Abstract
Adults with metabolic syndrome (MetS) have increased fasting arterial stiffness and altered central hemodynamics that contribute, partly, to increased cardiovascular disease (CVD) risk. Although insulin affects aortic wave reflections in healthy adults, the effects in individuals with MetS are unclear. We hypothesized that insulin stimulation would reduce measures of pressure waveforms and hemodynamics in people with MetS. Thirty-five adults with obesity (27 women; 54.2 ± 6.0 yr; 37.1 ± 4.8 kg/m2) were selected for MetS (ATP III criteria) following an overnight fast. Pulse wave analysis was assessed using applanation tonometry before and after a 2-h euglycemic-hyperinsulinemic clamp (90 mg/dL, 40 mU/m2/min). Deconvolution analysis was used to decompose the aortic waveform [augmentation index corrected to heart rate of 75 beats/min (AIx@75); augmentation pressure (AP)] into backward and forward pressure components. Aerobic fitness (V̇o2max), body composition (DXA), and blood biochemistries were also assessed. Insulin significantly reduced augmentation index (AIx@75, 28.0 ± 9.6 vs. 23.0 ± 9.9%, P < 0.01), augmentation pressure (14.8 ± 6.4 vs. 12.0 ± 5.7 mmHg, P < 0.01), pulse pressure amplification (1.26 ± 0.01 vs. 0.03 ± 0.01, P = 0.01), and inflammation [high-sensitivity C-reactive protein (hsCRP): P = 0.02; matrix metallopeptidase 7 (MMP-7): P = 0.03] compared to fasting. In subgroup analyses to understand HTN influence, there were no insulin stimulation differences on any outcome. V̇o2max, visceral fat, and blood potassium correlated with fasting AIx@75 (r = −0.39, P = 0.02; r = 0.41, P = 0.03; r = −0.53, P = 0.002). Potassium levels were also associated with insulin-mediated reductions in AP (r = 0.52, P = 0.002). Our results suggest insulin stimulation improves indices of aortic reflection in adults with MetS.
NEW & NOTEWORTHY This study is one of the first to investigate the effects of insulin on central and peripheral hemodynamics in adults with metabolic syndrome. We provide evidence that insulin infusion reduces aortic wave reflection, potentially through a reduction in inflammation and/or via a potassium-mediated vascular response.
Keywords: arterial compliance, augmentation index, insulin stimulation, metabolic syndrome
INTRODUCTION
Approximately 34% of adults in the United States have metabolic syndrome (MetS) (1), which is a cluster of risk factors that include increased blood pressure, glucose, triglycerides, waist circumference, as well as low HDL cholesterol. Having at least three of five of these MetS criteria places people at high risk for developing type 2 diabetes and cardiovascular disease (2). Insulin plays a key role in not only maintaining blood glucose levels but also in promoting arterial compliance (3). Arterial compliance of blood vessels is an important mechanical property that contributes to regulation of blood pressure, flow, and hemodynamic load on the heart (3, 4). While pulse-wave velocity is considered the gold standard for noninvasive assessment of arterial stiffness (5), augmentation index has been considered an indirect surrogacy measure, although it more specifically is a measure of aortic pulse waveforms using oscillometric brachial pressure waveforms (6, 7). In fact, the augmentation index is determined from components of the pressure waveform, including the maximum systolic pressure minus pressure at the inflection end point and divided by total pulse pressure (8). In addition to this pulse wave analysis (9), separation analysis of the backward and forward components of the aortic pressure waveform is essential since these velocity waveforms relate to changes in aortic pressure and load as well as peripheral arterial compliance (3, 10). Based on previous research (11, 12), there is reason to believe insulin may impact central and peripheral hemodynamics differently, but few data exist.
Inflammation is considered an important mediator of arterial hemodynamics (5). For instance, C-reactive protein (CRP) is associated with MetS and CVD, in part, by decreasing arterial compliance and vasodilation (13, 14). Matrix metallopeptidase 1 (MMP-1) and matrix metallopeptidase 7 (MMP-7) are also implicated in remodeling of the endothelial cell membrane via vascular wall thickness and elastin breakdown (13). Interestingly, aerobic fitness as determined by maximal oxygen consumption (V̇o2max) relates to low inflammation independent of body weight, thereby conferring protection of arterial stiffness (14, 15). However, the influence of adiposity, inflammation, and/or fitness on insulin-mediated changes in aortic waveforms and central hemodynamics is unclear. Despite insulin acutely lowering augmentation index and pulse wave velocity in healthy individuals (16), there is reduced endothelial responsiveness in some (16, 17) but not all studies (18, 19) of adults with obesity, MetS, type 1 diabetes, and type 2 diabetes. It is worth noting that some people were on antihypertensive and/or glucose lowering medication in these insulin infusion studies, and this could have masked and/or enhanced responses to insulin (9, 18, 20). Further, in the only study examining adults with MetS (17), there were no measures of central pressure or wave reflection to understand how insulin modifies reflective pressure waves of small muscular arteries to influence central pressures on the heart (21). Because insulin is a key hormone regulating cardiometabolic health as well as the target of several diabetes-related drug therapies, we sought to primarily examine the role of insulin on augmentation index and central hemodynamics in people with MetS. We secondarily explored the role of HTN classification (on or off medication) in subgroup analysis to gain insight toward insulin-stimulation in MetS HTN phenotypes. We hypothesized that insulin would favorably affect indices of aortic wave reflection and central hemodynamics, and these changes would corelate with inflammation and fitness.
METHODS
Subjects
Thirty-five adults (n = 27 women; 54.2 ± 6.0 yr; 37.1 ± 4.8 kg/m2) with MetS were recruited for this cross-sectional study via social media and/or newspaper flyers from the Charlottesville, VA community. Subjects underwent medical examination that included a resting ECG as well as urine and blood chemistry analysis. Subjects were excluded if smoking, physically active (exercise >60 min/wk), weight unstable (>2 kg last 3 mo), or diagnosed with the following disease states: type 2 diabetes, cardio-pulmonary dysfunction, renal, hepatic, immune dysfunction, and/or cancer. Subjects were also excluded if taking medications considered to impact insulin sensitivity (e.g., metformin, SGLT-2 inhibitors, fish oils, and other dietary supplements). Participants were classified as having at least three of five MetS ATP III criteria (22). Subgroup analyses were then performed, and people were classified as: 1) normotensive (NT), 2) hypertensive (HTN), or 3) on antihypertensive medication (HTN + meds). At the screening visit, average blood pressure for the subgroups was defined (NT: 116.8 ± 2.3/71.5 ± 2.8; HTN: 138.5 ± 1.8/80.3 ± 2.2; HTN + meds: 128.5 ± 4.6/74.1 ± 2.0 mmHg). All subjects provided written and verbal informed consent as approved by the University of Virginia Institutional Review Board.
