Abstract
Ataxia-telangiectasia mutated (ATM) kinase deficiency exacerbates heart dysfunction late after myocardial infarction. Here, we hypothesized that ATM deficiency modulates Western-type diet (WD)-induced cardiac remodeling with an emphasis on functional and biochemical parameters of the heart. Weight gain was assessed in male wild-type (WT) and ATM heterozygous knockout (hKO) mice on weekly basis, whereas cardiac functional and biochemical parameters were measured 14 wk post-WD. hKO-WD mice exhibited rapid body weight gain at weeks 5, 6, 7, 8, and 10 versus WT-WD. WD decreased percent fractional shortening and ejection fraction, and increased end-systolic volumes and diameters to a similar extent in both genotypes. However, WD decreased stroke volume, cardiac output, peak velocity of early ventricular filling, and aortic ejection time and increased isovolumetric relaxation time (IVRT) and Tei index versus WT-NC (normal chow). Conversely, IVRT, isovolumetric contraction time, and Tei index were lower in hKO-WD versus hKO-NC and WT-WD. Myocyte apoptosis and hypertrophy were higher in hKO-WD versus WT-WD. WD increased fibrosis and expression of collagen-1α1, matrix metalloproteinase (MMP)-2, and MMP-9 in WT. WD enhanced AMPK activation, while decreasing mTOR activation in hKO. Akt and IKK-α/β activation, and Bax, PARP-1, and Glut-4 expression were higher in WT-WD versus WT-NC, whereas NF-κB activation and Glut-4 expression were lower in hKO-WD versus hKO-NC. Circulating concentrations of IL-12(p70), eotaxin, IFN-γ, macrophage inflammatory protein (MIP)-1α, and MIP-1β were higher in hKO-WD versus WT-WD. Thus, ATM deficiency accelerates weight gain, induces systolic dysfunction with increased preload, and associates with increased apoptosis, hypertrophy, and inflammation in response to WD.
NEW & NOTEWORTHY Ataxia-telangiectasia mutated (ATM) kinase deficiency in humans associates with enhanced susceptibility to ischemic heart disease. Here, we provide evidence that ATM deficiency accelerates body weight gain and associates with increased cardiac preload, hypertrophy, and apoptosis in mice fed with Western-type diet (WD). Further investigations of the role of ATM deficiency in WD-induced alterations in function and biochemical parameters of the heart may provide clinically applicable information on treatment and/or nutritional counseling for patients with ATM deficiency.
Keywords: apoptosis, ATM, fibrosis, heart, Western-type diet
INTRODUCTION
Chronic consumption of Western-type diet (WD) induces inflammation, oxidative stress, and mitochondrial dysfunction in skeletal as well as cardiac muscle (1–3). WD associates with hemodynamic and structural alterations of the heart leading to a clinical syndrome called obesity cardiomyopathy (4, 5). As metabolic demand increases with continually expanding adipose tissue, hyperdynamic circulation with increased blood volume and hemodynamic overload occurs (6, 7). Preload and afterload cardiac dysfunction can occur due to continual adaptive compensation for changes in blood volume (8, 9). In animal models, left ventricular (LV) hypertrophy, increased arrhythmic events, pump failure, atherosclerosis, biventricular stiffness, and increased fibrosis have been noted with diet-induced obesity (5, 10–12).
Ataxia-telangiectasia mutated (ATM) kinase is generally activated in response to double-strand DNA (dsDNA) breaks, oxidative damage, and other genotoxic stressors (13–16). Although the main function of ATM is to maintain genomic stability through redox sensing, coordination of DNA damage repair, and facilitation of cell cycle progression in response to dsDNA breaks, ATM is also suggested to play a pivotal role in metabolism, vesicle transport, and mitochondrial function (13–16). Disruption of the ATM gene results in a complex multisystem disorder called ataxia-telangiectasia (A-T), which associates with neurological, immunological, endocrinological, and cardiovascular abnormalities (15, 17–19). Individuals with an ATM mutation in one allele (A-T carriers) constitute 1.4%–2% of the general population and exhibit enhanced susceptibility to cancer and ischemic heart disease (16). Previously, we provided evidence that ATM deficiency in mice associates with increased cardiac fibrosis, myocyte hypertrophy, and exacerbation of heart dysfunction 28 days following myocardial infarction (MI) (20). However, there are no reports investigating the role of ATM deficiency in WD-induced cardiac remodeling. Here, we hypothesized that ATM deficiency modulates WD-induced cardiac remodeling with a focus on functional and biochemical parameters of the heart. The data presented here suggest that ATM deficiency associates with rapid weight gain, systolic dysfunction with increased preload, and exacerbated cardiac remodeling in terms of hypertrophy and apoptosis in response to WD. It also affects WD-induced circulating levels of cytokines/chemokines and expression/activation of protein associated with fibrosis, metabolism, and inflammation.
MATERIALS AND METHODS
Vertebrate Animals and Diets
This study conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). East Tennessee State University Committee on Animal Care approved all protocols used in this study. Breeding pairs of ATM-deficient mice (129S6/SvEvTac) were purchased from Jackson Laboratory (Stock No. 002753). The homozygous KO mice are infertile and die ∼2 mo of age. Therefore, ATM heterozygous knockout (hKO; deficient) mice were used for breeding providing us with littermate WT and hKO mice. Primers suggested by the Jackson Laboratory were used to genotype the mice using PCR. Six-week-old male WT and hKO mice were placed on a normal chow (NC; Envigo 8604) or Western-type diet (WD; Envigo TD 88137) for 14 wk. The energy composition of NC is 32% kcal protein, 14% kcal fat, 54% kcal carbohydrate, and 4% sugar (by weight). The energy composition of WD is 15.2% kcal protein, 42.0% kcal fat, 42.7% kcal carbohydrate, and 34% sugar (by weight). All mice were kept on a 12-h:12-h dark/light cycle with food and water available ad libitum.
Fasting Glucose Levels
NC and WD mice from both genotypes were fasted for 12 h. The tail was then gently nicked using a 5-mm Goldenrod lancet. Approximately 1 µL of blood sample was collected from the tail vein, and blood glucose was measured using a ReliOn monitoring system.
Echocardiography
Structural and functional parameters of the heart were measured 14 wk post-WD using a Vevo 1100 imaging system (VisualSonics, Fujifilm) equipped with a 22- to 55-MHz MS550D transducer (21, 22). For this, mice were anesthetized using a mixture of isoflurane (2%) and oxygen (0.6 L/min). Heating pad was used to maintain body temperature ∼37°C. M-mode recordings, obtained using transthoracic short axis view at midpapillary level, were used to measure/calculate heart rate, %ejection fraction (%EF), %fractional shortning (%FS), LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), stroke volume, and cardiac output. Doppler tracings, acquired from apical four-chamber view, were used to measure peak velocity of early ventricular filling (E wave), aortic ejection time (AET), isovolumetric relaxation time (IVRT; measured from the aortic valve closure to the mitral valve opening), and isovolumetric contraction time (IVCT; measured from the closing of the mitral valve to the opening of the aortic valve) (22). Tei index, also known as myocardial performance index, was calculated as: IVRT + IVCT/AET.
Morphometric Analysis
Mice were weighed on a weekly basis until 14-wk diet completion. Mice were anesthetized using a mixture of isoflurane (2%) and oxygen (0.6 L/min). The heart was excised through an opening of the diaphragm region. The heart was perfused with Krebs–Henseleit buffer to ensure blood clearance, arrested in diastole using 16 mM KCl, and weighed. Epidermal skin layer was removed postmortem, and subcutaneous and visceral fat were collected and weighed. Tibia length was measured using Vernier calipers (Monostat). The heart was then divided into two transverse sections (base/mid and apex) and embedded in paraffin. Midcardiac transverse sections (5 µm thick) were stained with Masson’s trichrome staining to measure fibrosis and septal wall hypertrophy. For fibrosis, ten separate septal images from each heart were analyzed using Nikon NIS software as previously described (20). Percent fibrosis was calculated by dividing the total fibrosis area by the total tissue area of each image and multiplying by 100. To measure septal wall hypertrophy, six separate transverse measurements were averaged from each heart. To examine lipid deposition, cryosections (10-µm-thick) of the heart were stained with oil red O. Ten separate septal images from each heart were analyzed using Nikon NIS software as described (20).
