Myocardial infarction (MI) remains the most common cause for heart failure worldwide (1). After the first week of birth, cardiomyocytes are unable to regenerate, and after ischemic injury, the heart undergoes fibrotic remodeling. In response to the ischemic event, immune cells, including macrophages, begin to remove the necrotic debris and stimulate deposition of an extracellular matrix (ECM)-rich scar (2). Recent transcriptomic analyses have suggested that with age, the transition from neonatal yolk sac macrophages to classical macrophages is stimulating adverse fibroblast-based cardiac remodeling after MI. Yolk sac-derived macrophages predominate in the neonate hearts and are more receptive to growth factors that affect cardiomyocyte regeneration. The molecular mechanisms that govern the change with age and drive fibrosis via inflammation are poorly understood.
In this issue of the American Journal of Physiology-Heart and Circulatory Physiology, Whitehead et al. (3) potentiated the cross talk mechanisms that occur between macrophages and cardiac fibroblasts and explore how this mechanism contributes to heart remodeling after MI injury using genomic data from multiple data sets. Through transcriptomic analysis of regenerative (P1) and nonregenerative (P8) mouse hearts before and after infarction, they found differences in expression of genes that control damage-associated molecular pattern (DAMP) signaling, ECM deposition, and chemokine profiles. A major strength of this study is the analysis of the many variables of cardiac injury and healing. Because these processes are multifactorial, it is important to account for the multiple affecting variables. Interestingly, the P1 and P8 hearts had similar expression profiles immediately after infarct, but the P1 hearts eventually returned to baseline whereas the P8 hearts formed a new homeostatic baseline. This led to the conclusion that signal transducer and activator of transcription 3 (STAT3) signaling occurs earlier in the regenerative (P1) hearts resulting in resolution of inflammation and an attenuation in leukocyte recruitment compared with the nonregenerating (P8) hearts.
Another critical difference between the early- and later-stage hearts lies in the gap junctions. Early-stage hearts express higher levels of connexin proteins, which is vital in the formation of gap junctions. When damage to the heart occurs in the P1 group, the intact gap junctions allow for DAMPs to diffuse through the cells more efficiently, leading to more degradation of the signals, a lower concentration gradient, and less inflammatory response overall. This overexpression of gap junctions also allows for more nutrient flow and communication between the cardiomyocytes, which could play a role in the heightened ability to regenerate in these hearts. Matrix metalloproteinases (MMPs) including MMP-7 increase after MI and have been shown to induce adverse electrical remodeling via cleavage of the connexin-43 COOH-terminal domain (4). Seeing that MMPs have also been linked to adverse remodeling with age (5, 6), it is possible that an age-induced increase in MMP activity may be facilitating in a loss of connexins and thus facilitating in DAMP signaling.
Whitehead et al. outlines two different macrophages that differ by their gene expression profiles and presence during specific stages of development. Yolk sac-derived macrophages are present in the P1 neonate hearts and play a role in the increased regenerative capacity seen early in development. Conversely, bone marrow-derived macrophages are present during later stages of development. The myocardium of neonates does not recruit a significant amount of bone marrow-derived macrophages after MI, which is a fundamental difference compared with that in the adult heart (7). This decrease in bone marrow-derived macrophages protects the neonate myocardium from prolonged inflammation and matrix remodeling seen in adult hearts. The signal that triggers this switch in the macrophage profile is still not known, but it is hypothesized that using the inherent protective effects of resident, embryonic-derived macrophages after MI may stimulate some aspects of cardiac repair.
Delving deeper into the specific cellular regulators, the authors analyzed genomic data from cardiac fibroblasts from regenerative (P1) hearts and found they were predominated by STAT3 and growth factor signaling, whereas cardiac fibroblasts from nonregenerative (P8) hearts were predominated by Toll-like receptor (TLR) and NF-κB signaling. This upregulation of TLR and NF-κB with age alone may lead to an increased inflammatory response during remodeling that could negatively affect the repair process. In addition, P8 cardiac fibroblasts expressed more leukocyte adhesion molecules, which may facilitate in leukocyte recruitment after MI. Similarly, Mouton et al. demonstrated an upregulation in genes that regulated T-cell activation/differentiation and macrophage chemotaxis in fibroblasts isolated from day 1 infarcts (8). Whether or not the switch from STAT3 to TLR and NF-κB signaling in cardiac fibroblasts is facilitating in the loss of protective effects with age after MI still needs to be evaluated.
Although the authors do a thorough job of answering the article’s scientific question, this study does have a few limitations. The first limitation of this study is the age of the hearts used for the analysis. The authors used P8 mouse hearts as a model for an aging heart. Although the cardiomyocytes of this age have been indicated to have less regenerative capacity than the P1 hearts, P8 hearts are still neonate and may not be the best to model an adult heart. Cardiac age plays a huge role in the ability of the heart to heal after damage, especially because of the reduced activity and efficiency of the immune system as the body ages. With this information in mind, it is therefore important to consider the effect of cardiac age when making conclusions about immune-related mechanisms of cardiac remodeling.