Metabolic Syndrome Characterization
After an overnight fast, blood was drawn to assess fasting blood glucose (FG; ≥100 mg/dL), high-density lipoprotein (HDL; men: <40 mg/dL; women: <50 mg/dL), and triglycerides (≥150 mg/dL). Waist circumference (WC; men: ≥102; women: ≥88 cm) was measured up to 3 times using a plastic tape measure ∼2 cm above the umbilicus and averaged. Blood pressure (systolic ≥130 mmHg and/or diastolic ≥85 mmHg) was recorded after 5 min of rest in a seated position with feat positioned flat on the floor. Blood pressure, including heart rate and pulse pressure, were obtained a total of three times with 1–2 min rest in between at each visit and the average was recorded. Individuals on statins (NT = 1 and HTN + meds = 1) were considered to have elevated lipids, whereas those on angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and/or diuretics were considered to have HTN, respectively. An ATP III score was calculated from the sum of risk factors and a sex-specific MetS severity Z-score was calculated, as reported previously (23, 24) as: Z-scoreMen = [(40 − HDL)/8.9] + [(TGs − 150)/69.0] + [(FG − 100)/17.8] + [(WC − 102)/11.5] + [(MAP − 100)/10.1], and Z-scoreWomen = [(50 − HDL)/14.5] + [(TG − 150)/69.0] + [(FG − 100)/17.8] + [(WC − 88)/12.1] + [(MAP − 100)/10.1] (23). Mean arterial pressure (MAP) was calculated as MAP = [2/3*diastolic blood pressure (DBP)] + [1/3*systolic blood pressure (SBP)].
Body Composition and Aerobic Fitness
Body mass was measured on a digital scale and height was recorded without shoes using a wall-mounted stadiometer (Hamburg, Germany) to determine body mass index (BMI). Total fat mass and fat-free mass were measured by dual-energy X-ray absorptiometry (DXA; Horizon DXA System; Hologic, Marlborough, MA). Visceral adipose tissue (VAT) mass was estimated from DXA, and subcutaneous fat (SubQ) was obtained by subtracting VAT from android fat. V̇o2max was assessed using a modified Astrand incremental treadmill protocol with indirect calorimetry (Carefusion, Vmax Encore, Yorba Linda, CA). Individuals needed to have at least three of the four criteria to be considered at V̇o2max: 1) ≥1.1 respiratory exchange ratio, 2) within 10 beats of estimated heart rate max, 3) V̇o2 change ≤150 mL/min, and/or 4) volitional fatigue (≥17 rating of perceived exertion).
Metabolic Control
Subjects were instructed to refrain from alcohol, caffeine, medications, and strenuous physical activity for 24 h before the study visit. Food was provided to standardize diet via resting metabolic rate from indirect calorimetry and a physical activity factor of 1.2. A low-fat American Heart Association (AHA) diet was used and consisted of 55% carbohydrates, 15% protein, and 30% fat, with <10% from saturated fat.
Euglycemic-Hyperinsulinemic Clamp
After an approximate 10-h overnight fast, subjects reported to the Clinical Research Unit. Intravenous catheters were placed in the antecubital and hand veins for infusion and blood collection, respectively. A primed (250 mU/m/min) constant infusion (40 mU/m/min) of human recombinant insulin was administered via peristaltic infusion pumps (Harvard Apparatus Pump 22; Harvard Apparatus, Holliston, MA) for 120 min and a variable infusion of glucose was provided to maintain a plasma glucose of 90 mg/dL. Plasma glucose was collected every 5 min to determine the appropriate glucose infusion rate. Inflammatory markers [high-sensitivity (hs)-CRP, MMP-1, and MMP-7] were measured at 0 and 120 min, and plasma lactate (estimation of nonoxidative glucose metabolism) and insulin were determined at 0, 90, 105, and 120 min. Peripheral insulin sensitivity was defined as the mean glucose metabolized infusion rate during 90–120 min (M-value) divided by ambient insulin concentrations.
Pulse Waveform Analysis
Aortic waveform and hemodynamic measurements were recorded at 0 and 120 min of the euglycemic hyperinsulinemic clamp using applanation tonometry by way of the SphygmoCor XCEL system (AtCor Medical, Itasca, IL) to determine vascular responses at fasting and during steady state insulin conditions. All measurements occurred while participants were resting semisupine in a temperature-controlled room. A blood pressure cuff was placed on the upper left arm and measurements were averaged from three trials over 10 min. Augmentation index corrected for heart rate of 75 beats/min (AIx@75), augmentation pressure (AP), brachial systolic (bSBP) and diastolic blood pressure (bDBP), central systolic (cSBP) and diastolic blood pressure (cDBP), brachial and central pulse pressure (bPP and cPP), and brachial and central mean arterial pressure (bMAP and cMAP) were measured. Central forward pressure (Pf), backward pressure (Pb), and reflection magnitude were also characterized through deconvolution analysis. Pulse pressure amplification (PPA) was calculated as a ratio (brachial PP/central PP).
Biochemical Analysis
Fasting blood was sent to our medical laboratory for analysis of potassium, sodium, white blood cells, as well as high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), and triglycerides (TG). Plasma glucose obtained during the clamp was analyzed by a glucose oxidase assay (YSI Instruments 2700, Yellow Springs, OH), whereas plasma lactate was analyzed by a lactate oxidase assay (YSI Instruments 2700, Yellow Springs, OH). Other samples were centrifuged at 4°C for 10 min at 1,500 g and stored at −80°C until later analysis. Insulin was assessed by radioimmunoassay and hs-CRP was determined by chemiluminescent assay (Millipore, Billerica, MA). MMP-1 and MMP-7 were tested by ELISA (R&D Systems, INC, Minneapolis, MN).
