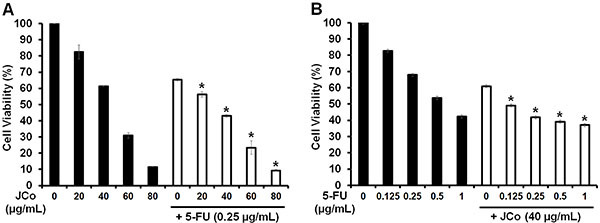
To analyze whether JCo extract had a synergistic, additive, or antagonistic effect when administered in combination with 5-FU, HT-29 cells were treated with JCo extract (0-80 μg/mL) combined with 0.25 μg/mL 5-FU or with 5-FU (0-1 μg/mL) combined with 40 μg/mL JCo extract. After 72 h of treatment, cell viability was determined by the MTT assay. As shown in Figure 2, the viability of CRC cells was lower after treatment with JCo extract combined with 0.25 μg/mL 5-FU (42.94±0.69%) than that after treatment with JCo extract alone (61.30±0.17%). In contrast, cell viability decreased after treatment with 5-FU combined with 40 μg/mL JCo extract (41.82±1.45%) compared to that after treatment with 5-FU alone (67.94±0.35%). The synergistic, additive, or antagonistic effects of the drug combinations were determined using the Chou-Talalay method (32). The CI was 0.79, suggesting a synergistic effect of the combination of JCo extract and 5-FU (CI<1) after a treatment period of 72 h.
Figure 2. Juniperus communis (JCo) extract enhances the sensitivity of colorectal cancer cells to 5-fluorouracil (5-FU). HT-29 cells were treated with (A) JCo extract (0, 20, 40, 60, and 80 μg/mL) alone or JCo extract in combination with 0.25 μg/mL 5-FU or with (B) 5-FU (0, 0.125, 0.25, 0.5, and 1 μg/mL) alone or 5-FU in combination with 40 μg/mL JCo extract for 72 h. Cell viability was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Data are reported as means±SD. *P<0.05 vs single drug in the combination group. JCo extract in combination with 5-FU displayed a synergistic effect (CI<1) (ANOVA).
JCo extract induced cell cycle arrest at the G0/G1 phase in CRC cells
CRC cells treated with JCo extract were collected and stained with PI. Cell cycle distribution was then analyzed by measuring FL2 intensity using a flow cytometer (Figure 3A). The results showed that the percentage of G0/G1 phase cells increased from 51.66±0.19% to 68.86±0.47% within 48 h of JCo extract treatment, while the percentages of S and G2/M phase cells decreased from 18.34±0.2 to 8.48±0.07% and 29.99±0.48 to 22.66±0.66%, respectively (Figure 3B).
Figure 3. Juniperus communis (JCo) extract induces G0/G1 arrest and regulates the expression of cell cycle-associated proteins in HT-29 cells. A, HT-29 cells were treated with 65 μg/mL JCo extract for 0, 6, 12, 24, and 48 h, stained with propidium iodide, and analyzed for FL2 intensity by flow cytometry. B, Cell cycle distribution (G0/G1, S, and G2/M phases) in JCo extract-treated cells was analyzed using Kaluza Flow Cytometry Analysis software. Data are reported as means±SD. *P<0.05 vs control with a significant increase, #P<0.05 vs control with a significant decrease (ANOVA). C, The expression of cell cycle-associated proteins in JCo extract-treated cells was determined by western blotting.
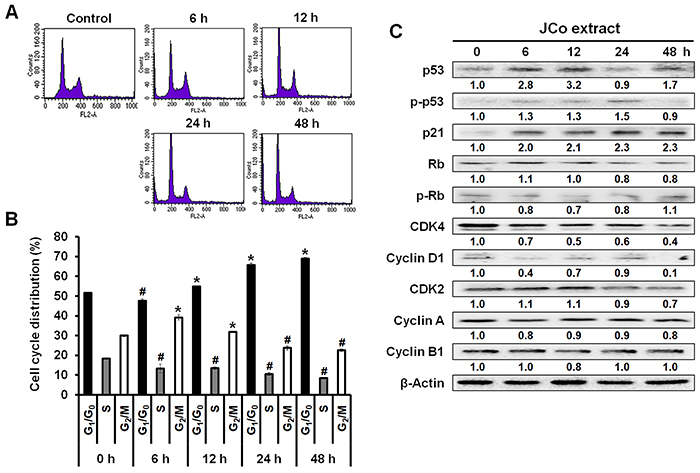
In addition, JCo extract regulated the expression of proteins related to the cell cycle, including cell cycle regulators (p53, p-p53, and p21), tumor suppressors (Rb and p-Rb), and regulatory molecules involved in the G0/G1 phase (CDK4 and cyclin D1, Figure 3C). Taken together, these results suggested that the tumor growth inhibition effect of JCo extract was associated with the induction of cell cycle arrest via the regulation of cell cycle-related protein expression.