Western Blot Analysis
Cardiac lysates were prepared in RIPA buffer [10 mM Tris-HCl (pH 7.2), 158 mM NaCl, 1 mM EGTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and 0.2 mM phenylmethylsulfonyl fluoride] supplemented with Halt Protease Inhibitor Cocktail. Equal amounts of proteins (50 μg) were resolved by SDS-PAGE and transferred to PVDF membranes. All membranes were blocked with 5% nonfat dry milk and incubated overnight with primary antibodies against ATM (1:500, Cat. No. sc23921, Santa Cruz), MMP-9 (1:1,000, Cat. No. AB19016, Millipore), MMP-2 (1:500, Cat. No. MAB3308, Millipore), BAX (1:1,000, Cat. No. SC7480, Santa Cruz), PARP-1 (1:1,000, Cat. No. 9542, Cell Signaling), p-Akt (ser473; 1:1,000, Cat. No. 9271S, Cell Signaling), total Akt (1:1,000, Cat. No. 9272S, Cell Signaling), p-NF-κB (ser-536; 1:1,000, Cat. No. 3033S, Cell Signaling), total NF-кB (1:1,000, Cat. No. 6956S, Cell Signaling), p-IKK α/β (ser180/ser181; 1:500, Cat. No. 2681S, Cell Signaling), total IKK-α (1:1,000, Cat. No. 61294S, Cell Signaling), Glut-4 (1:500, Cat. No. sc7938, Santa Cruz), p-mTOR (ser-2448; 1:1,000, Cat. No. 5536S, Cell Signaling), total mTOR (1:1,000, Cat. No. 2983S, Cell Signaling), p-AMPK (thr-172; 1:1,000, Cat. No. 2535S, Cell Signaling), total AMPK (1:1,000, Cat. No. 5832S, Cell Signaling), and collagen-1α1 (1:500, Cat. No. 72026, Cell Signaling). The immune complexes were detected using appropriate secondary antibodies and chemiluminescent reagents. There was no significant difference in GAPDH levels among the groups; therefore, protein loading in each lane was normalized using GAPDH immunostaining (1:10,000; Cat. No. 32233, Santa Cruz). Band intensities were quantified using ImageQuant LAS 500 imaging system (GE Healthcare) (23). For all phosphoproteins, total protein expression was normalized using GAPDH immunostaining. This analysis showed no significant difference in the expression of total proteins (Figs. 9 and 10). Therefore, phosphoprotein band intensities were normalized using GAPDH immunostaining. Western blot analysis for ATM was performed using 6% SDS-PAGE, and Ponceau S staining was used to indicate protein loading in each lane.
Terminal Deoxynucleotidyl Transferase Nick End Labeling Assay
TUNEL assay was performed according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, Roche) (24). Tissue sections (5 µm thick) were also stained with rhodamine-conjugated wheat germ agglutinin (WGA, Rl-1022, Vector) to visualize myocytes and Hoechst 33258 (10 μM; Sigma) to visualize nuclei. Hoechst-positive stained nuclei served as an index to count the total number of nuclei. Apoptotic myocytes were identified by TUNEL-positive staining clearly seen within WGA-stained cells and Hoechst positively stained nuclei. The index of myocyte apoptosis was calculated as the percentage myocyte apoptotic nuclei/total nuclei. Total cardiac cell apoptosis was measured by counting the TUNEL-positive and Hoechst-positive nuclei. The index of cardiac cell apoptosis was calculated as the percentage of total apoptotic nuclei/total nuclei.
Myocyte Cross-Sectional Area
WGA-stained cross sections of the heart were used to measure myocyte cross-sectional area. For this, suitable myocytes utilized for analysis included myocytes with a circular border with centrally localized nuclei. Images were obtained with the EVOS M7000 imaging system, and myocyte cross-sectional area was quantified with Nikon NIS software as previously described (20).
Cholesterol and Triglyceride Assay
Total cholesterol and triglyceride levels from nonhemolyzed serum were measured according to manufacturer’s instructions (Pointe Scientific). Sample to reagent ratio was diluted to 1:1,000, and serum standards provided by the manufacturer were used to verify the validity of reaction. Samples were read at 500 nm wavelength using a BioTek PowerWave XS2 microplate spectrophotometer. Cholesterol and triglyceride levels were determined as: absorbance (sample)/absorbance (standard) multiplied by the concentration of standard.
Serum Cytokine and Chemokine Assay
The Bio-Plex Pro Mouse Cytokine 23-Plex Assay was used to measure circulating cytokine and chemokine concentrations according to the manufacturer’s instructions (Bio-Rad). The following cytokines/chemokines were assessed: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 subunit p40 [IL-12(P40)], IL-12(p70), IL-13, IL-17, eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon (IFN)-γ, keratinocyte chemoattractant (KC), monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, macrophage inflammatory protein (MIP)-1β, regulated upon activation, normal T cell expressed, and secreted (RANTES), and TNF-α.
Statistical Analysis
Data are expressed as means ± SE. Data were analyzed using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test or two-tailed Student’s t test. P values of <0.05 were considered to be significant.
RESULTS
ATM Expression
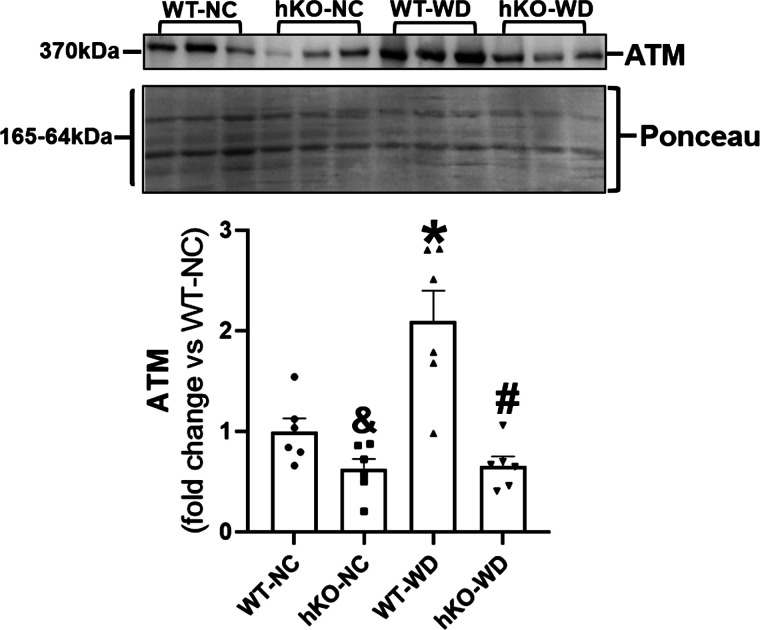
ATM expression increases in response to many different stimuli including oxidative stress, β-adrenergic receptor (β-AR) stimulation, and MI (15, 25). Western blot analysis of heart lysates using anti-ATM antibodies showed that ATM protein levels (expression) are ∼50% lower in hKO-NC group versus WT-NC. WD significantly increased ATM expression (>2.0-fold) in WT group versus WT-NC. However, no increase in ATM expression was observed in hKO-WD group, and ATM expression remained significantly lower in hKO-WD versus WT-WD (Fig. 1).
Figure 1.
WD increases ATM expression in the myocardium of WT mice. Total heart lysates were analyzed by Western blot using anti-ATM antibodies. Ponceau S staining indicates protein loading in each lane. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, #P < 0.05 vs. WT-WD, n = 6. ATM, ataxia-telangiectasia mutated; NC, normal chow; WD, Western-type diet; WT, wild type.
Morphometric Analyses
Adiposity is commonly observed in mice fed with high-fat diets (10). To assess differences in diet-induced weight gain, mice were weighed on weekly basis. The starting body weights (week 0) of 6-wk-old mice were not significantly different between the two genotypes (WT, 21.2 ± 0.6 g; hKO, 20.1 ± 0.7 g; P = NS; n = 6–8). Weekly measurement of weight gain versus week 0 showed a steady increase in weight gain in all four groups. However, weight gain was significantly greater in the WD groups versus their respective NC starting week 3, which continued until week 14 (Fig. 2). Interestingly, hKO-WD group exhibited rapid weight gain and weight gain was significantly greater in hKO-WD at weeks 5, 6, 7, 8, and 10 versus WT-WD. Although weight gain increased steadily from weeks 11–14 in both WD groups, there was no significant difference between the two WD groups. At weeks 12–14, there was no significant increase in weight gain in hKO-WD versus week 10 (P = NS; n = 8). On the other hand, weight gain was significantly greater at weeks 13 and 14 versus week 10 in WT-WD group (P < 0.05; n = 16). At week 14, the increase in body weight, subcutaneous fat weight, and visceral fat weight were not significantly different between the two NC or WD groups (Table 1). Heart weights remained unchanged among the four groups. Both WD groups exhibited significant decrease in heart weight-to-body weight (HW/BW) ratio versus their respective NC groups. However, no difference in HW/BW ratio was observed between the two WD groups. Heart weight-to-tibia length ratio is commonly used as a measure of hypertrophy (26). This ratio was found to be slightly, but significantly, higher in hKO-NC versus WT-NC. However, the ratio remained unchanged between the two WD groups and was not significantly different versus their NC counterparts. Total cholesterol levels were significantly increased in the two WD groups versus their respective NC groups with no significant difference between the two WD groups. Triglyceride levels trended higher in WD versus NC groups; however, the differences did not reach statistical significance. Oil red O staining showed no discernable deposition of lipids in the heart 14 wk post-WD (data not shown). Fasting blood glucose tended to be higher WT-WD versus WT-NC (P = 0.05). However, it was significantly higher in WT-WD group versus hKO-WD (mg/dL; WT-NC, 108.6 ± 7.1; hKO-NC, 90.1 ± 3.5; WT-WD, 136.4 ± 10.5; hKO-WD, 97.9 ± 5.98#; #P < 0.05 vs. WT-WD; n = 7–10; Table 1).
Figure 2.
WD-induced weight gain with time. A: visceral (left) and subcutaneous (right) adipose distribution after 14 wk on NC or WD. B: weight gain for normal chow (NC) and Western-type diet (WD) groups from wk 0 to wk 14. $P < 0.05 vs. hKO-NC, *P < 0.05 vs. WT-NC, #P < 0.05 vs. WT-WD, @P < 0.05 vs. wk 10, n = 8–16. hKO, heterozygous knockout; WD, Western-type diet; WT, wild type.
Table 1.