Statistical Analysis
Data were analyzed using SPSS (IBM, V. 25.0). Nonnormally distributed data determined by Shapiro–Wilk test were log-transformed for analysis to minimize heterogeneity. Paired t tests were used to understand the effect of insulin compared to fasting on arterial function in the whole group. For exploratory analysis, one-way ANOVAs were utilized to understand fasting and insulin-stimulated (i.e., delta; clamp minus fasting) on outcomes across HTN subgroups. A pairwise comparison was performed if a main effect was identified from the ANOVA. Fasting outcomes were used as a covariate when statistically significant groups effects were noted to understand independent insulin-stimulated effects. Due to technical issues, some blood samples were not able to be analyzed. Pearson correlations were used to determine relationships between outcomes. Significance was accepted as P ≤ 0.05, and data are expressed as mean ± standard error of the mean (SE).
RESULTS
Subject Characteristics
Overall, participants had an average ATP III score of 3.54 ± 0.11 and MetS Z-score of 3.17 ± 0.33 (Table 1). In addition, individuals had low cardiorespiratory fitness (V̇o2max: 22.5 ± 0.7 mL/kg/min) and obesity (body fat: 43.1 ± 0.9%, Table 1). There were no differences in anthropometrics, fitness, blood lipids or white blood cells among HTN subgroups (Table 1). However, HTN + meds presented with higher ATP III criteria and MetS z-scores than NT (both P < 0.05; Table 1).
Table 1.
Anthropometric and clinical labs
| Whole Group | NT | HTN | HTN + Meds | Subgroup P Value | |
|---|---|---|---|---|---|
| Subjects, n (women/men) | 35 (27/8) | 12 (10/2) | 12 (9/3) | 11 (8/3) | — |
| Postmenopausal, n | 15 | 7 | 5 | 3 | — |
| Age, yr | 54.2 ± 1.0 | 53.7 ± 1.7 | 53.4 ± 1.1 | 55.6 ± 2.2 | 0.66 |
| Metabolic Z-score | 3.17 ± 0.3 | 1.98 ± 0.4 | 3.21 ± 0.4 | 4.44 ± 0.6† | 0.009 |
| ATP III score | 3.54 ± 0.1 | 3.25 ± 0.1 | 3.33 ± 0.1† | 4.09 ± 0.2† | 0.004 |
| Body composition | |||||
| Weight, kg | 106 ± 2.8 | 100 ± 4.5 | 108 ± 3.4 | 112 ± 6.2 | 0.28 |
| BMI, kg/m2 | 37.1 ± 0.8 | 35.8 ± 1.1 | 37.2 ± 1.3 | 38.2 ± 1.6 | 0.45 |
| Total body fat, % | 43.1 ± 0.9 | 42.4 ± 1.1 | 43.2 ± 1.7 | 43.5 ± 2.1 | 0.89 |
| Lean mass, kg | 57.0 ± 1.8 | 54.2 ± 3.3 | 55.7 ± 1.2 | 60.6 ± 3.5 | 0.35 |
| VAT volume, cm2 | 194 ± 10.2 | 175 ± 9.7 | 210 ± 20.4 | 198 ± 20.2 | 0.41 |
| Aerobic fitness | |||||
| V̇o2max, mL/kg/min | 22.5 ± 0.7 | 24.0 ± 1.2 | 22.3 ± 1.1 | 21.0 ± 1.1 | 0.25 |
| Clinical labs | |||||
| HbA1C, % | 5.63 ± 0.07 | 5.58 ± 0.08 | 5.69 ± 0.17 | 5.62 ± 0.11 | 0.85 |
| TC, mg/dL | 204 ± 5.6 | 200 ± 12.0 | 202 ± 7.3 | 210 ± 8.9 | 0.74 |
| HDL cholesterol, mg/dL | 44.7 ± 1.5 | 47.3 ± 3.1 | 44.0 ± 2.1 | 42.4 ± 2.0 | 0.43 |
| LDL cholesterol, mg/dL | 133 ± 4.4 | 130 ± 8.8 | 127 ± 6.3 | 142 ± 6.5 | 0.41 |
| Triglycerides, mg/dL* | 154 ± 12.4 | 136 ± 16.7 | 168 ± 28.1 | 158 ± 15.2 | 0.50 |
| Potassium, mmol/L | 4.05 ± 0.05 | 4.15 ± 0.07 | 3.87 ± 0.06 | 4.10 ± 0.11 | 0.11 |
| Sodium, mmol/L | 139 ± 0.3 | 140 ± 0.6 | 139 ± 0.4 | 138 ± 0.4 | 0.055 |
| WBC, L | 6.43 ± 0.2 | 6.52 ± 0.3 | 6.81 ± 10.3 | 5.93 ± 0.3 | 0.21 |
| Hematocrit, %* | 41.1 ± 0.8 | 42.2 ± 0.7 | 39.5 ± 2.1 | 41.4 ± 1.3 | 0.41 |
| Lymphocytes, % | 28.4 ± 1.1 | 30.3 ± 1.4 | 26.7 ± 2.0 | 28.1 ± 1.9 | 0.41 |
| Monocytes, % | 7.51 ± 0.3 | 7.85 ± 0.4 | 7.17 ± 0.6 | 7.49 ± 0.5 | 0.72 |
| Eosinophil, % | 2.74 ± 0.2 | 2.51 ± 0.2 | 2.68 ± 0.2 | 3.03 ± 0.5 | 0.65 |
| Basophils, %* | 0.71 ± 0.04 | 0.70 ± 0.08 | 0.72 ± 0.07 | 0.70 ± 0.07 | 0.96 |
| Neutrophils, %* | 60.6 ± 1.2 | 58.5 ± 1.4 | 62.6 ± 2.4 | 60.6 ± 2.0 | 0.43 |
Values are means ± SE; n, number of subjects. BMI, body mass index; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; HTN, hypertensive; HTN + Meds, on antihypertensive medication; LDL, low-density lipoprotein; NT, normotensive; VAT, visceral adipose tissue volume; V̇o2max, peak oxygen consumption; WBC, white blood cells.
*Data were log transformed for statistical analysis. †P < 0.05, significant difference from NT.