JCo extract triggered cell apoptosis by regulating the caspase cascade
The percentage of cells in the SubG1 phase (apoptosis peak) significantly increased from 5.61±0.19 to 20.70±0.09% after treatment with JCo extract (Figure 4A). To determine whether this increase was due to the induction of cell apoptosis by JCo extract, we used the TUNEL assay to detect apoptosis in JCo extract-treated cells. The data revealed that JCo extract-treated cells were TUNEL-positive and showed apoptotic morphologies, including anoikis, chromatin condensation, and DNA fragmentation, as well as the presence of apoptotic bodies (Figure 4B).
Figure 4. Effects of Juniperus communis (JCo) extract on the extrinsic and intrinsic apoptotic pathways in HT-29 cells. A, The percentage of SubG1 phase cells after JCo extract treatment was analyzed by flow cytometry. Data are reported as means±SD. *P<0.05 vs control (ANOVA). B, Cell apoptosis was determined after treatment with 65 μg/mL JCo extract for 48 h by TUNEL assay (scale bar 50 μm). The apoptotic morphologies included anoikis, chromatin condensation, DNA fragmentation, and the appearance of apoptotic bodies (arrow). C, The protein expression levels of the components of the extrinsic and intrinsic apoptotic pathways in JCo extract-treated cells were analyzed by western blotting. D, HT-29 cells pretreated with 1 μM Z-DEVD-FMK (caspase-3 inhibitor) for 2 h were treated with 65 μg/mL JCo extract for 24 h, and caspase-3 activation was determined by western blotting.
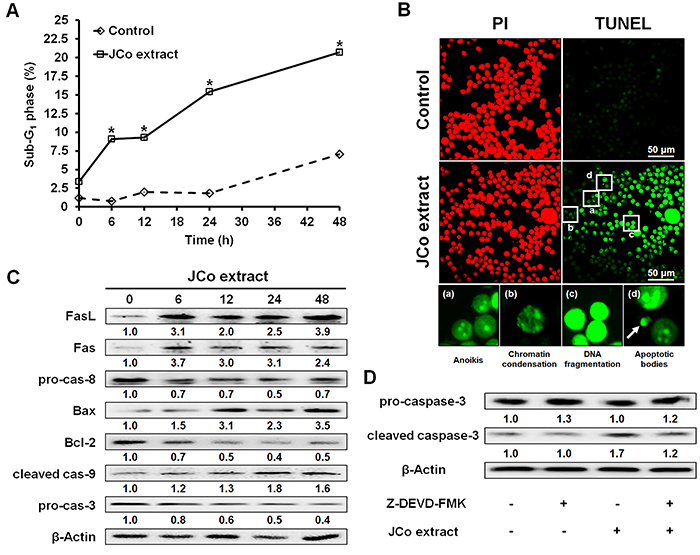
Next, to identify the apoptotic pathway activated by treatment with JCo extract in HT-29 cells, we performed western blotting. We found that JCo extract activated both the extrinsic (FasL, Fas, and pro-caspase-8) and intrinsic (Bax, Bcl-2, and cleaved caspase-9) apoptotic pathways (Figure 4C). Furthermore, JCo extract-induced activation of caspase-3 was blocked by the pretreatment of cells with a caspase-3 inhibitor (Figure 4D). Together, these results indicated that JCo extract activated the extrinsic and intrinsic pathways of apoptosis to trigger apoptosis and tumor cell death.