Morphometric and biochemical measurements
| Parameters | WT-NC | hKO-NC | WT-WD | hKO-WD |
|---|---|---|---|---|
| Body weight, g | 27.1 ± 1.3 | 27.7 ± 0.7 | 31.4 ± 1.3* | 31.9 ± 1.2$ |
| Weight gain, g | 7.6 ± 1.0 | 7.2 ± 0.6 | 11.1 ± 0.9* | 10.7 ± 0.9$ |
| Heart weight, mg | 146 ± 3 | 152 ± 3 | 142 ± 5 | 141 ± 4 |
| Heart weight/body weight, mg/g | 5.6 ± 0.2 | 5.5 ± 0.1 | 4.5 ± 0.2* | 4.4 ± 0.2$ |
| Heart weight/tibia length, mg/mm | 10.5 ± 0.2 | 11.3 ± 0.2& | 10.3 ± 0.3 | 10.6 ± 0.3 |
| Subcutaneous fat, mg | 550 ± 70 | 530 ± 60 | 1163 ± 120* | 1190 ± 200$ |
| Abdominal fat, mg | 840 ± 100 | 880 ± 90 | 2160 ± 170* | 2390 ± 210$ |
| Total cholesterol, mg/dL | 81.9 ± 8.7 | 66.9 ± 2.6 | 148.2 ± 3.9* | 158.3 ± 5.2$ |
| Triglycerides, mg/dL | 44.5 ± 6.5 | 54.8 ± 6.2 | 59.9 ± 10.4 | 68.1 ± 10.2 |
| Fasting glucose, mg/dL | 108.6 ± 7.1 | 90.1 ± 3.5 | 136.4 ± 10.5 | 97.9 ± 6.0# |
Values are means ± SE, n = 7–10. hKO, heterozygous knockout; NC, normal chow; WD, Western-type diet; WT, wild type. *P < 0.05 vs. WT-NC; &P < 0.05 vs. WT-NC; $P < 0.05 vs. hKO-NC; #P < 0.05 vs. WT-WD.
Echocardiographic Measurements
M-mode echocardiography revealed no difference in the parameters between the two NC groups. WD decreased %FS and %EF, and increased LVESD and LVESV to a similar extent in both genotypes versus their NC counterparts. Heart rate, LVEDD, and LVEDV remained unchanged among the four groups. Interestingly, a significant decrease in stroke volume and cardiac output was observed in WT-WD versus WT-NC (Table 2). Pulsed wave Doppler analysis revealed that E wave is significantly lower in hKO-NC versus WT-NC. WD led to a significant decrease in E wave in WT-WD, not in hKO-WD group (mm/s; WT-NC, 656.8 ± 41.1; hKO-NC, 526.9 ± 40.8&; WT-WD, 536.1 ± 29.5*; hKO-WD, 544.4 ± 19.0; &P < 0.05 vs. WT-NC; *P < 0.05 vs. WT-NC; n = 9–10; Fig. 3, A and B). AET was significantly lower in WT-WD versus WT-NC. WD had no effect on AET in hKO group, and AET was significantly lower in WT-WD versus hKO-WD (Fig. 3C). IVCT and IVRT were not different between the two NC groups. However, IVCT was significantly lower in hKO-WD versus hKO-NC and WT-WD groups (Fig. 3D). IVRT was significantly increased in WT-WD versus WT-NC. Conversely, IVRT was significantly lower in hKO-WD versus hKO-NC and was found to be significantly lower in hKO-WD versus WT-WD (Fig. 3E). Tei index (an indicator of global cardiac function) was significantly higher in WT-WD versus WT-NC, whereas Tei index was significantly lower in hKO-WD versus hKO-NC and was found to be significantly lower in hKO-WD versus WT-WD (Fig. 3F).
Table 2.
M-Mode echocardiographic parameters
| Parameters | WT-NC | hKO-NC | WT-WD | hKO-WD |
|---|---|---|---|---|
| Heart rate, beats/min | 367 ± 9 | 344 ± 10 | 352 ± 14 | 370 ± 12 |
| %EF | 63.71 ± 1.77 | 66.18 ± 2.14 | 52.81 ± 1.44* | 52.26 ± 1.53$ |
| %FS | 34.19 ± 1.39 | 36.13 ± 1.68 | 26.69 ± 0.90* | 26.40 ± 0.95$ |
| LVESD, mm | 2.47 ± 0.06 | 2.41 ± 0.11 | 2.77 ± 0.08* | 2.84 ± 0.08$ |
| LVEDD, mm | 3.76 ± 0.05 | 3.77 ± 0.11 | 3.78 ± 0.09 | 3.85 ± 0.10 |
| LVESV, µL | 22.05 ± 1.28 | 21.19 ± 2.4 | 29.44 ± 2.19* | 31.00 ± 2.14$ |
| LVEDV, µL | 60.85 ± 1.94 | 61.78 ± 4.70 | 62.13 ± 3.66 | 64.97 ± 4.04 |
| Stroke volume, µL | 38.79 ± 1.67 | 40.59 ± 2.84 | 32.69 ± 1.95* | 33.97 ± 2.35 |
| Cardiac output, mL/min | 14.20 ± 0.55 | 13.81 ± 0.84 | 11.58 ± 0.89* | 12.47 ± 0.78 |
Values are means ± SE, n = 10. %EF, %ejection fraction; %FS, %fractional shortning; hKO, heterozygous knockout; LVEDD, LV end-diastolic diameter; LVEDV, LV end-diastolic volume; LVESD, LV end-systolic diameter; LVESV, LV end-systolic volume; NC, normal chow; WD, Western-type diet; WT, wild type. *P < 0.05 vs. WT-NC; $P < 0.05 vs. hKO-NC.
Figure 3.
WD-induced changes in Doppler flow parameters of the heart. Indices of Doppler flow parameters: E wave, aortic ejection time (AET), isovolumetric contraction time (IVCT), isovolumetric relaxation time (IVRT), and Tei index were measured/calculated using pulsed wave Doppler echocardiographic images after 14 wk on normal chow (NC) or Western-type diet (WD). Representative Doppler tracings for each group (A); E wave (B); AET (C); IVCT (D); IVRT (E); and Tei index (F). &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, $P < 0.05 vs. hKO-NC, #P < 0.05 vs. WT-WD, n = 9–10. hKO, heterozygous knockout; WD, Western-type diet; WT, wild type.
Figure 4.
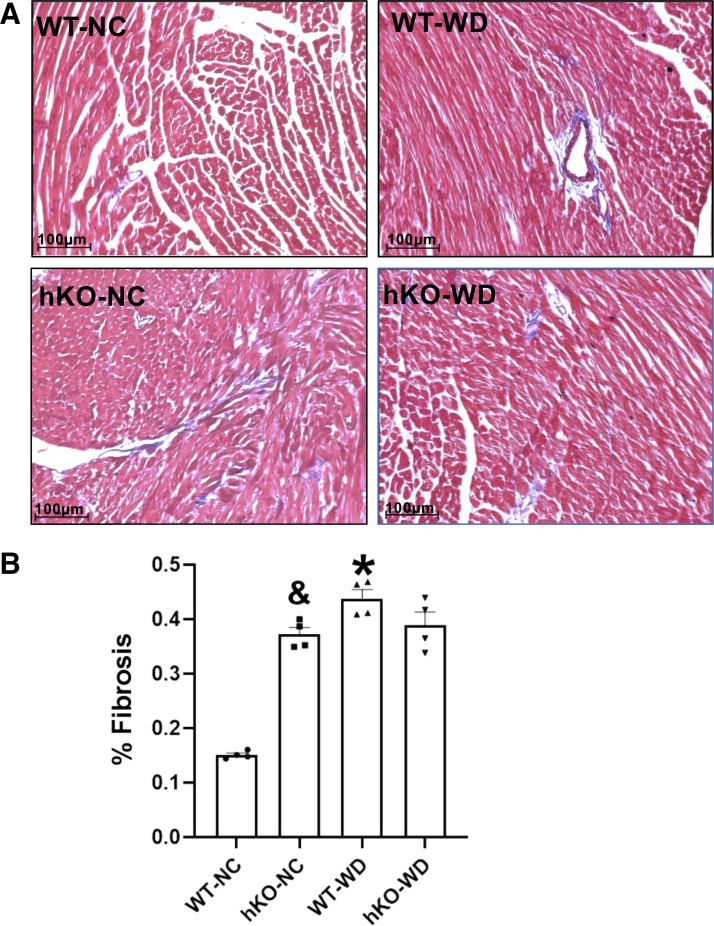
Analysis of fibrosis in the heart. A: representative images obtained from Masson’s trichrome-stained sections of the heart. Blue staining indicates fibrosis, while red staining indicates live tissue. B: quantitative measurements of fibrosis. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, n = 4. hKO, heterozygous knockout; WD, Western-type diet; NC, normal chow; WT, wild type.
Fibrosis, Apoptosis, and Hypertrophy
Increased cardiac fibrosis, myocyte apoptosis, and myocardial dysfunction are often observed with high-fat diets (5, 10, 27, 28). Percent fibrosis was significantly higher in hKO-NC versus WT-NC. WD significantly increased percent fibrosis in WT, not in hKO group (Fig. 4, A and B). Myocyte and total cell apoptosis was significantly higher in hKO-NC versus WT-NC. WD increased myocyte apoptosis in both genotypes. However, the increase in myocyte and total cell apoptosis was significantly greater in hKO-WD versus WT-WD (Fig. 5, A–C). Cardiac hypertrophy as measured by increased myocyte cross-sectional area and septal wall width was higher in hKO-NC versus WT-NC. Myocyte cross-sectional area and septal wall width were significantly increased in both WD groups versus their respective NC groups. However, myocyte cross-sectional area and septal wall width were significantly greater in hKO-WD versus WT-WD (Fig. 6, A–C).