Insulin Sensitivity and Inflammation
Participants had an average peripheral insulin sensitivity value of 0.028 ± 0.0, and there was no difference among HTN subgroups (P = 0.72, Table 2). Fasting glucose was similar among subgroups (P = 0.79) and was decreased to ∼90 mg/dL by design (Table 2). Although HTN status did not differentially impact fasting (P = 0.46) or steady-state insulin concentrations during the clamp (P = 0.47), NT individuals had a greater increase in insulin concentrations from 0 min to 120 min compared to HTN and HTN + meds (P = 0.01, Table 2). There were no differences in fasting values of lactate (P = 0.41), hsCRP (P = 0.47), MMP-1 (P = 0.68), or MMP-7 (P = 0.13, Table 2) among subgroups. Although insulin administration comparably increased lactate (n = 30; 0.73 ± 0.0 vs. 0.82 ± 0.0 mM, P < 0.05), there was a reduction in circulating hsCRP (n = 30; 7.29 ± 0.9 vs. 6.74 ± 0.9 mg/L, P = 0.02) and MMP-7 (n = 26; 3.25 ± 0.2 vs. 3.14 ± 0.2 mg/L, P = 0.03). However, there was no change in MMP-1 (n = 31; 4.78 ± 0.6 vs. 5.52 ± 0.6 ng/mL, P = 0.22).
Table 2.
Insulin sensitivity and inflammation
| Whole Group | NT | HTN | HTN + Meds | Subgroup P Value | |
|---|---|---|---|---|---|
| Glucose metabolism | |||||
| GIR (90–120 min), mg/kg/min | 2.29 ± 0.2 | 2.36 ± 0.4 | 2.21 ± 0.2 | 2.31 ± 0.4 | 0.96 |
| M value (90–120 min) | 2.3 ± 0.2 | 2.5 ± 0.3 | 2.3 ± 0.2 | 2.3 ± 0.4 | 0.90 |
| Insulin sensitivity (90–120 min)* | 0.028 ± 0.0 | 0.024 ± 0.0 | 0.027 ± 0.0 | 0.034 ± 0.0 | 0.72 |
| Glucose, mg/dL | |||||
| 0 min | 105.8 ± 2.2 | 106.1 ± 4.0 | 103.7 ± 2.7 | 107.7 ± 4.6 | 0.79 |
| Δ | −11.6 ± 2.9 | −11.4 ± 6.7 | −9.95 ± 2.8 | −13.8 ± 5.0 | 0.82 |
| Glucose steady-state (90–120 min), mg/dL | 89.2 ± 4.0 | 88.4 ± 3.8 | 90.5 ± 4.0 | 88.7 ± 3.7 | 0.40 |
| Insulin, µU/mL | |||||
| 0 min | 20.9 ± 2.1 | 18.0 ± 2.2 | 24.9 ± 4.6 | 21.3 ± 4.5 | 0.46 |
| Δ | 71.2 ± 5.1^ | 88.7 ± 6.5 | 56.2 ± 9.8† | 60.8 ± 6.3† | 0.01 |
| Insulin steady state (90–120 min), µU/mL | 92.1 ± 4.0^ | 103 ± 3.7 | 85.4 ± 8.2 | 83.2 ± 7.8 | 0.07 |
| Lactate, mmol/L* | |||||
| 0 min | 0.73 ± 0.0 | 0.69 ± 0.0 | 0.70 ± 0.0 | 0.79 ± 0.0 | 0.41 |
| Δ | 0.09 ± 0.0^ | 0.11 ± 0.0 | 0.11 ± 0.0 | 0.05 ± 0.0 | 0.62 |
| Lactate steady state (90–120 min), mmol/L* | 0.83 ± 0.0 | 0.84 ± 0.0 | 0.75 ± 0.0 | 0.89 ± 0.0 | 0.20 |
| Inflammation | |||||
| hsCRP, mg/L | |||||
| 0 min | 7.2 ± 0.9 | 6.7 ± 1.5 | 7.4 ± 2.1 | 7.7 ± 1.1 | 0.47 |
| Δ | −0.34 ± 0.1^ | −0.53 ± 0.2 | −0.50 ± 0.2 | −0.01 ± 0.3 | 0.23 |
| MMP-1, ng/mL* | |||||
| 0 min | 4.7 ± 0.6 | 5.5 ± 1.0 | 3.4 ± 0.5 | 5.2 ± 1.3 | 0.68 |
| Δ | 0.58 ± 0.2 | 0.62 ± 0.5 | 0.92 ± 0.5 | 0.22 ± 0.2 | 0.81 |
| MMP-7, ng/mL | |||||
| 0 min | 3.3 ± 0.2 | 2.7 ± 0.3 | 3.2 ± 0.3 | 4.0 ± 1.4 | 0.13 |
| Δ | −0.04 ± 0.2^ | 0.55 ± 0.5 | −0.50 ± 0.1 | −0.20 ± 0.0 | 0.13 |
Values are means ± SE. GIR, glucose infusion rate over 90–120 min; hsCRP, high-sensitivity C-reactive protein; HTN, hypertensive; HTN + Meds, on antihypertensive medication; insulin sensitivity, glucose metabolized over 90–120 min divided by ambient insulin; M value, glucose metabolized over 90–120 min; MMP, matrix metalloproteinase; NT, normotensive. One-way ANOVA was used to understand HTN influence across subgroups. A pairwise comparison was performed if a significant main effect was identified from the ANOVA.
*Raw values are presented, but data were log transformed for statistical analysis. ^P < 0.05, compared with 0 min. †P < 0.05, significant difference from NT.
Pulse Waveform Analysis and Central Hemodynamics
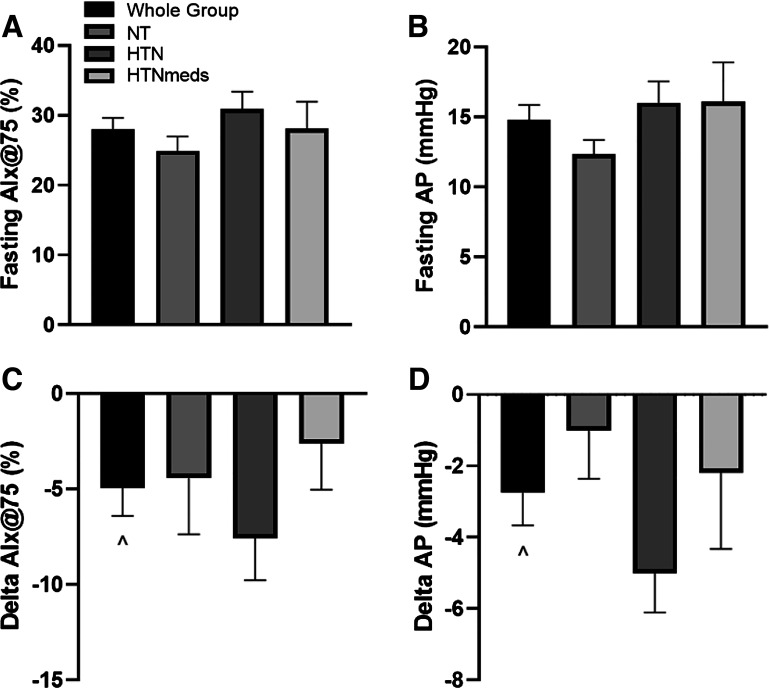
HTN had higher fasting measurements of bSBP (P < 0.05), bDBP (P < 0.05), HR (P < 0.05), cSBP (P < 0.05), cDBP (P < 0.05), and cMAP (P < 0.05) compared to both NT and HTN + meds as well as higher fasting cPP (P < 0.05) and Pf (P < 0.05) compared to NT. Insulin infusion did not alter central or brachial blood pressure measures, HR, PP, MAP, Pf, or reflection magnitude (Table 3). However, insulin reduced AIx@75 (P = 0.002, Fig. 1), AP (P = 0.002, Fig. 1), PPA (P = 0.01, Table 3), and Pb (P = 0.02, Table 3), independent of hypertension status.