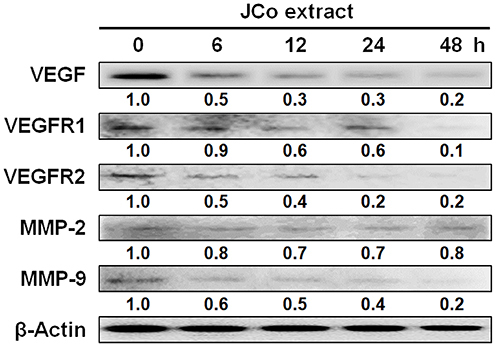
JCo extract downregulated the expression of angiogenesis- and metastasis-associated proteins
The effects of JCo extract on angiogenesis and metastasis were determined by western blotting. The results showed that JCo extract treatment decreased the protein expression levels of the autocrine angiogenesis-associated proteins VEGF, VEGFR1, and VEGFR2 and the metastasis-associated protein MMP-9 (Figure 5). Therefore, JCo extract may inhibit tumor proliferation, angiogenesis, and metastasis by downregulating the expression of these associated proteins.
Figure 5. Expression of proteins involved in autocrine angiogenesis and metastasis in Juniperus communis (JCo) extract-treated HT-29 cells. HT-29 cells were treated with 65 μg/mL JCo extract for 0, 6, 12, 24, and 48 h, and the expressions of vascular endothelial growth factor (VEGF), VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2), matrix metalloproteinase (MMP)-2, and MMP-9 were analyzed by western blotting.
Effects of JCo extract on CRC in tumor-bearing mice
To analyze tumor growth suppression in vivo, CRC mouse models bearing tumors were established. The results showed a significantly lower tumor volume in mice treated with JCo extract (703±192.80 mm3) than that in the vehicle group (1459.84±144.14 mm3) at day 25 (Figure 6A). The survival rate was 100% in the JCo extract treatment group but only 25% in the vehicle group (Figure 6B). Thus, JCo extract suppressed tumor growth and prolonged life expectancy in animals.
Figure 6. Effect of Juniperus communis (JCo) extract on the inhibition of CT-26 tumors in a BALB/c mouse model. Subcutaneous tumor-bearing mice were treated with 200 mg/kg JCo extract (sc) once every 2 days for 40 days. A and B, Tumor volumes were calculated once every 2 days, and the mice were sacrificed