Figure 5.
ATM deficiency exacerbates apoptosis in the heart in response to Western-type diet. A: representative images of TUNEL (green), WGA (red), and Hoechst (blue) stained hearts. B: quantitative analysis of myocyte apoptosis. C: quantitative analysis of cardiac cell apoptosis. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, $P < 0.05 vs. hKO-NC, #P < 0.05 vs. WT-WD, n = 4. ATM, ataxia-telangiectasia mutated; hKO, heterozygous knockout; NC, normal chow; TUNEL, terminal deoxynucleotidyl transferase nick end labeling; WD, Western-type diet; WGA, wheat germ agglutinin; WT, wild type.
Figure 6.
ATM deficiency exacerbates myocyte and septal hypertrophy in response to Western-type diet. A: representative images of wheat germ agglutinin (WGA)-stained cross sections of the heart depicting myocytes. B: quantitative analysis of myocyte cross-sectional area. C: quantitative analysis of septal hypertrophy using Masson’s trichrome-stained hearts. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, $P < 0.05 vs. hKO-NC, #P < 0.05 vs. WT-WD, n = 4. ATM, ataxia-telangiectasia mutated; hKO, heterozygous knockout; NC, normal chow; WD, Western-type diet; WT, wild type.
Expression of Collagen-1α1, MMP-2, and MMP-9
WD and ATM deficiencies independently associate with increased cardiac fibrosis (12, 20, 24, 29). Excessive extracellular matrix deposition causes cardiac scar formation and stiffness. Chronic high-fat diets associate with increased collagen-1 production and exacerbated cardiac fibrosis (30). Western blot analysis of heart lysates showed that the expression of collagen-1α1 is significantly higher in hKO-NC versus WT-NC group. WD increased collagen-1α1 expression in WT-WD versus WT-NC (Fig. 7A). However, no significant increase in collagen-1α1 expression was observed in hKO-WD versus hKO-NC. Matrix metalloproteinase (MMP)-2 and MMP-9 play a critical role in extracellular matrix turnover and remodeling (31). Protein levels of MMP-2 and MMP-9 were higher in hKO-NC versus WT-NC group. MMP-2 and MMP-9 protein levels remained unchanged in hKO-WD versus hKO-NC. Conversely, a significant increase in MMP-2 and MMP-9 protein levels was observed in WT-WD versus WT-NC (Fig. 7, B and C).
Figure 7.
Expression of collagen-1α1 (Col-1α1), MMP-2, and MMP-9 in the heart. Heart lysates were analyzed by Western blots using anti-Col-1α1 (A), MMP-2 (B), and MMP-9 (C) antibodies. Top panels exhibit immunostaining for Col-1α1, MMP-2, and MMP-9. Bottom panels exhibit quantitative analyses normalized to GAPDH. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, n = 6–9. hKO, heterozygous knockout; MMP, matrix metalloproteinase; NC, normal chow; WD, Western-type diet; WT, wild type.
Activation of Proteins Related to Apoptosis and Energy Metabolism
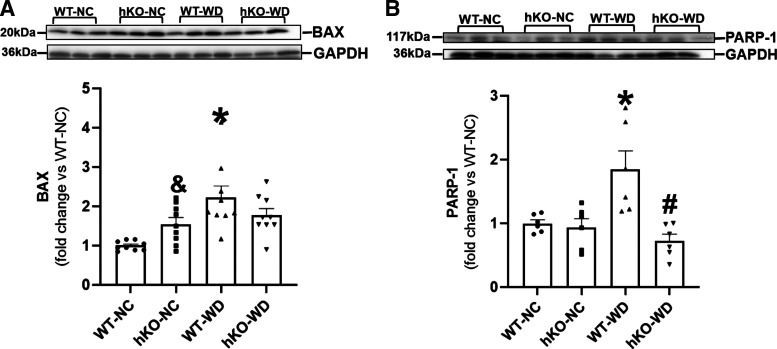
Bax is a pro-apoptotic protein that modulates mitochondrial membrane potential to increase the release of cytochrome C (24). Bax protein levels were higher in hKO-NC versus WT-NC. WD increased Bax expression in WT-WD versus WT-NC. However, no significant increase in Bax expression was observed in hKO-WD versus hKO-NC (Fig. 8A). Full-length PARP-1 (117 kDa) plays an important role in DNA repair (32). WD increased PARP-1 expression in WT group. Interestingly, no increase in PARP-1 protein levels was observed in hKO-WD versus hKO-sham, and PARP-1 protein levels were significantly lower in hKO-WD versus WT-WD (Fig. 8B). AMPK is a sensor for alterations in energy metabolism (33). AMPK activation was significantly higher in hKO-NC versus WT-NC. WD had no effect on AMPK activation in both genotypes versus their NC groups. However, AMPK activation remained higher in hKO-WD versus WT-WD (Fig. 9A). mTOR is a critical regulator of cell growth, metabolism, and cell survival. Dysregulation of mTOR signaling associates with obesity (34). mTOR activation was higher in hKO-NC versus WT-NC group. mTOR activation remained unchanged in WT-WD versus WT-NC. However, mTOR activation was significantly lower in hKO-WD versus hKO-NC (Fig. 9B). Activation of Akt, a master regulator of apoptosis and cell metabolism (35), was significantly higher in hKO-NC versus WT-NC group (Fig. 9C). Akt activation was significantly higher in WT-WD mice versus WT-NC, whereas Akt activation remained unchanged in hKO-WD versus hKO-NC. Akt activation upregulates Glut-4, which facilitates glucose uptake (16). Similar to Akt activation, Glut-4 protein levels were significantly higher in hKO-NC versus WT-NC. WD increased Glut-4 expression in WT group. Conversely, a decrease in Glut-4 expression was observed in hKO-WD versus hKO-NC. In addition, Glut-4 expression was significantly lower in hKO-WD versus WT-WD (Fig. 9D).
Figure 8.
Expression of Bax and PARP-1 in the heart. Heart lysates were analyzed by Western blots using anti-BAX (A) and PARP-1 (B) antibodies. Top panels exhibit immunostaining for BAX and PARP-1. Bottom panels exhibit quantitative analyses normalized to GAPDH. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, #P < 0.05 vs. WT-WD, n = 6–9. Western blot membrane used in Fig. 8B was also probed for total Akt in Fig. 9C. GAPDH in Fig. 8B was also used to normalize total Akt signal in Fig. 9C as the same membrane was probed for PARP-1, total Akt, and GAPDH. hKO, heterozygous knockout; NC, normal chow; WD, Western-type diet; WT, wild type.
Figure 9.
Phosphorylation of AMPK, mTOR, and Akt and expression of GLUT-4. Heart lysates were analyzed by Western blots using anti-p-AMPK and total AMPK (A), p-mTOR and total mTOR (B), p-Akt and total Akt (C), and Glut-4 (D) antibodies. Bar graphs exhibit quantitative analyses normalized to GAPDH. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, #P < 0.05 vs. WT-WD, $P < 0.05 vs. hKO-NC, n = 9 for phosphoproteins and Glut-4; n = 3 for total proteins. hKO, heterozygous knockout; NC, normal chow; WD, Western-type diet; WT, wild type.
Inflammatory Mediators
ATM deficiency is shown to associate with increased inflammation (36). When activated, IKK and NF-кB are primary regulators of the inflammatory response (37). Activation of NF-кB and IKK α/β (upstream regulator of NF-кB) was significantly higher in hKO-NC versus WT-NC. NF-кB activation remained unchanged in WT-WD versus WT-NC, whereas a significant decrease in NF-кB activation was observed in hKO-WD versus hKO-NC (Fig. 10A). WD induced a significant increase in IKK α/β activation in WT group (vs. WT-NC). However, IKK α/β activation remained unchanged in hKO-WD versus hKO-NC (Fig. 10B).
Figure 10.
Phosphorylation of NF-кB and IKK α/β. Heart lysates were analyzed by Western blots using anti-p-NF-кB and total NF-кB (A), and p-IKK α/β and total IKK α (B) antibodies. Bar graphs exhibit quantitative analyses normalized to GAPDH. &P < 0.05 vs. WT-NC, *P < 0.05 vs. WT-NC, $P < 0.05 vs. hKO-NC, n = 8–9 for phosphoproteins; n = 3 for total proteins. hKO, heterozygous knockout; NC, normal chow; WD, Western-type diet; WT, wild type.
Serum Cytokine/Chemokine Levels
Chronic inflammation commonly associates with prolonged high-fat diets and obesity (38, 39). Interestingly, patients with A-T exhibit elevated circulating concentrations of cytokines (40). Circulating levels of 23 cytokines/chemokines were not significantly different between the two NC groups and remained unchanged in WT-WD versus WT-NC. However, serum concentrations of IL-12(p70), MIP-1α, and MIP-1β were significantly higher in hKO-WD versus hKO-NC. In addition, serum concentrations of IL-12(p70), eotaxin, IFN-γ, MIP-1α, and MIP-1β were significantly higher in hKO-WD versus WT-WD (Table 3).
Table 3.