The article mainly focuses on the mechanisms of heart remodeling in regard to aging and damaged hearts, but an important factor to consider is the effect that sex can have on this process. The article neither mentions the sex of the mice in which the data came from nor how their sex could influence the conclusions of the study. Sex, specifically the presence or absence of estrogen, has been shown to have a high degree of influence on ECM remodeling after cardiac injury (9). As the heart ages or after cardiac damage, male hearts tend to become more hypertrophic when compared with women, indicating a possible role for sex hormones in the remodeling process (10). Regarding the conclusions made in this article, questions arise on the ability to translate these findings across the sexes. Sex hormones play a vital role in the regulation of gene expression within the body as a whole and have the capacity to influence the inflammatory response in regard to aging and injury, so analyzing the influence of these hormones on immune signaling post-MI is vital.
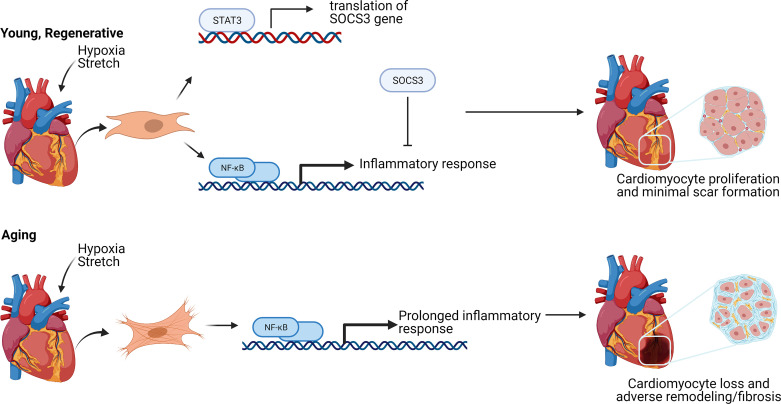
The study concludes induction of the NF-κB pathway is stronger in the nonregenerative hearts that creates a more robust inflammatory response postinfarction (Fig. 1). In regenerative hearts, acute induction of STAT3 limits NF-κB, reducing its effects. Although the study states that improper dosing of NF-κB could be fatal, further analysis of those detrimental effects as well as how to limit them could be a promising step toward a treatment option for cardiac damage. The study also concludes that pharmacological inhibition of TLR/tissue growth factor-β receptor (TGFβR)/hyaluronan-mediated motility receptor (RHAMM) ligands provides the most promise for clinical translation. Further study on the effects of inhibiting these ligands, particularly low-molecular-weight hyaluronic acid in postnatal and adult hearts, is still needed to test this conclusion.
Figure 1.
Overview of the NF-κB pathway in young and aging hearts after stress or damage. Both P1, regenerative hearts, and P8, nonregenerative hearts, express NF-κB immediately after myocardial infraction (MI). Neonate P1 hearts trigger the signal transducer and activator of transcription 3 (STAT3) pathway, resulting in the expression of suppressor of cytokine signaling 3 (SOCS3). SOCS3 inhibits the activity of NF-κB, stopping the inflammatory pathway prematurely and resulting in minimal scar formation. Aging hearts experience a prolonged NF-κB response leading to more fibrosis after damage. Created with BioRender.com and published with permission.
In summary, the study by Whitehead et al. (3) highlights the importance of age-specific changes in the cardiac macrophage and fibroblast populations. Understanding how these cell-specific mechanisms alter with age could provide possible therapeutics for cardiovascular disease.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant HL145817, American Heart Association Innovator Project IPA35260039, and Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award IK2BX003922.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHORS CONTRIBUTIONS
B.W. and K.Y.D.-P. prepared figures; B.W., D.B., and K.Y.D.-P. drafted manuscript; B.W., D.B., and K.Y.D.-P. edited and revised manuscript; B.W., D.B., and K.Y.D.-P approved final version of manuscript.
REFERENCES
- 1.Roger VL. Epidemiology of heart failure. Circ Res 113: 646–659, 2013. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML, Han HC. Building a better infarct: modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol 85: 229–239, 2015. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitehead AJ, Engler AJ. Regenerative cross talk between cardiac cells and macrophages. Am J Physiol Heart Circ Physiol 320, 2021. doi: 10.1152/ajpheart.00056.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113: 2919–2928, 2006. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 5.Bonnema DD, Webb CS, Pennington WR, Stroud RE, Leonardi AE, Clark LL, McClure CD, Finklea L, Spinale FG, Zile MR. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail 13: 530–540, 2007. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin Y-F, Lindsey ML. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 96: 444–455, 2012. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 111: 16029–16034, 2014. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouton AJ, Ma Y, Rivera Gonzalez OJ, Daseke MJ 2nd, Flynn ER, Freeman TC, Garrett MR, DeLeon-Pennell KY, Lindsey ML. Fibroblast polarization over the myocardial infarction time continuum shifts roles from inflammation to angiogenesis. Basic Res Cardiol 114: 6, 2019. doi: 10.1007/s00395-019-0715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piro M, Della Bona R, Abbate A, Biasucci LM, Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol 55: 1057–1065, 2010. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141, 1997. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]