when the tumor volume exceeded 1500 mm3. C-E, Protein expression levels of proliferating cell nuclear antigen (PCNA), vascular endothelial growth factor (VEGF), VEGF receptor 1 (VEGFR1), VEGF receptor 2 (VEGFR2), matrix metalloproteinase (MMP)-2, MMP-9, and cleaved caspase-3 were detected by immunohistochemistry and scored using the Quickscore method (scale bars 100 μm). JCo extract-induced cell apoptosis was measured using the TUNEL assay. Data are reported as means±SD. *P<0.05 vs vehicle (ANOVA or t-test).
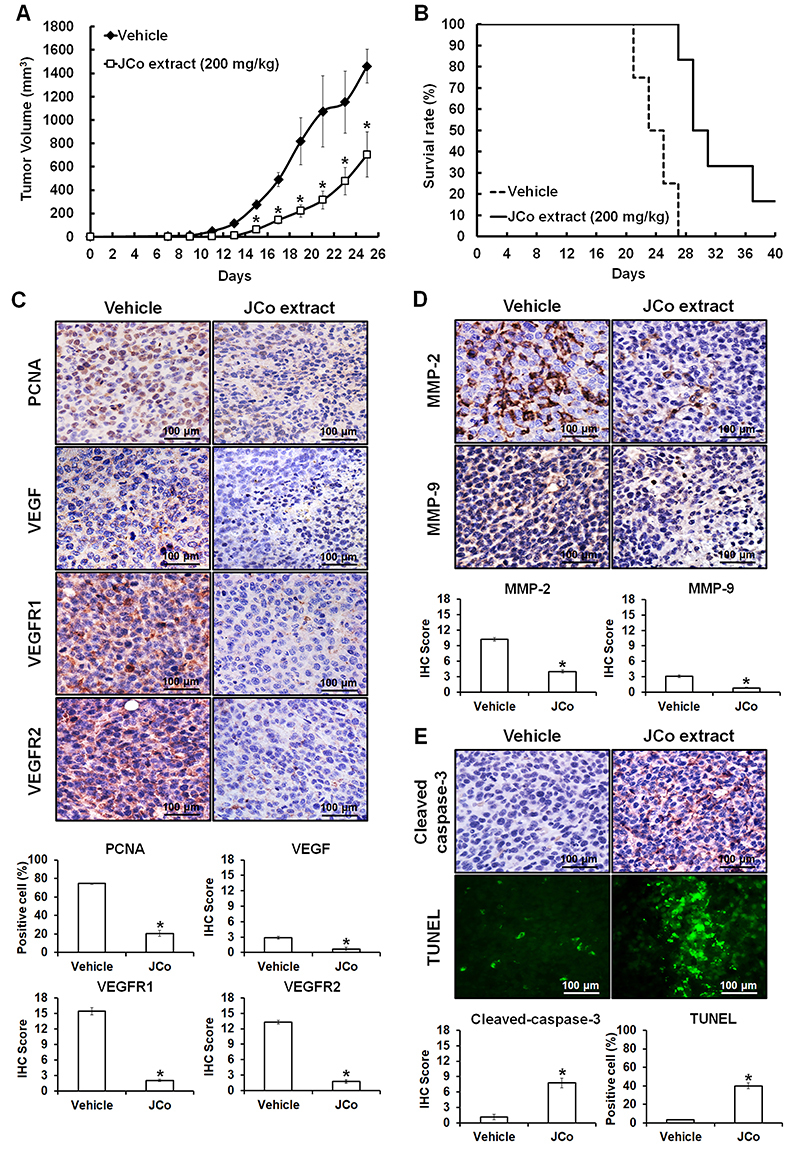
Next, we investigated the effects of JCo extract on major regulatory proteins, including cell proliferation markers (PCNA), angiogenesis proteins (VEGF/VEGFR1/VEGFR2), metastasis proteins (MMP-2/MMP-9), and apoptosis proteins (cleaved caspase-3), in vivo by IHC analysis. The results showed that JCo extract decreased the protein expression levels of PCNA (27.6%), VEGF (24.1%), VEGFR1 (13.3%), VEGFR2 (13.4%), MMP-2 (39.1%), and MMP-9 (26.4%) and increased the expression of cleaved caspase-3 (692.1%) compared to those in the vehicle group (values in the vehicle group were regarded as 100%) (Figure 6C-E). The TUNEL positivity rate was 3.2±0.33% in the vehicle group and 40±3.33% in the JCo treatment group, indicating that JCo extract triggered increased apoptosis (Figure 6E). These results suggested that JCo extract suppressed tumor development through the inhibition of tumor proliferation and induction of cell apoptosis.
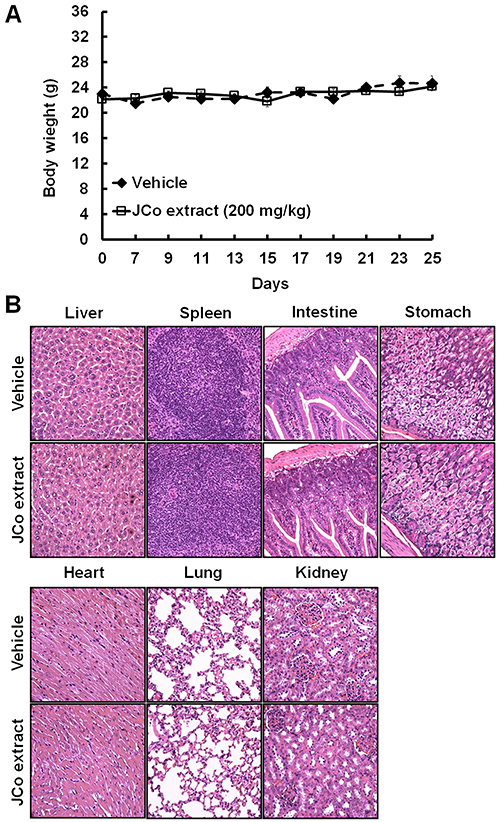
In animal studies, no obvious loss of body weight or damage to the liver, spleen, intestine, stomach, heart, lung, and kidney were observed in the JCo extract treatment group compared to that in the vehicle group (Figure 7A and B), suggesting that the therapeutic course of JCo extract was well tolerated.