Serum cytokine/chemokine concentrations
| Cytokine/Chemokine | WT-NC | hKO-NC | WT-WD | hKO-WD |
|---|---|---|---|---|
| IL-1α | 6.1 ± 0.3 | 7.3 ± 1.4 | 6.1 ± 1.3 | 8.0 ± 1.1 |
| IL-1β | 5.3 ± 0.6 | 5.2 ± 0.2 | 5.6 ± 0.2 | 5.4 ± 0.7 |
| IL-2 | 1.8 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.1 | 2.4 ± 0.3 |
| IL-3 | 1.9 ± 0.0 | 3.0 ± 1.2 | 1.4 ± 0.3 | 3.3 ± 1.1 |
| IL-4 | 1.7 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.2 | 1.8 ± 0.3 |
| IL-5 | 4.8 ± 0.6 | 6.1 ± 1.8 | 4.5 ± 0.1 | 4.9 ± 0.2 |
| IL-6 | 2.7 ± 0.4 | 5.8 ± 4.1 | 3.3 ± 0.3 | 3.2 ± 0.2 |
| IL-9 | 11.6 ± 1.0 | 12.1 ± 0.7 | 11.2 ± 1.1 | 18.4 ± 3.0 |
| IL-10 | 23.5 ± 5.4 | 16.2 ± 1.2 | 19.5 ± 0.6 | 20.3 ± 2.0 |
| IL-12 (p40) | 174.5 ± 21.6 | 268.4 ± 71.8 | 147.1 ± 32.5 | 171.5 ± 35.1 |
| IL-12 (p70) | 107.9 ± 20.5 | 88.6 ± 6.5 | 81.0 ± 9.4 | 111.9 ± 4.3$,# |
| IL-13 | 48.1 ± 6.0 | 51.4 ± 2.4 | 85.4 ± 21.6 | 64.7 ± 6.3 |
| IL-17A | 42.7 ± 9.0 | 102.0 ± 50.0 | 23.4 ± 3.5 | 46.0 ± 14.0 |
| Eotaxin | 322.2 ± 51.5 | 369.4 ± 66.4 | 206.8 ± 14.7 | 318.7 ± 26.9# |
| G-CSF | 97.3 ± 25.2 | 119.0 ± 34.1 | 112.0 ± 52.3 | 85.3 ± 12.3 |
| GM-CSF | 27.5 ± 2.3 | 26.6 ± 0.7 | 27.2 ± 0.2 | 28.8 ± 1.4 |
| IFN-γ | 21.4 ± 2.6 | 20.9 ± 1.7 | 20.1 ± 1.7 | 33.5 ± 4.6# |
| KC | 19.3 ± 1.0 | 19.6 ± 1.2 | 25.9 ± 6.3 | 21.3 ± 1.9 |
| MCP-1 | 73.3 ± 5.5 | 79.1 ± 8.5 | 73.6 ± 3.5 | 76.5 ± 4.5 |
| MIP-1α | 2.3 ± 0.3 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.66 ± 0.1$,# |
| MIP-1β | 33.0 ± 2.2 | 33.8 ± 1.8 | 32.6 ± 2.6 | 44.8 ± 3.1$,# |
| RANTES | 35.0 ± 3.5 | 41.2 ± 5.7 | 59.7 ± 14.8 | 39.5 ± 5.0 |
| TNF-α | 44.2 ± 7.4 | 88.5 ± 53.7 | 35.5 ± 2.6 | 48.7 ± 7.0 |
Values are means ± SE (in pg/mL), n = 4–5. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; hKO, heterozygous knockout; IFN, interferon; MCP-1, monocyte chemotactic protein; MIP-1α, macrophage inflammatory protein, MIP-1β, macrophage inflammatory protein; NC, normal chow; RANTES, regulated upon activation, normal T cell expressed, and secreted; WD, Western-type diet; WT, wild type. $P< 0.05 vs. hKO-NC, #P< 0.05 vs. WT-WD.
DISCUSSION
Approximately 60%–70% of US population consumes WD (41, 42). A-T carriers, make up a substantial portion of the general population (∼1.4%–2.0%), are more susceptible to ischemic heart disease (16). Thus, ∼2–4 million A-T carriers consume WD. This study investigated the cardiac impact of WD during ATM deficiency using ATM-deficient mice. The main findings of the study are that ATM deficiency 1) associates with initial rapid weight gain; 2) induces systolic dysfunction with increased preload; 3) increases myocyte and cardiac cell apoptosis; 4) induces myocyte hypertrophy; 5) induces alterations in the expression and activation of signaling molecules associated with remodeling, metabolism, and inflammation; and 6) increases circulating levels of cytokines/chemokines.
ATM expression is shown to increase in response to a multitude of stimuli including genotoxic stress, cardiac injury, and oxidative stress (16). Our previous work has shown increased ATM expression in the heart following β-AR receptor stimulation (25). Increased ATM expression in the heart was also observed in the noninfarct and infarct LV regions of the heart 1 and 3 days post-MI (24). Here, ATM protein levels were ∼50% lower in the myocardium of ATM-deficient hearts when compared to their normal counterparts, confirming ATM deficiency in hKO group. WD increased ATM expression (by >2-fold) in the myocardium of WT, but not hKO mice. This WD-mediated increase in ATM expression in WT-WD heart may relate to changes in oxidative stress, inflammation, and/or sympathetic nerve activity (11, 43, 44).
Increased adiposity is often observed with WD feeding (12, 45). Lack of ATM is shown to decrease adipocyte maturation and diet-induced fat accumulation (46). Diet-induced body weight gain and fat accumulation in A-T carriers in a time-dependent manner has not yet been established. Using a mouse model, this study provides evidence that ATM deficiency associates with rapid weight gain in response to WD versus their normal counterparts. The increase in body weight gain became apparent at week 5 on WD and stayed higher until 10 wk on WD. Thereafter, body weight gain tended to be higher in ATM-deficient group; however, no significant differences in body weight gain were observed at weeks 11–14 between the two WD groups. At week 14 post-WD, there was no difference in fat (subcutaneous and visceral) accumulation, cardiac lipid deposition, and serum levels of cholesterol and triglycerides between the two WD groups. Based on the observation of initial rapid weight gain, it is speculated that ATM deficiency may initially augment adipocyte maturation leading to accelerated weight gain. However, persistent deficiency of ATM may interfere with adipocyte maturation and function. Obesity and insulin resistance independently associate with heart failure (6, 47). Interestingly, fasting blood glucose levels were significantly higher in WT-WD versus hKO-WD. This observation points toward the possibility that WT mice may have developed a level of insulin resistance 14 wk post-WD.
Increased fat accumulation and body mass index associate with adaptations in cardiovascular structure and function (5, 10, 12). The severity of cardiac dysfunction is often proportional to the duration of WD feeding and the degree of obesity (6, 10). A diet high in fat and sugar for 8 wk is shown to impair systolic and diastolic parameters of the heart in mice (48). Tei index is considered as an index of global heart function capable of estimating combined systolic and diastolic performance. Volume overload, reduction in arterial compliance, and ventricular diastolic stiffening are predicted to decrease Tei index, whereas increased afterload due to higher systemic resistance, reduction in end-systolic elastance, and impairment of LV relaxation are predicted to increase Tei index (49). Alterations in LV dimensions are another common consequence of adiposity (7, 50). Cardiac structural alterations can alter the filling, relaxation, and distensibility of the left ventricle (8, 51). Consequently, diastolic dysfunction is a common feature in patients with obesity, significantly contributes to the development of heart failure, and independently increases the risk of mortality (52). The degree of LV dysfunction is linked to the duration of obesity and associated changes in preload or afterload (53). Continual increase in systemic resistance can cause afterload dysfunction with alterations in Doppler parameters such as decreased E wave and AET, and prolongation of IVRT (49). Obesity-associated hyperdynamic circulation causes change in the filling and relaxation in preload (9). Consequently, as the heart continually compensates for changes in volume, preload and afterload dysfunction occurs (8, 9, 53). The data presented here demonstrate that WD induces systolic dysfunction as observed by decreased %FS and %EF, and increased LVESD and LVESV in WT mice. However, these parameters are equally affected by ATM deficiency. On the other hand, decreased E wave, AET, stroke volume, and cardiac output, and increased IVRT and Tei index were only observed in WT-WD, suggesting that WD induces systolic dysfunction with concomitant increase in afterload in WT mice. In contrast, AET was significantly higher, whereas IVCT, IVRT, and Tei index were significantly lower in hKO-WD when compared to the WT-WD group, suggesting that ATM deficiency induces systolic dysfunction with concomitant increase in preload in response to WD. It is interesting to note that the E wave is significantly smaller in hKO-NC versus WT-NC. WD did not significantly alter E wave in ATM-deficient hearts. However, it decreased IVCT and IVRT which may suggest increased cardiac contractility during ATM deficiency. Patients with A-T, with mutations in both alleles, exhibit severe dysfunction in autonomic nervous system (54). Therefore, increased contractility in hKO and preload during ATM deficiency in response to WD may relate to the changes in autonomic nervous system. Future investigations related to the measurement of arterial and LV blood pressures in a time-dependent manner are needed to confirm the afterload- and preload-associated changes in the heart in response to WD.