Table 3.
Effect of insulin on peripheral and central hemodynamics
| Whole Group | NT | HTN | HTN + Meds | Subgroup P Value | |
|---|---|---|---|---|---|
| Peripheral | |||||
| bSBP, mmHg* | |||||
| 0 min | 136 ± 2.7 | 129 ± 2.7 | 149 ± 4.8† | 129 ± 3.6‡ | 0.001 |
| Δ | −2.0 ± 1.9 | 0.83 ± 1.8 | −8.9 ± 3.5 | 2.1 ± 3.2 | 0.86 |
| bDBP, mmHg | |||||
| 0 min | 80.9 ± 1.5 | 79.0 ± 1.7 | 88.5 ± 2.5† | 74.6 ± 2.2‡ | 0.001 |
| Δ | −0.91 ± 1.0 | −2.0 ± 1.1 | −3.4 ± 1.4 | 3.0 ± 2.3 | 0.31 |
| bPP* | |||||
| 0 min | 54.6 ± 1.7 | 49.5 ± 1.7 | 60.1 ± 3.2 | 54.1 ± 2.8 | 0.03 |
| Δ | −1.17 ± 1.4 | 2.83 ± 2.0 | −5.5 ± 2.6 | −0.81 ± 2.1 | 0.39 |
| bMAP* | |||||
| 0 min | 99.1 ± 1.8 | 95.6 ± 1.9 | 109 ± 3.1† | 92.6 ± 2.4‡ | <0.01 |
| Δ | −1.30 ± 1.2 | −1.05 ± 1.0 | −5.25 ± 2.0 | 2.72 ± 2.4 | 0.51 |
| Central | |||||
| HR, beats/min | |||||
| 0 min | 63.1 ± 1.5 | 61.4 ± 2.6 | 69.7 ± 1.9† | 57.5 ± 2.0‡ | <0.01 |
| Δ | 1.97 ± 1.1 | −0.45 ± 1.7 | 1.3 ± 1.7 | 5.0 ± 2.3 | 0.10 |
| cSBP, mmHg* | |||||
| 0 min | 125 ± 2.4 | 120 ± 2.4 | 137 ± 4.2† | 119 ± 3.4‡ | 0.001 |
| Δ | −3.28 ± 1.6 | −0.33 ± 1.5 | −10.0 ± 2.9 | 0.90 ± 2.9 | 0.64 |
| cDBP, mmHg* | |||||
| 0 min | 80.6 ± 2.1 | 80.4 ± 1.7 | 89.5 ± 2.6† | 71.0 ± 4.3‡ | <0.01 |
| Δ | 0.65 ± 1.6 | −2.3 ± 1.1 | −3.08 ± 1.5 | 8.0 ± 4.0 | 0.13 |
| cPP* | |||||
| 0 min | 43.3 ± 1.4 | 38.7 ± 1.4 | 47.1 ± 2.5† | 43.9 ± 2.4 | 0.04 |
| Δ | −2.0 ± 1.2 | 2.4 ± 1.8 | −6.0 ± 1.7 | −2.5 ± 4.0 | 0.06 |
| cMAP* | |||||
| 0 min | 98.3 ± 1.9 | 94.5 ± 1.9 | 108 ± 3.3† | 91.2 ± 2.5‡ | <0.001 |
| Δ | −1.6 ± 1.3 | −1.4 ± 1.2 | −5.6 ± 2.2 | 2.4 ± 2.5 | 0.54 |
| Pf, mmHg | |||||
| 0 min | 27.9 ± 1.1 | 24.6 ± 1.3 | 31.6 ± 1.9† | 27.2 ± 1.9 | 0.03 |
| Δ | −0.97 ± 0.8 | 0.77 ± 1.5 | −3.5 ± 1.3 | −0.13 ± 1.0 | 0.54 |
| Pb, mmHg | |||||
| 0 min | 19.4 ± 0.7 | 17.3 ± 0.5 | 21.8 ± 1.4 | 19.1 ± 1.3 | 0.053 |
| Δ | −1.3 ± 0.6^ | −0.40 ± 0.7 | −2.9 ± 0.9 | −0.49 ± 1.1 | 0.12 |
| Reflection magnitude | |||||
| 0 min | 67.9 ± 2.1 | 72.7 ± 4.2 | 63.7 ± 2.5 | 67.2 ± 3.9 | 0.25 |
| Δ | −2.9 ± 1.9 | −5.0 ± 2.8 | −2.6 ± 3.7 | −0.90 ± 3.3 | 0.71 |
| PPA | |||||
| 0 min | 1.26 ± 0.01 | 1.28 ± 0.03 | 1.27 ± 0.01 | 1.23 ± 0.02 | 0.44 |
| Δ | 0.03 ± 0.01^ | −0.00 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.07 |
Values are means ± SE. bDBP, brachial diastolic blood pressure; bMAP, brachial mean arterial pressure; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; cDBP, central diastolic blood pressure; cMAP, central mean arterial pressure; cPP, central pulse pressure; cSBP, central systolic blood pressure; HR, heart rate; HTN, hypertensive; HTN + Meds, on antihypertensive medication; NT, normotensive; Pb, backward pressure; Pf, forward pressure; PPA, pulse pressure amplification.
*Data were log transformed for statistical analysis. ^P < 0.05, compared with 0 min. †P < 0.05, significant difference from NT. ‡P < 0.05, significant difference from HTN.
Figure 1.