Figure 7. Effects of Juniperus communis (JCo) extract on mouse body weight and vital organs. A, The body weights of tumor-bearing mice were recorded once every 2 days after JCo extract treatment for 40 days. Data are reported as means±SD. B, The vital organs were collected and analyzed by hematoxylin-eosin (HE) staining. No significant differences between the JCo extract and vehicle groups were noted in terms of body weight and histological morphology (scale bars, 100 μm).
Discussion
Accumulating evidence suggests that many herbal extracts and mixtures have anticancer and chemopreventive effects based on the disruption of the cell cycle and induction of apoptosis (34,35). JCo is a well-known plant with a long history of use in traditional Chinese medicine and herbal medicine. While JCo has shown anticancer effects against lung cancer, breast cancer, neuroblastoma, liver cancer, and colon cancer, the molecular mechanisms underlying these effects are not yet clearly understood. Our results demonstrated that JCo extract inhibited tumor cell growth in vitro and in vivo and enhanced the survival rate of tumor-bearing mice. In addition, JCo extract combined with 5-FU had a synergistic effect in CRC cells. In the context of molecular mechanisms, our results showed that JCo extract induced cell cycle arrest and activated the extrinsic and intrinsic apoptotic pathways to trigger tumor cell death. Furthermore, JCo extract treatment decreased the expression of proteins associated with autocrine angiogenesis and metastasis. A similar mechanism of action was detected in the animal model using IHC staining. Importantly, JCo extract exhibited lower cytotoxicity against normal cells and little or no organ damage in mice treated with JCo extract for 40 days.
The cell cycle plays an important role in the regulation of cell proliferation, division, and growth and is a target of many cancer therapeutic drugs (36). A recent study reported that the treatment of A549, MCF7, TK6, and U937 human cell lines with JCo extract (made from branches and leaves) resulted in the enhanced accumulation of G2/M phase cells (23). Another study showed that treatment with JCo extract (made from berries) increased the percentages of cells in the G2, M, and G0 phases and led to cell death in liver and colon carcinomas and myosarcoma (24). In our study, treatment with JCo extract upregulated p53 protein expression and downregulated p21 protein expression. p21 is a cyclin-dependent kinase inhibitor that regulates different phases of the cell cycle. The protein expression levels of CDK4/cyclin D1, which regulate the G1/S transition, were reduced after treatment with JCo extract, which triggered the accumulation of G0/G1 phase cells.
Apoptosis, a form of programmed cell death, is an important physiological process that balances cell formation and cell death without inducing an inflammatory response (37). Moreover, the induction of apoptosis is an important therapeutic strategy in cancer treatment. Apoptosis is mediated by two major pathways, the extrinsic (death receptor) and intrinsic (mitochondrial disruption) apoptotic pathways, which involve the activation of caspase-8 and caspase-9, respectively, to trigger caspase-3 activation (30,35). Therefore, we performed western blotting to identify the apoptotic pathway activated by JCo extract in CRC cells. Our results revealed that both the extrinsic (FasL, Fas, and pro-caspase-8) and intrinsic (Bax, Bcl-2, and cleaved caspase-9) apoptotic pathways were activated by JCo extract, finally resulting in apoptosis and morphological changes, including anoikis, chromatin condensation, DNA fragmentation, and the appearance of apoptotic bodies.
To investigate the anticancer effect of JCo extract and the underlying mechanism in vivo, a CT-26 tumor-bearing mouse model was established. The mean tumor volume was significantly reduced and the survival rate was significantly increased in the JCo extract treatment group compared to those in the vehicle group. IHC staining showed that JCo extract inhibited cell proliferation, autocrine angiogenesis, and metastasis and induced apoptosis in tumor-bearing mice; these findings are consistent with those obtained in the in vitro assays. Moreover, there was no significant difference between the JCo extract treatment and vehicle groups in terms of body weight and the histological morphology of the liver, spleen, intestine, stomach, heart, lungs, and kidneys after 40 days of treatment. These results indicated that JCo extract exhibited anti-proliferative, anti-angiogenic, and anti-metastatic activities and triggered apoptosis in CRC cells both in vitro and in vivo, without displaying obvious cytotoxicity against normal cells or organs at a low and well-tolerated dose.