Consumption of high-fat diet associates with increased cardiac fibrosis, myocyte apoptosis, and myocardial dysfunction (5, 10, 27, 28). Afterload-associated dysfunction is suggested to correlate with increased fibrosis in the heart (55). Further, increased levels of toxic lipid byproducts can induce cardiac myocyte death due to apoptosis (10, 56). Compensative hypertrophy can also occur in obesity models due to volume overload (5, 10). Although hypertrophy is beneficial during the early stages of cardiac remodeling, it can lead to increased myocyte apoptosis and cardiac dysfunction (57). ATM deficiency independently associates with increased cardiac fibrosis and hypertrophy at basal levels and following β-AR stimulation and MI (20, 24, 29). ATM deficiency also associates with increased apoptosis in the infarct LV region 1 and 3 days post-MI (24). Here, we observed that WD increases myocardial fibrosis, and myocyte hypertrophy and apoptosis in both genotypes. However, the increase in myocyte hypertrophy and apoptosis was significantly greater in ATM-deficient hearts in response to WD when compared to their WT counterpoints. Akt plays a significant role in regulation of cardiac cell apoptosis and hypertrophy. Transgenic mice studies provide evidence that a short-term activation of Akt associates with modest cardiac growth, whereas a chronic activation of Akt associates with excessive hypertrophy (58). Therefore, increased activation of Akt in WT-WD (vs. WT-NC) may help explain the observed increase in hypertrophic response in this group. Conversely, Akt activation was higher in hKO-NC versus WT-NC with no significant change in Akt activation in response to WD in hKO-WD (Fig. 9C), suggesting that a chronic activation of Akt may play a role in exaggerated hypertrophic response in ATM-deficient hearts at basal levels and in response to WD.
Although ATM deficiency associated with increased basal fibrosis and collagen-1α1 expression, no significant increase in fibrosis or collagen-1α1 was observed in ATM-deficient hearts in response to WD. Thus, increased myocyte apoptosis and hypertrophy may help explain systolic dysfunction in the two WD groups, whereas increased fibrosis in WT-WD group (vs. WT-NC) may relate to increased afterload in WT-WD group. It was interesting to observe that increase in fibrosis in WT-WD group was associated with increased expression of MMP-2 and MMP-9. MMP-2 and MMP-9 play a significant role in myocardial remodeling process by affecting the structure as well as the function in response to cardiac injury and increased hemodynamic load (31). Generally, increased expression of MMPs is anticipated to associate with decreased fibrosis. However, deposition of fibrosis involves an intricate relationship between collagen synthesis, degradation, and deposition. This is shown by the observation that myocyte-specific expression of MMP-2 associates with hypertrophy, extensive fibrosis, and systolic dysfunction (59). In aging mice, deletion of MMP-9 attenuates myocardial fibrosis and diastolic dysfunction (60). Future investigations involving the analysis of other components of fibrosis may help understand the role of WD and ATM deficiency in myocardial fibrosis.
Bax, a pro-apoptotic protein, modulates mitochondrial membrane potential to increase the release of cytochrome C (24). Ablation of Bax plays a cardioprotective role in myocardial ischemia/reperfusion injury (61). Here, ATM-deficient heart exhibited enhanced basal apoptosis and Bax expression. WD augmented apoptosis and Bax expression in WT group, suggesting that increased Bax expression may play a role in increased apoptosis in WT-WD group. However, WD did not induce a significant increased Bax expression in hKO-WD, suggesting involvement of Bax-independent mechanism in this group. PARP-1 serves as a DNA damage sensor and critical DNA repair regulator. Apoptotic stimuli induce PARP-1 cleavage via the activation of caspases (32). WD increased protein levels of intact PARP-1 (117 kDa) only in WT group. However, there was no increase in intact PARP-1 expression in hKO-WD group. No increase in PARP-1 expression in hKO-WD suggests interference in DNA damage response and/or enhanced PARP-1 cleavage, leading to enhanced apoptosis in hKO-WD versus WT-WD. However, further investigations are needed to clarify the role of PARP-1 in ATM-mediated cardiac cell apoptosis.
Akt, AMPK, and mTOR are critical regulators of cell growth, metabolism, and survival and are commonly dysregulated in animal models of obesity (34, 62, 63). Acute activation of Akt associates with enhanced activation of Glut-4, whereas a chronic activation decreases Glut-4 expression in the heart (63). Diets high in fat decrease activation of AMPK and promote mTOR activation in the heart (62, 64, 65). Transgenic mice overexpressing a kinase dead isoform of AMPK exhibit exaggerated high-fat, diet-induced cardiac hypertrophy and contractile dysfunction with concurrent decrease in Akt activation and Glut-4 expression, and enhanced mTOR activation (65). Here, we observed greater activation of Akt, AMPK, and mTOR, and expression of Glut-4 in hKO-NC versus WT-NC. In our previous report using MI as a model, hKO-deficient sham animals did not exhibit activation of Akt or AMPK, whereas mTOR activation was lower (23). The reasons for these discrepant findings may include the age of the mice and/or performance of sham surgeries in the MI model. The age of mice in this study is ∼5 mo, whereas the previous study used mice aged ∼4 mo. In addition, the sham mice in MI model underwent surgical preparation and sham ligation of the coronary artery. The observed increase in Akt activation and Glut-4 in hKO-NC (vs. WT-NC) suggests the possibility of a metabolic shift toward glucose utilization in ATM-deficient hearts. WD significantly increased Akt activation and Glut-4 expression in WT group. In ATM-deficient hearts, WD had no effect on Akt activation, whereas Glut-4 expression was lower when compared to hKO-NC and WT-WD groups. These changes suggest that ATM-deficient hearts may be experiencing decreased insulin production/sensitivity and reduced metabolic flexibility. In response to WD, ATM-deficient hearts exhibited enhanced AMPK activation and decreased mTOR activation. Since AMPK deficiency exacerbates contractile function (65) and inhibition of mTOR (C1) improves ventricular function and reduces volume overload-induced hypertrophy (66), alterations in the activation of AMPK and mTOR may play a crucial role in genotype-specific differences in diastolic dysfunction in response to WD.
Approximately, two-thirds of patients with A-T have immunological abnormalities. This includes a reduction in the release of B and T cells, low levels of immunoglobulin, and poor antibody production (17, 67–69). Elevated circulating concentrations of cytokines have also been reported with patients with A-T (40). The inflammatory response is an essential component of host defense and tissue repair. Although acute inflammation is beneficial, chronic inflammation has been linked to cardiovascular disease (38). High-fat diet and obesity associate with chronic inflammation (38, 39). NF-кB and IKK are central inflammatory regulators, and increased NF-кB activation has been observed with high-fat diet (37, 70). Here, activation of NF-кB and IKK α/β was significantly higher in hKO-NC group versus WT-NC, suggesting that ATM deficiency associates with increased myocardial inflammation under basal conditions. WD induced a significant increase in IKK α/β activation in WT-WD versus WT-NC. Conversely, WD failed to activate IKK α/β in hKO-WD versus hKO-NC, while decreasing NF-кB activation. In addition, ATM deficiency is associated with increased circulating levels of inflammatory mediators such as IL-12(p70), eotaxin, IFN-γ, MIP-1α, and MIP-1β in response to WD. The increase in the pro-inflammatory IL-12(p70) has been observed during heart failure and has the potential to enhance production of IFN-γ in T cells (71, 72). An increase in IFN-γ is suggested to play a role in inflammation, insulin sensitivity, apoptosis, and cardiac remodeling (73–75). Furthermore, MIP-1α and MIP-1β are important chemotactic chemokines, which play a role in the inflammatory response (76). Increase in MIP-1β has also been linked to the development of adiposity, and IFN-γ can upregulate MIP-1α in the heart (73, 77). Eotaxin, an eosinophil-specific chemokine, is an important inflammatory mediator. Circulating levels of eotaxin are shown to be higher in patients with ischemic heart disease (78). In MI model, ATM deficiency is associated with delayed inflammatory response and decreased dilative remodeling in the heart 1 day post-MI (24). Collectively, these data suggest that ATM deficiency plays a critical role in modulation of inflammatory response. However, future investigations are warranted to clarify the mechanism by which ATM deficiency modulates WD-induced inflammation and heart function.
In summary, the data presented here provide evidence that WD induces systolic dysfunction with increased afterload in WT group as observed by an increase in Tei index, prolongation of IVRT, and decrease in AET, E wave, stroke volume, and cardiac output. On the other hand, ATM deficiency induces systolic dysfunction with increased preload as observed by decreased Tei index, IVCT, and IVRT. Further, ATM deficiency plays an important role in alterations in WD-induced fibrosis, apoptosis, hypertrophy, and metabolic and inflammatory signaling proteins. It should be emphasized that cardiac structural and functional changes may vary with the duration of WD feeding/obesity (53, 56). Here, all the observations, except body weights, were made 14 wk post-WD. WD feeding time of 14 wk was chosen based on the observations that WD-induced obesity in mice occurs within 12–16 wk (79–81). Additional time points are needed to elucidate the progression of cardiac dysfunction during ATM deficiency in response to WD. In addition, sex-specific differences are known to influence glucose and lipid metabolism, and cardiac energy metabolism and function (82). Therefore, complementary investigations should be performed using age-matched female mice.