Aortic waveform reflections before and in response to insulin stimulation: fasting AIx@75 (A), fasting AP (B), changes in AIx@75 (C), and changes in AP (D). ^Compared to 0 min, P < 0.05. AIx@75, augmentation index corrected to heart rate of 75 beats/min; AP, augmentation pressure; HTN, hypertensive; HTNmeds, on antihypertensive medication; NT, normotensive.
Correlations
VAT volume was associated with increased fasting measures of AP (r = 0.48, P = 0.01), Pb (r = 0.54, P < 0.01), and AIx@75 (r = 0.41, P = 0.03). V̇o2max was inversely associated with fasting AIx@75 (r = −0.39, P = 0.02) as well as AP (r = −0.38, P = 0.03) whereas MetS z-score positively correlated with fasting AIx@75 (r = 0.34, P = 0.04). Potassium levels were also associated with lower fasting AIx@75 (r = −0.53, P = 0.002) and fasting AP (r = −0.43, P = 0.01) as well as lower insulin-stimulated changes in AP (r = 0.52, P = 0.002). In addition, fasting Pf was associated with lower clamp-derived insulin sensitivity (r = −0.48, P < 0.01).
DISCUSSION
The primary finding of the current study is that insulin reduces AIx@75 and AP in individuals with MetS. Furthermore, insulin was shown to significantly reduce Pb and raise PPA compared to fasting. Taken together, our results support the hypothesis that insulin is vasoactive at the arterial level in adults with MetS. In fact, our findings support prior work in MetS adults (18) and suggest HTN medication does not blunt insulin responses in these people with MetS. However, it is important to recognize that this index of aortic wave reflection was lowered compared to fasting in lean healthy adults with but not people with type 1 or type 2 diabetes (19). Importantly, no subject in the current study had diabetes and fasting glucose/HbA1c were only mildly elevated. In line with the literature, it is possible our findings suggest that blood glucose at certain thresholds is deleterious for the ability of insulin to effectively impact aortic wave reflection. Regardless, our findings highlight that insulin can reduce AIx@75 and AP in people with obesity and MetS risk factors without overt diabetes. This appears to be of clinical relevance since PPA increased with insulin. A rise in PPA highlights that central blood pressure was lower relative to peripheral blood pressure, thereby decreasing cardiac workload and potentially lowering risk of end organ damage (25).
There are several mechanisms that could explain how insulin reduced AIx@75. One reason may relate to the role of excess adiposity on arterial stiffness (3). Elevated body fat, particularly located in the visceral region, has been proposed to exacerbate arterial stiffness through an inflammatory-mediated mechanism that promotes decreased arterial compliance, elasticity, and vasodilation (14, 16, 19, 26). In the present study, VAT did correlate with fasting but not insulin-stimulated AIx@75. Nonetheless, these results confirm that VAT is related to fasting aortic wave reflections but has no direct influence on insulin action on the vasculature. In line with these VAT findings, our results suggest that insulin reduced hsCRP as well as MMP-7. Although we did not directly measure vascular components of arterial compliance or elasticity, these inflammatory data may be important given studies have suggested that hsCRP and MMP-7 contribute to arterial stiffness via elastic degradation (13) and increased aortic tone (27). Similarly, V̇o2max was also associated with only fasting AIx@75, which is consistent with prior work (15). Thus, the relevance of increasing aerobic fitness for insulin action on blood vessel stiffness remains to be tested in prospective trials. Regardless, aerobic fitness has been linked to elevated nonoxidative glucose disposal. This could be relevant to endothelial function since lactate has recently been shown to act as a signal for angiogenesis (28). We report, like others (29), that lactate levels increased during insulin stimulation. Despite this observation, we did not identify any correlation between the rise in circulating lactate and reductions in aortic wave reflections. However, lactate tracers were not used, and we cannot confirm circulating lactate was mainly due to production despite insulin being expected to suppress endogenous glucose production.
Another possibility would be to expect decreased vascular resistance due to enhanced vasodilation by insulin stimulating NO (30). Although direct measures of NO-mediated arterial diameter and/or blood flow were not assessed in the current study, wave deconvolution analysis allows for the estimation of wave reflection, which is associated with arterial resistance (31). The current data show that insulin stimulation reduced the backward wave in aortic pressure in adults with MetS. A decrease in the backward reflected wave suggests increased elasticity of the vascular wall that typically would counteract with the forward wave produced at the aorta (5). Therefore, reductions in AIx@75 observed herein may be attributed to decreased impedance in the arterial tree as opposed to changes in left ventricular ejection fraction (Pf), sympathetic innervation (HR), or central blood pressure. However, we acknowledge that higher fasting blood potassium levels were associated with decreased fasting AIx@75 and AP. This would be consistent with potassium having favorable effects on reducing myocardial ejection in relation to backward wave reflection (32) as well as increased flow mediated dilation (33) through either a NO mechanism (34, 35) or NO-independent action (36–39). Interestingly, we observed that high fasting potassium was related to attenuated declines in AP following insulin infusion. We do not have a readily available explanation for such a finding, but this could partly reflect that higher potassium levels were linked to lower fasting AP. In turn, insulin was less effective at lowering AP due to a “floor effect.” Alternatively, insulin treatment can lower circulating potassium (40, 41), and this may have created a counterregulatory response in some people to blunt AP declines with insulin.
In the subgroup analysis, we observed no differential effect of insulin on AIx@75, AP, or Pb. This may seem somewhat surprising at first, but it is worth acknowledging that insulin sensitivity was similar across groups. As previously mentioned, AIx@75 is influenced by NO (28). Because insulin acts on NO in the vascular via activation of endothelial NO synthase (eNOS) (34), it is possible that the comparable glucose infusion rates after accounting for ambient insulin during the clamp minimized differences across groups. Although these results may help with understandings in precision medicine, our results should be interpreted with caution. The current power for the variable AIx@75 is 0.21 and for AP is 0.37, suggesting 54 and 32 subjects per group would be needed to detect differences. Furthermore, differences in medication half-lives may have impacted our results, despite approximate 24-h washouts. As current research suggests hypertensive populations present insulin resistance and these people were only screened for HTN phenotyping, it is important for the field to analyze how hypertension status impacts the effect of insulin on vascular health.