Previous studies have demonstrated that JCo contains many pure natural compounds with anticancer effects. For example, deoxypodophyllotoxin isolated from JCo is a potent inducer of caspase-dependent apoptosis mediated by the mitochondrial (intrinsic) pathway and also inhibits cell survival via the MAPK/ERK and NFκB signaling pathways in malignant breast cancer cells (38). Another study showed that podophyllotoxin and deoxypodophyllotoxin isolated from selected Juniperus species are effective against leukemia cell lines (39). Imbricatolic acid isolated from the methanolic extract of JCo (made from berries) induces the accumulation of G1 phase cells and the downregulation of cyclins A, D1, and E1 in CaLu-6 cells (40). In this study, the major components of JCo extract with molecular weights less than 500 Da included α-pinene (27.8%), carane (14.3%), d-limonene (10.7%), and terpinolene (7.4%), as determined using gas chromatography-mass spectrometry (GC-MS) and the National Institute of Standards and Technology and Wiley library databases. Previous studies have shown that α-pinene induces cell cycle arrest in the G2/M phase in human hepatoma cell lines (25), has a synergistic effect against non-small-cell lung carcinoma when combined with paclitaxel (26), and induces apoptosis and confers anti-metastatic protection with respect to melanoma (27). d-limonene induces autophagy in SH-SY5Y neuroblastoma cells (28), inhibits angiogenesis and metastasis, induces cell death in human colon cancer cells (29), and induces cell apoptosis via a caspase-dependent mitochondrial pathway in human leukemia cells (30). Terpinolene, a common component of plants such as sage and rosemary, inhibits cell proliferation by decreasing the protein expression level of AKT1 in K562 cells (31). The results of these studies indicate that JCo extract contains anticancer compounds and that this extract shows potential as a future anticancer agent or an adjuvant therapeutic agent in CRC therapy.
In conclusion, our results demonstrated that JCo extract suppressed CRC cell growth both in vitro and in vivo and also showed a synergistic effect in combination with 5-FU. Therefore, JCo extract may serve as a potential therapeutic agent for the treatment of CRC.
Acknowledgments
This research was supported by grants from the Chung Shan Medical University Hospital Foundation, Taiwan (No. CSH-2013-A-025 and CSH-2014-A-019) and the Chung Shan Medical University Foundation, Taiwan (No. CSMU/PU-103-2). Flow cytometry (FACScan) was performed at the Instrument Center of Chung Shan Medical University, which is supported by the National Science Council, the Ministry of Education, and Chung Shan Medical University.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Can J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang P, Cartwright C, Chan D, Ding J, Felix E, Pan Y, et al. Anticancer activity of fish oils against human lung cancer is associated with changes in formation of PGE2 and PGE3 and alteration of Akt phosphorylation. Mol Carcinog. 2014;53:566–577. doi: 10.1002/mc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall JL, Haller DG, de Gramont A, Hochster HS, Lenz HJ, Ajani JA, et al. Adjuvant therapy for stage II and III colon cancer: consensus report of the international society of gastrointestinal oncology. Gastrointest Cancer Res. 2007;1:146–154. [PMC free article] [PubMed] [Google Scholar]
- 5.Smitha KT, Anitha A, Furuike T, Tamura H, Nair SV, Jayakumar R. In vitro evaluation of paclitaxel loaded amorphous chitin nanoparticles for colon cancer drug delivery. Colloids Surf B Biointerfaces. 2013;104:245–253. doi: 10.1016/j.colsurfb.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 6.Ashwanikumar N, Kumar NA, Nair SA, Kumar GSV. Dual drug delivery of 5-fluorouracil (5-FU) and methotrexate (MTX) through random copolymeric nanomicelles of PLGA and polyethylenimine demonstrating enhanced cell uptake and cytotoxicity. Colloids Surf B Biointerfaces. 2014;122:520–528. doi: 10.1016/j.colsurfb.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Granci V, Cai F, Lecumberri E, Clerc A, Dupertuis YM, Pichard C. Colon cancer cell chemosensitisation by fish oil emulsion involves apoptotic mitochondria pathway. Br J Nutr. 2013;109:1188–1195. doi: 10.1017/S000711451200308X. [DOI] [PubMed] [Google Scholar]