GRANTS
This work was supported by Merit Review Awards I01BX004045 and I01BX002332 from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development; National Heart, Lung, and Blood Institute Grant R15HL141947; and funds from the Institutional Research and Improvement account (to K.S.) and C06RR0306551.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.W., M.S., and K.S. conceived and designed research; M.C.W., S.D., P.L.S., R.M., B.A.C., and D.P.T. performed experiments; M.C.W. and R.M. analyzed data; M.C.W. and K.S. interpreted results of experiments; M.C.W. prepared figures; M.C.W. drafted manuscript; M.C.W., S.D., P.L.S., B.A.C., D.P.T., M.S., and K.S. edited and revised manuscript; M.C.W., S.D., P.L.S., R.M., B.A.C., D.P.T., M.S., and K.S. approved final version of manuscript.
REFERENCES
- 1.Littlejohns B, Pasdois P, Duggan S, Bond AR, Heesom K, Jackson CL, Angelini GD, Halestrap AP, Suleiman M-S. Hearts from mice fed a non-obesogenic high-fat diet exhibit changes in their oxidative state, calcium and mitochondria in parallel with increased susceptibility to reperfusion injury. PLoS One 9: e100579, 2014. doi: 10.1371/journal.pone.0100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu J, Lloyd SG. High-fat, low-carbohydrate diet alters myocardial oxidative stress and impairs recovery of cardiac function after ischemia and reperfusion in obese rats. Nutr Res 33: 311–321, 2013. doi: 10.1016/j.nutres.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117, 2011. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: Epidemiology, pathophysiology, clinical manifestations, and management. Transl Res 164: 345–356, 2014. doi: 10.1016/j.trsl.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Bhatheja S, Panchal HB, Ventura H, Paul TK. Obesity cardiomyopathy: pathophysiologic factors and nosologic reevaluation. Am J Med Sci 352: 219–222, 2016. doi: 10.1016/j.amjms.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Ebong IA, Goff DC, Rodriguez CJ, Chen H, Bertoni AG. Mechanisms of heart failure in obesity. Obes Res Clin Pract 8: e540–e548, 2014. doi: 10.1016/j.orcp.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozakova M, Morizzo C, Bianchi V, Marchetti S, Federico G, Palombo C. Hemodynamic overload and intra-abdominal adiposity in obese children: relationships with cardiovascular structure and function. Nutr Metab Cardiovasc Dis 26: 60–66, 2016. doi: 10.1016/j.numecd.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Russo C, Jin Z, Homma S, Rundek T, Elkind MV, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol 57: 1368–1374, 2011. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasan RS. Cardiac function and obesity. Heart 89: 1127–1129, 2003. doi: 10.1136/heart.89.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 88: 389–419, 2008. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone S, Lavie CJ, Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc 92: 266–279, 2017. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Gonçalves N, Silva AF, Rodrigues PG, Correia E, Moura C, Eloy C, Roncon-Albuquerque R, Falcão-Pires I, Leite-Moreira AF. Early cardiac changes induced by a hypercaloric Western-type diet in subclinical obesity. Am J Physiol Hear Circ Physiol 310: 655–666, 2016. doi: 10.1152/ajpheart.00684.2015. [DOI] [PubMed] [Google Scholar]
- 13.Blignaut M, Loos B, Botchway SW, Parker AW, Huisamen B. Ataxia-telangiectasia mutated is located in cardiac mitochondria and impacts oxidative phosphorylation. Sci Rep 9: 4782, 2019. doi: 10.1038/s41598-019-41108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guleria A, Chandna S. ATM kinase: much more than a DNA damage responsive protein. DNA Repair (Amst) 39: 1–20, 2016. doi: 10.1016/j.dnarep.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Thrasher P, Singh M, Singh K. Ataxia-telangiectasia mutated kinase: role in myocardial remodeling. J Rare Dis Res Treat 2: 32–37, 2017. [PMC free article] [PubMed] [Google Scholar]
- 16.Wingard MC, Frasier CR, Singh M, Singh K. Heart failure and diabetes: role of ATM. Curr Opin Pharmacol 54: 27–35, 2020. doi: 10.1016/j.coph.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr 144: 505–516, 2004. doi: 10.1016/j.jpeds.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 18.Rothblum-Oviatt C, Wright J, Lefton-Greif MA, McGrath-Morrow SA, Crawford TO, Lederman HM. Ataxia telangiectasia: a review. Orphanet J Rare Dis 11: 159–170, 2016. doi: 10.1186/s13023-016-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 14: 197–210, 2013. doi: 10.1038/nrm3546. [DOI] [PubMed] [Google Scholar]
- 20.Daniel LL, Scofield SLC, Thrasher P, Dalal S, Daniels CR, Foster CR, Singh M, Singh K. Ataxia telangiectasia-mutated kinase deficiency exacerbates left ventricular dysfunction and remodeling late after myocardial infarction. Am J Physiol Heart Circ Physiol 311: H445–H452, 2016. doi: 10.1152/ajpheart.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalal S, Zha Q, Singh M, Singh K. Osteopontin-stimulated apoptosis in cardiac myocytes involves oxidative stress and mitochondrial death pathway: role of a pro-apoptotic protein. Mol Cell Biochem 418: 1–11, 2016. doi: 10.1007/s11010-016-2725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scofield SLC, Dalal S, Lim KA, Thrasher PR, Daniels CR, Peterson JM, Singh M, Singh K. Exogenous ubiquitin reduces inflammatory response and preserves myocardial function 3 days post-ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 316: 617–628, 2019. doi: 10.1152/ajpheart.00654.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thrasher PR, Scofield SLC, Dalal S, Crawford CC, Singh M, Singh K. Ataxia telangiectasia mutated kinase deficiency impairs the autophagic response early during myocardial infarction. Am J Physiol Heart Circ Physiol 315: H48–H57, 2018. doi: 10.1152/ajpheart.00042.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniel LL, Daniels CR, Harirforoosh S, Foster CR, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase delays inflammatory response in the heart following myocardial infarction. J Am Heart Assoc 3: e001286, 2014. doi: 10.1161/JAHA.114.001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster CR, Singh M, Subramanian V, Singh K. Ataxia telangiectasia mutated kinase plays a protective role in β-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Mol Cell Biochem 353: 13–22, 2011. doi: 10.1007/s11010-011-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin FCP, Spurgeon HA, Rakusan K. Use of tibial length to quantify cardiac hypertrophy: Application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. doi: 10.1152/ajpheart.1982.243.6.h941. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Tang R, Ouyang S, Ma F, Liu Z, Wu J. Folic acid prevents cardiac dysfunction and reduces myocardial fibrosis in a mouse model of high-fat diet-induced obesity. Nutr Metab (Lond) 14: 61, 2017. doi: 10.1186/s12986-017-0224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ternacle J, Wan F, Sawaki D, Surenaud M, Pini M, Mercedes R, Ernande L, Audureau E, Dubois-Rande JL, Adnot S, Hue S, Czibik G, Derumeaux G. Short-term high-fat diet compromises myocardial function: A radial strain rate imaging study. Eur Heart J Cardiovasc Imaging 18: 1283–1291, 2017. doi: 10.1093/ehjci/jew316. [DOI] [PubMed] [Google Scholar]
- 29.Foster CR, Zha Q, Daniel LL, Singh M, Singh K. Lack of ATM induces structural and functional changes in the heart: role in β-adrenergic receptor-stimulated apoptosis. Exp Physiol 97: 506–515, 2011. doi: 10.1113/expphysiol.2011.061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahraoui A, Dewachter C, De Medina G, Naeije R, Bouguerra SA, Dewachter L. Myocardial structural and biological anomalies induced by high fat diet in Psammomys obesus gerbils. PLoS One 11: e0148117, 2016. doi: 10.1371/journal.pone.0148117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87: 1285–1342, 2007. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Szabó C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev 25: 235–260, 2007. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Liu J, Lu Q, Ren D, Sun X, Rousselle T, Tan Y, Li J. AMPK: a therapeutic target of heart failure, not only metabolism regulation. Biosci Rep 39: BSR20181767, 2019. doi: 10.1042/BSR20181767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Z, Zhang W. Role of mTOR in glucose and lipid metabolism. Int J Mol Sci 19: 2043–2014, 2018. doi: 10.3390/ijms19072043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail 13: 825–829, 2011. doi: 10.1093/eurjhf/hfr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaki-Dizaji M, Akrami SM, Azizi G, Abolhassani H, Aghamohammadi A. Inflammation, a significant player of ataxia–telangiectasia pathogenesis? Inflamm Res 67: 559–570, 2018. doi: 10.1007/s00011-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 37.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther 2: 17023, 2017. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, Xu K. Inflammatory links between high fat diets and diseases. Front Immunol 9: 2649, 2018. doi: 10.3389/fimmu.2018.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, Mcardle HJ, Kremer BHA, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC, Sinclair H. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr 114: 999–1012, 2015. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harbort CJ, Soeiro-Pereira PV, Von Bernuth H, Kaindl AM, Costa-Carvalho BT, Condino-Neto A, Reichenbach J, Roesler J, Zychlinsky A, Amulic B. Neutrophil oxidative burst activates ATM to regulate cytokine production and apoptosis. Blood 126: 2842–2851, 2015. doi: 10.1182/blood-2015-05-645424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 288: 1–8, 2017. [PubMed] [Google Scholar]
- 42.Office of Disease Prevention and Health Promotion. Current Eating Patterns in the United States—2015-2020 Dietary Guidelines—health.gov (Online). Diet. Guidel. Chapter 2. https://health.gov/dietaryguidelines/2015/guidelines/chapter-2/current-eating-patterns-in-the-united-states/[2019 Aug 26].