The current study has limitations that warrant discussion. Although the current study utilizes generally accepted methods of wave reflections and separation analysis, it should be acknowledged that the gold standard methodologies of arterial stiffness include pulse wave velocity and measuring central pressures and flow simultaneously and directly. Thus, interpreting these data to suggest insulin lowers arterial stiffness should be made with caution, and further work is warranted to assess arterial compliance and/or elasticity. No weight matched control population without MetS was utilized to examine the differences in insulin action compared to individuals with MetS. However, previous work has shown that insulin reduces arterial stiffness to a smaller extent in individuals with obesity, MetS, and type 2 diabetes than lean controls (11, 16, 17, 42). In addition, the present study comprised mainly middle-aged females. Therefore, these results may not be generalizable to other populations across the lifespan. Correlations do not equal causation and additional studies are needed to elucidate mechanisms by which insulin affects large conduit and muscular or resistance arterioles. Nevertheless, a strength of this study is that a low-fat AHA-based diet was provided the day before investigations to minimize influence of nutrition on vascular insulin responses. Moreover, groups were similar in age, BMI, body fat, aerobic fitness, and metabolic insulin sensitivity. This highlights that differences seen in indices of arterial stiffness are unique to insulin in people with MetS.
In summary, insulin reduces AIx@75 and AP in individuals with MetS. Although insulin stimulation had no effect on central or brachial blood pressure measures, there was a significant increase in PPA and reduction in Pb as well as inflammatory processes as reflected by hsCRP and MMP-7. Together, these findings highlight that insulin acts to modify aortic wave reflection among individuals with MetS. Future studies are needed to examine the effects of insulin across the arterial tree in people with varying hypertension status to improve design of optimal treatments that reduce CVD risk in individuals with insulin resistance.
DATA AVAILABILITY
Data used to support this study may be requested upon reasonable request from the corresponding author.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL130296 (to S.K.M.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.K.M. conceived and designed research; B.L.D., E.M.H., S.L.M., and S.K.M. performed experiments; B.L.D. and E.M.H. analyzed data; B.L.D., E.M.H., S.L.M., and S.K.M. interpreted results of experiments; B.L.D. prepared figures; B.L.D., E.M.H., and S.K.M. drafted manuscript; B.L.D., E.M.H., S.L.M., and S.K.M. edited and revised manuscript; B.L.D., E.M.H., S.L.M., and S.K.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the nursing staff of the Clinical Research Unit for technical assistance; Lisa Farr, from the Exercise Physiology Core Lab, for fitness testing aide; the Ligand Research Assay Core Lab; as well as the dedicated research assistants of the Applied Metabolism and Physiology Lab and participants for their effort. We also thank, in particular, Dr. Eugene J. Barrett and Linda Jahn for training and assistance with AIx@75 measures.
REFERENCES
- 1.Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Prevalence of the metabolic syndrome in the United States, 2003-2012. JAMA 313: 1973–1974, 2015. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Sherling DH, Perumareddi P, Hennekens CH. Metabolic syndrome. J Cardiovasc Pharmacol Ther 22: 365–367, 2017. doi: 10.1177/1074248416686187. [DOI] [PubMed] [Google Scholar]
- 3.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery C, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papaioannou TG, Protogerou AD, Stergiopulos N, Vardoulis O, Stefanadis C, Safar M, Blacher J. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age Dordr Neth 36: 9661, 2014. doi: 10.1007/s11357-014-9661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirwany NA, Zou M. Arterial stiffness: a brief review. Acta Pharmacol Sin 31: 1267–1276, 2010. doi: 10.1038/aps.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens 16: 2079–2084, 1998. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 7.Lemogoum D, Flores G, Van den Abeele W, Ciarka A, Leeman M, Degaute JP, van de Borne P, Van BL. Validity of pulse pressure and augmentation index as surrogate measures of arterial stiffness during beta-adrenergic stimulation. J Hypertens 22: 511–517, 2004. doi: 10.1097/00004872-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM, Stone K, McCoy SM. Impact of prolonged sitting on peripheral and central vascular health. Am J Cardiol 123: 260–266, 2019. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Laurent S. Surrogate measures of arterial stiffness. Hypertension 47: 325–326, 2006. doi: 10.1161/01.HYP.0000200701.43172.9a. [DOI] [PubMed] [Google Scholar]
- 10.Palatini P, Casiglia E, Gąsowski J, Głuszek J, Jankowski P, Narkiewicz K, Saladini F, Stolarz-Skrzypek K, Tikhonoff V, Van Bortel L, Wojciechowska W, Kawecka-Jaszcz K. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag 7: 725–739, 2011. doi: 10.2147/VHRM.S25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamminen M, Westerbacka J, Vehkavaara S, Yki-Järvinen H. Insulin-induced decreases in aortic wave reflection and central systolic pressure are impaired in type 2 diabetes. Diabetes Care 25: 2314–2319, 2002. doi: 10.2337/diacare.25.12.2314. [DOI] [PubMed] [Google Scholar]
- 12.Westerbacka J, Seppälä-Lindroos A, Yki-Järvinen H. Resistance to acute insulin induced decreases in large artery stiffness accompanies the insulin resistance syndrome. J Clin Endocrinol Metab 86: 5262–5268, 2001. doi: 10.1210/jcem.86.11.8047. [DOI] [PubMed] [Google Scholar]
- 13.Aroor AR, Jia G, Sowers JR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Regul Integr Comp Physiol 314: R387–R398, 2018. doi: 10.1152/ajpregu.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wedell-Neergaard A-S, Krogh-Madsen R, Petersen GL, Hansen ÅM, Pedersen BK, Lund R, Bruunsgaard H. Cardiorespiratory fitness and the metabolic syndrome: roles of inflammation and abdominal obesity. PloS One 13: e0194991, 2018. doi: 10.1371/journal.pone.0194991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jae SY, Heffernan KS, Fernhall B, Oh YS, Park WH, Lee M-K, Choi Y-H. Association between cardiorespiratory fitness and arterial stiffness in men with the metabolic syndrome. Diabetes Res Clin Pract 90: 326–332, 2010. doi: 10.1016/j.diabres.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Westerbacka J, Yki-Järvinen H. Arterial stiffness and insulin resistance. Semin Vasc Med 2: 157–164, 2002. doi: 10.1055/s-2002-32039. [DOI] [PubMed] [Google Scholar]