- 8.Yao Q, Fu T, Wang LU, Lai Y, Wang Y, Xu C, et al. Role of autophagy in the omega-3 long chain polyunsaturated fatty acid-induced death of lung cancer A549 cells. Oncol Lett. 2015;9:2736–2742. doi: 10.3892/ol.2015.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Chai H, Kinghorn AD. The continuing search for antitumor agents from higher plants. Phytochem Lett. 2010;3:1–8. doi: 10.1016/j.phytol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Peng Z, Su C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int J Mol Sci. 2015;16:10888–10906. doi: 10.3390/ijms160510888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindseil KU, Jakupovic J, Wolf D, Lavayre J, Leboul J, van der Pyl D. Pure compound libraries; a new perspective for natural product based drug discovery. Drug Discov Today. 2001;6:840–847. doi: 10.1016/S1359-6446(01)01856-6. [DOI] [PubMed] [Google Scholar]
- 13.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 14.Moein MR, Ghasemi Y, Moein S, Nejati M. Analysis of antimicrobial, antifungal and antioxidant activities of Juniperus excelsa M. B subsp. Polycarpos (K. Koch) Takhtajan essential oil. Pharmacognosy Res. 2010;2:128–131. doi: 10.4103/0974-8490.65505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiritikar KR BB. Dehradun. India:: International Book Distributors; 1994. Indian Medicinal Plants. 2nd ed. [Google Scholar]
- 16.Tunon H, Olavsdotter C, Bohlin L. Evaluation of anti-inflammatory activity of some Swedish medicinal plants. Inhibition of prostaglandin biosynthesis and PAF-induced exocytosis. J Ethnopharmacol. 1995;48:61–76. doi: 10.1016/0378-8741(95)01285-L. [DOI] [PubMed] [Google Scholar]
- 17.Ved A, Gupta A, Rawat AK. Antioxidant and hepatoprotective potential of phenol-rich fraction of Juniperus communis Linn. Leaves. Pharmacog Mag. 2017;13:108–113. doi: 10.4103/0973-1296.197648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elshafie HS, Caputo L, De Martino L, Grulova D, Zheljazkov VZ, De Feo V, et al. Biological investigations of essential oils extracted from three Juniperus species and evaluation of their antimicrobial, antioxidant and cytotoxic activities. J Appl Microbiol. 2020;129:1261–1271. doi: 10.1111/jam.14723. [DOI] [PubMed] [Google Scholar]
- 19.Fierascu I, Ungureanu C, Avramescu SM, Cimpeanu C, Georgescu MI, Fierascu RC, et al. Genoprotective, antioxidant, antifungal and anti-inflammatory evaluation of hydroalcoholic extract of wild-growing Juniperus communis L. (Cupressaceae) native to Romanian southern sub-Carpathian hills. BMC Complement Altern Med. 2018;18:3. doi: 10.1186/s12906-017-2066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banerjee S, Singh H, Chaterjee TK. Evaluation of anti-diabetic and anti-hyperlipidemic potential of methanolic extracts of Juniperus communis Linn. Intl J Pharma Biosci. 2013;4:10–17. [Google Scholar]
- 21.Gao HW, Huang XF, Yang TP, Chang KF, Yeh LW, Hsieh MC, et al. Juniperus communissuppresses melanoma tumorigenesis by inhibiting tumor growth and inducing apoptosis. Am J Chin Med. 2019;47:1171–1191. doi: 10.1142/S0192415X19500605. [DOI] [PubMed] [Google Scholar]
- 22.Lee CC, Hsiao CY, Lee SC, Huang XF, Chang KF, Lee MS, et al. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci Rep. 2020;40:BSR20202083. doi: 10.1042/BSR20202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollio A, Zarrelli A, Romanucci V, Di Mauro A, Barra F, Pinto G, et al. Polyphenolic profile and targeted bioactivity of methanolic extracts from Mediterranean ethnomedicinal plants on human cancer cell lines. Molecules. 2016;21:395. doi: 10.3390/molecules21040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayazit V. Cytotoxic effects of some animal and vegetable extracts and some chemicals on liver and colon carcinoma and myosarcoma. Saudi Medical J. 2004;25:156–163. [PubMed] [Google Scholar]