- 43.Schwartz JH, Young JB, Landsberg L. Effect of dietary fat on sympathetic nervous system activity in the rat. J Clin Invest 72: 361–370, 1983. doi: 10.1172/JCI110976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients 11: 2579–2522, 2019. doi: 10.3390/nu11112579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang CY, Liao JK. A mouse model of diet-induced obesity and insulin resistance. Methods Mol Biol 821: 421–433, 2012. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takagi M, Ogawa Y, Correspondence SM. ATM regulates adipocyte differentiation and contributes to glucose homeostasis. Cell Reports 10: 957–967, 2015. doi: 10.1016/j.celrep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin 8: 609–617, 2012. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carbone S, Mauro AG, Mezzaroma E, Kraskauskas D, Marchetti C, Buzzetti R, Van Tassell BW, Abbate A, Toldo S. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol 198: 66–69, 2015. doi: 10.1016/j.ijcard.2015.06.136. [DOI] [PubMed] [Google Scholar]
- 49.Inuzuka R, Kuwata S, Kurishima C, Liang F, Sughimoto K, Senzaki H. Influence of cardiac function and loading conditions on the myocardial performance index - theoretical analysis based on a mathematical model. Circ J 80: 148–156, 2015. doi: 10.1253/circj.CJ-15-0598. [DOI] [PubMed] [Google Scholar]
- 50.Shiou YL, Huang IC, Lin HT, Lee HC. High fat diet aggravates atrial and ventricular remodeling of hypertensive heart disease in aging rats. J Formos Med Assoc 117: 621–631, 2018. doi: 10.1016/j.jfma.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Galderisi M. Diastolic dysfunction and diastolic heart failure: diagnostic, prognostic and therapeutic aspects. Cardiovasc Ultrasound 3: 9, 2005. doi: 10.1186/1476-7120-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kossaify A. Clinical medicine insights: cardiology original research impact of overweight and obesity on left ventricular diastolic function and value of tissue Doppler echocardiography. Clin Med Insights Cardiol 7: 43–50, 2013. doi: 10.4137/CMC.S11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis 56: 391–400, 2014. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Tubani L, Donato G, Perciaccante A, Baratta L, Fiorentini A, Fiorilli M. Autonomic dysfunction in patients with ataxia-telangiectasia. Clin Neurophysiol 117: 1630–1631, 2006. doi: 10.1016/j.clinph.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Toischer K, Rokita AG, UnsöLd B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuß L, Gupta SN, Schmidt K, Lehnart SE, KrüGer M, Linke WA, Backs J, Regitz-Zagrosek V, SchäFer K, Field LJ, Maier LS, Hasenfuss G. Differential cardiac remodeling in preload versus afterload. Circulation 122: 993–1033, 2010. doi: 10.1161/CIRCULATIONAHA.110.943431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wende AR, Dale Abel E. Lipotoxicity in the heart. Biochim Biophys Acta 1801: 311–319, 2010. doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.González A, Ravassa S, López B, Moreno MU, Beaumont J, San José G, Querejeta R, Bayés-Genís A, Díez J. Myocardial remodeling in hypertension toward a new view of hypertensive heart disease. Hypertension 72: 549–558, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11125. [DOI] [PubMed] [Google Scholar]
- 58.Walsh K. Akt signaling and growth of the heart. Circulation 113: 2032–2034, 2006. doi: 10.1161/CIRCULATIONAHA.106.615138. [DOI] [PubMed] [Google Scholar]
- 59.Bergman MR, Teerlink JR, Mahimkar R, Li L, Zhu BQ, Nguyen A, Dahi S, Karliner JS, Lovett DH. Cardiac matrix metalloproteinase-2 expression independently induces marked ventricular remodeling and systolic dysfunction. Am J Physiol Heart Circ Physiol 292: H1847–H1860, 2007. doi: 10.1152/ajpheart.00434.2006. [DOI] [PubMed] [Google Scholar]
- 60.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 96: 444–455, 2012. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoehhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol 284: H2351–H2359, 2003. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 62.Lyons CL, Roche HM. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int J Mol Sci 19: 3092–3017, 2018. doi: 10.3390/ijms19103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsui T, Nagoshi T, Hong E-G, Luptak I, Hartil K, Li L, Gorovits N, Charron MJ, Kim JK, Tian R, Rosenzweig A. Effects of chronic Akt activation on glucose uptake in the heart. Am J Physiol Metab 290: E789–E797, 2006. doi: 10.1152/ajpendo.00564.2004. [DOI] [PubMed] [Google Scholar]
- 64.Liang L, Shou X-L, Zhao H-K, Ren G-Q, Wang J-B, Wang X-H, Ai W-T, Maris JR, Hueckstaedt LK, Ma A-Q, Zhang Y. Antioxidant catalase rescues against high fat diet-induced cardiac dysfunction via an IKKβ-AMPK-dependent regulation of autophagy. Biochim Biophys Acta 1852: 343–352, 2015. doi: 10.1016/j.bbadis.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 65.Turdi S, Kandadi MR, Zhao J, Huff AF, Du M, Ren J. Deficiency in AMP-activated protein kinase exaggerates high fat diet-induced cardiac hypertrophy and contractile dysfunction. J Mol Cell Cardiol 50: 712–722, 2011. doi: 10.1016/j.yjmcc.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mtor signaling in the cardiovascular system. Circ Res 122: 489–505, 2018. doi: 10.1161/CIRCRESAHA.117.311147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kraus M, Lev A, Simon AJ, Levran I, Nissenkorn A, Levi YB, Berkun Y, Efrati O, Amariglio N, Rechavi G, Somech R. Disturbed B and T cell homeostasis and neogenesis in patients with ataxia telangiectasia. J Clin Immunol 34: 561–572, 2014. doi: 10.1007/s10875-014-0044-1. [DOI] [PubMed] [Google Scholar]
- 68.Vacchio MS, Olaru A, Livak F, Hodes RJ. ATM deficiency impairs thymocyte maturation because of defective resolution of T cell receptor α locus coding end breaks. Proc Natl Acad Sci USA 104: 6323–6328, 2007. doi: 10.1073/pnas.0611222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Warren R, Domm W, Yee M, Campbell A, Malone J, Wright T, Mayer-Pröschel M, O’Reilly MA. Ataxia-telangiectasia mutated is required for the development of protective immune memory after influenza A virus infection. Am J Physiol Lung Cell Mol Physiol 317: L591–L601, 2019. doi: 10.1152/ajplung.00031.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-κB signaling in reporter mice. Genes Nutr 4: 215–222, 2009. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mallat Z, Heymes C, Corbaz A, Logeart D, Alouani S, Cohen‐Solal A, Seidler T, Hasenfuss G, Chvatchko Y, Shah AM, Tedgui A. Evidence for altered interleukin (IL)‐18 pathway in human heart failure. FASEB J 18: 1752–1754, 2004. doi: 10.1096/fj.04-2426fje. [DOI] [PubMed] [Google Scholar]
- 72.Rottinghaus EK, Vesosky B, Turner J. Interleukin-12 is sufficient to promote antigen-independent interferon-β production by CD8 T cells in old mice. Immunology 128: e679, 2009. doi: 10.1111/j.1365-2567.2009.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levick SP, Goldspink PH. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev 19: 227–236, 2014. doi: 10.1007/s10741-013-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Rourke RW, White AE, Metcalf MD, Winters BR, Diggs BS, Zhu X, Marks DL. Systemic inflammation and insulin sensitivity in obese IFN-γ knockout mice. Metabolism 61: 1152–1161, 2012. doi: 10.1016/j.metabol.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sidles SJ, Xiong Y, Young MRI, La RA. High-fat diet alters immunogenic properties of circulating and adipose tissue-associated myeloid-derived CD45+DDR2+ cells. Mediators Inflamm 2019: 1648614, 2019. doi: 10.1155/2019/1648614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhavsar I, Miller CS, Al-Sabbagh M. Macrophage inflammatory protein-1 alpha (MIP-1 alpha)/CCL3: as a biomarker. In: General Methods in Biomarker Research and their Applications. Dordrecht, NL: Springer International Publishing, 2015, p. 223–249. [Google Scholar]
- 77.Surmi BK, Webb CD, Ristau AC, Hasty AH. Absence of macrophage inflammatory protein-1α does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. Am J Physiol Endocrinol Metab 299: E437–E445, 2010. doi: 10.1152/ajpendo.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Economou E, Tousoulis D, Katinioti A, Stefanadis C, Trikas A, Pitsavos C, Tentolouris C, Toutouza MG, Toutouzas P. Chemokines in patients with ischaemic heart disease and the effect of coronary angioplasty. Int J Cardiol 80: 55–60, 2001. doi: 10.1016/S0167-5273(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 79.Lang P, Hasselwander S, Li H, Xia N. Effects of different diets used in diet-induced obesity models on insulin resistance and vascular dysfunction in C57BL/6 mice. Sci Rep 9: 19556, 2019. doi: 10.1038/s41598-019-55987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roberts NW, González-Vega M, Berhanu TK, Mull A, García J, Heydemann A. Successful metabolic adaptations leading to the prevention of high fat diet-induced murine cardiac remodeling. Cardiovasc Diabetol 14: 127, 2015. doi: 10.1186/s12933-015-0286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zeng H, Vaka VR, He X, Booz GW, Chen J-X. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med 19: 1847–1856, 2015. doi: 10.1111/jcmm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat Med 25: 1657–1666, 2019. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]