- 17.Jahn LA, Hartline L, Rao N, Logan B, Kim JJ, Aylor K, Gan L-M, Westergren HU, Barrett EJ. Insulin enhances endothelial function throughout the arterial tree in healthy but not metabolic syndrome subjects. J Clin Endocrinol Metab 101: 1198–1206, 2016. doi: 10.1210/jc.2015-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jatic Z, Skopljak A, Hebibovic S, Sukalo A, Rustempasic E, Valjevac A. Effects of different antihypertensive drug combinations on blood pressure and arterial stiffness. Med Arch Sarajevo Bosnia Herzeg 73: 157–162, 2019. doi: 10.5455/medarh.2019.73.157-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Järvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes 48: 821–827, 1999. doi: 10.2337/diabetes.48.4.821. [DOI] [PubMed] [Google Scholar]
- 20.Dudenbostel T, Glasser SP. Effects of antihypertensive drugs on arterial stiffness. Cardiol Rev 20: 259–263, 2012. doi: 10.1097/CRD.0b013e31825d0a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Rourke MF, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertens 11: 327–337, 1993. doi: 10.1097/00004872-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 22.National Cholesterol Education Program (NCEP ). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106: 3143–3421, 2002. [PubMed] [Google Scholar]
- 23.Heiston EM, Gilbertson NM, Eichner NZ, Malin SK. A low-calorie diet with or without exercise reduces postprandial aortic waveform in females with obesity. Med Sci Sports Exerc 53: 796–803, 2021.doi: 10.1249/MSS.0000000000002515. [3292549] [DOI] [PubMed] [Google Scholar]
- 24.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. β-Cell dysfunction is associated with metabolic syndrome severity in adults. Metab Syndr Relat Disord 12: 79–85, 2014. doi: 10.1089/met.2013.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nijdam M-E, Plantinga Y, Hulsen HT, Bos WJW, Grobbee DE, van der Schouw YT, Bots ML. Pulse pressure amplification and risk of cardiovascular disease. Am J Hypertens 21: 388–392, 2008. doi: 10.1038/ajh.2007.89. [DOI] [PubMed] [Google Scholar]
- 26.Diamant M, Lamb HJ, van de Ree MA, Endert EL, Groeneveld Y, Bots ML, Kostense PJ, Radder JK. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J Clin Endocrinol Metab 90: 1495–1501, 2005. doi: 10.1210/jc.2004-1579. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation 119: 2480–2489, 2009. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Liu T, Guo H, Cui H, Li P, Feng D, Hu E, Huang Q, Yang A, Zhou J, Luo J, Tang T, Wang Y. Lactate potentiates angiogenesis and neurogenesis in experimental intracerebral hemorrhage. Exp Mol Med 50: 1–12, 2018. doi: 10.1038/s12276-018-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berhane F, Fite A, Daboul N, Al-Janabi W, Msallaty Z, Caruso M, Lewis MK, Yi Z, Diamond MP, Abou-Samra A-B, Seyoum B. Plasma lactate levels increase during hyperinsulinemic euglycemic clamp and oral glucose tolerance test. J Diabetes Res 2015: 1–7, 2015. doi: 10.1155/2015/102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron AD, Brechtel-Hook G, Johnson A, Cronin J, Leaming R, Steinberg HO. Effect of perfusion rate on the time course of insulin-mediated skeletal muscle glucose uptake. Am J Physiol Endocrinol Physiol 271: E1067–E1072, 1996. doi: 10.1152/ajpendo.1996.271.6.E1067. [DOI] [PubMed] [Google Scholar]
- 31.Wilenius M, Tikkakoski AJ, Tahvanainen AM, Haring A, Koskela J, Huhtala H, Kähönen M, Kööbi T, Mustonen JT, Pörsti IH. Central wave reflection is associated with peripheral arterial resistance in addition to arterial stiffness in subjects without antihypertensive medication. BMC Cardiovasc Disord 16: 131, 2016. doi: 10.1186/s12872-016-0303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GF. Triangulating the peaks of arterial pressure. Hypertension 48: 543–545, 2006. doi: 10.1161/01.HYP.0000238325.41764.41. [DOI] [PubMed] [Google Scholar]
- 33.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, Dalton RN, Kaski JC, MacGregor GA. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 55: 681–688, 2010. doi: 10.1161/HYPERTENSIONAHA.109.147488. [DOI] [PubMed] [Google Scholar]
- 34.Gijsbers L, Dower JI, Schalkwijk CG, Kusters YHAM, Bakker SJL, Hollman PCH, Geleijnse JM. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr 114: 1419–1426, 2015. doi: 10.1017/S0007114515002986. [DOI] [PubMed] [Google Scholar]
- 35.Oberleithner H, Callies C, Kusche-Vihrog K, Schillers H, Shahin V, Riethmüller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA 106: 2829–2834, 2009. doi: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colantuoni A, Lapi D, Paterni M, Marchiafava PL. Protective effects of insulin during ischemia-reperfusion injury in hamster cheek pouch microcirculation. J Vasc Res 42: 55–66, 2005. doi: 10.1159/000083092. [DOI] [PubMed] [Google Scholar]
- 37.Hasdai D, Best PJ, Cannan CR, Mathew V, Schwartz RS, Holmes DR, Lerman A. Acute endothelin-receptor inhibition does not attenuate acetylcholine-induced coronary vasoconstriction in experimental hypercholesterolemia. Arterioscler Thromb Vasc Biol 18: 108–113, 1998. doi: 10.1161/01.ATV.18.1.108. [DOI] [PubMed] [Google Scholar]
- 38.Izhar U, Hasdai D, Richardson DM, Cohen P, Lerman A. Insulin and insulin-like growth factor-I cause vasorelaxation in human vessels in vitro. Coron Artery Dis 11: 69–76, 2000. doi: 10.1097/00019501-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 39.McKay MK, Hester RL. Role of nitric oxide, adenosine, and ATP-sensitive potassium channels in insulin-induced vasodilation. Hypertension 28: 202–208, 1996. doi: 10.1161/01.hyp.28.2.202. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Vijayan A. Insulin for the treatment of hyperkalemia: a double-edged sword? Clin Kidney J 7: 239–241, 2014. doi: 10.1093/ckj/sfu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNicholas BA, Pham MH, Carli K, Chen CH, Colobong-Smith N, Anderson AE, Pham H. Treatment of hyperkalemia with a low-dose insulin protocol is effective and results in reduced hypoglycemia. Kidney Int Rep 3: 328–336, 2018. doi: 10.1016/j.ekir.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J-W, Lee D-C, Im J-A, Shim J-Y, Kim S-M, Lee H-R. Insulin resistance is associated with arterial stiffness independent of obesity in male adolescents. Hypertens Res 30: 5–11, 2007. doi: 10.1291/hypres.30.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to support this study may be requested upon reasonable request from the corresponding author.