- 25.Chen W, Liu Y, Li M, Mao J, Zhang L, Huang R, et al. Anti-tumor effect of alpha-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J Pharmacol Sci. 2015;127:332–338. doi: 10.1016/j.jphs.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Guo S, Liu X, Gao X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC) Drug Res (Stuttg) 2015;65:214–218. doi: 10.1055/s-0034-1377025. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JA, Massaoka MH, et al. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun. 2011;411:449–454. doi: 10.1016/j.bbrc.2011.06.176. [DOI] [PubMed] [Google Scholar]
- 28.Russo R, Cassiano MG, Ciociaro A, Adornetto A, Varano GP, Chiappini C, et al. Role of D-Limonene in autophagy induced by bergamot essential oil in SH-SY5Y neuroblastoma cells. PloS One. 2014;9:e113682. doi: 10.1371/journal.pone.0113682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chidambara Murthy KN, Jayaprakasha GK, Patil BS. D-limonene rich volatile oil from blood oranges inhibits angiogenesis, metastasis and cell death in human colon cancer cells. Life Sci. 2012;91:429–439. doi: 10.1016/j.lfs.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Ji J, Zhang L, Wu YY, Zhu XY, Lv SQ, Sun XZ. Induction of apoptosis by d-limonene is mediated by a caspase-dependent mitochondrial death pathway in human leukemia cells. Leuk Lymphoma. 2006;47:2617–24. doi: 10.1080/00268970600909205. [DOI] [PubMed] [Google Scholar]
- 31.Okumura N, Yoshida H, Nishimura Y, Kitagishi Y, Matsuda S. Terpinolene, a component of herbal sage, downregulates AKT1 expression in K562 cells. Oncol Lett. 2012;3:321–324. doi: 10.3892/ol.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao L, Wientjes MG, Au JL. Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin Cancer Res. 2004;10:7994–8004. doi: 10.1158/1078-0432.CCR-04-1087. [DOI] [PubMed] [Google Scholar]
- 33.Detre S, Jotti GS, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–878. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye YT, Zhong W, Sun P, Wang D, Wang C, Hu LM, et al. Apoptosis induced by the methanol extract of Salvia miltiorrhiza Bunge in non-small cell lung cancer through PTEN-mediated inhibition of PI3K/Akt pathway. J Ethnopharmacol. 2017;200:107–116. doi: 10.1016/j.jep.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 35.Sui Y, Li S, Shi P, Wu Y, Li Y, Chen W, et al. Ethyl acetate extract from Selaginella doederleinii Hieron inhibits the growth of human lung cancer cells A549 via caspase-dependent apoptosis pathway. J Ethnopharmacol. 2016;190:261–271. doi: 10.1016/j.jep.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Shukla S, Fu P, Gupta S. Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70-Bax interaction in prostate cancer. Apoptosis. 2014;19:883–894. doi: 10.1007/s10495-014-0971-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haanen C, Vermes I. Apoptosis and inflammation. Mediators Inflamm. 1995;4:5–15. doi: 10.1155/S0962935195000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benzina S, Harquail J, Jean S, Beauregard AP, Colquhoun CD, Carroll M, et al. Deoxypodophyllotoxin isolated from Juniperus communis induces apoptosis in breast cancer cells. Anticancer Agents Med Chem. 2015;15:79–88. doi: 10.2174/1871520614666140608150448. [DOI] [PubMed] [Google Scholar]
- 39.Och M, Och A, Ciesla L, Kubrak T, Pecio L, Stochmal A, et al. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm Biol. 2015;53:831–837. doi: 10.3109/13880209.2014.943246. [DOI] [PubMed] [Google Scholar]
- 40.De Marino S, Cattaneo F, Festa C, Zollo F, Iaccio A, Ammendola R, et al. Imbricatolic acid from Juniperus communis L. prevents cell cycle progression in CaLu-6 cells. Planta Med. 2011;77:1822–1828. doi: 10.1055/s-0030-1271104. [DOI] [PubMed] [Google Scholar]


