Abstract
PURPOSE
Studies have demonstrated that positron emission tomography/computed tomography (PET/CT) with Gallium-68 (68Ga)-labeled somatostatin analogues are effective at detecting metastatic disease in neuroendocrine tumors (NET), especially extrahepatic metastases. However, PET in combination with full-dose contrast-enhanced CT (ceCT) exposes patients to higher radiation (~25 mSv). The use of non-contrast-enhanced low-dose CT (ldCT) can reduce radiation to about 10 mSv and may avoid contrast-induced side effects. This study seeks to determine whether ceCT could be omitted from NET assessments.
METHODS
We retrospectively compared the performance of PET/ldCT versus PET/ceCT in 54 patients (26 male, 28 female) who had undergone a 68Ga-DOTATATE PET/CT. The selection criteria were as follows: available ldCT and ceCT, histologically confirmed NET, and follow-up of at least 6 months (median, 12.6 months; range, 6.1–23.2 months). The PET/ldCT and PET/ceCT images were analyzed separately. We reviewed metastases in the lungs, bones, and lymph nodes. The results were compared with the reference standard (clinical follow-up data).
RESULTS
The PET/ceCT scans detected 139 true-positive bone lesions compared with 140 lesions detected by the PET/ldCT scans, 106 true-positive lymph node metastases (PET/ceCT) compared with 90 metastases detected by the PET/ldCT scans, and 26 true-positive lung lesions (PET/ceCT) compared with 6 lesions detected by the PET/ldCT scans. The overall lesion-based sensitivity for full-dose PET/ceCT was 97%, specificity 86%, negative predictive value (NPV) 93%, and positive predictive value (PPV) 93%. The overall lesion-based sensitivity for PET/ldCT was 85%, specificity 73%, NPV 72%, and PPV 85%.
CONCLUSION
This study presents the first evidence that ceCT should not be omitted from extrahepatic staging using 68Ga-DOTATATE PET/CT in patients with NET. ceCT alone can be used as a follow-up to reduce radiation exposure when the patient has already undergone PET/ceCT and suffers from non-DOTATATE-avid NET.
Previous studies have shown the importance of DOTATOC, DOTATATE, and DOTANOC PET/CT imaging in the diagnosis and accurate staging of neuroendocrine tumors (NET) (1–3). The use of radiolabeled somatostatin analogs in PET/CT has become the standard protocol in NET staging. For many years, octreotide-scintigraphy was used for NET detection and assessment, but this practice has recently been replaced by combined, integrated PET/CT imaging with 68Ga-labelled somatostatin analogues. The new method yields higher spatial resolution and facilitates tracer uptake quantification, and 68Ga PET/CT has increasingly replaced the use of contrast-enhanced computed tomography (ceCT) alone. 68Ga PET/CT provides for precise staging and allows the physician to assess the feasibility of peptide receptor radionuclide therapy (4, 5). There is evidence that PET/ceCT can be beneficial for patients with NET and the ENETS guidelines, among others, recommend PET/ceCT for staging NETs (6–8). However, to date, there is no mandatory consensus on the appropriate 68Ga PET/CT protocol for assessing NET. A patient can undergo PET with non-contrast-enhanced low-dose CT (ldCT) or with full-dose ceCT. The diagnostic benefit of surplus ceCT has been assessed particularly for the detection and staging of 18F-fluorodeoxyglucose (FDG)- avid lymphoma (5) and NET abdominal lesions (9). The benefits of ceCT over PET/ldCT in the detection of extrahepatic metastases have not been analyzed with that kind of detail. As the ceCT method results in substantial radiation exposure (up to 25 mSv), depending on the type of CT machine, any potential dose reduction is desirable. While these levels of exposure are within the limits recommended by Huang et al. (10), they surpass those given by Persson et al. (11). In addition, contrast medium can cause adverse reactions such as hyperthyroidism and renal failure, so it should not be administered without cause, although current ESUR guidelines suggest that contrast media’s adverse effects have been widely overestimated (12). This study addresses whether ceCT is necessary for the detection and assessment of NET extrahepatic metastases and if PET/ldCT is sufficiently reliable.
Methods
Patients
This trial was approved by the local medical ethics committee (decision number EK 197/13). As the study design was retrospective, there was no need to obtain informed consent, and this requirement was waived by the ethics committee.
A total of 132 patients who had been diagnosed with NET and who had undergone a 68Ga-DOTATATE PET/ceCT scan between September 2011 and March 2013 were identified. The inclusion criteria were histological sampling and proof of NET; patient availability for at least six months of follow-up; and ceCT conducted as part of the examination. After these criteria were applied, 54 patients were included in the study (26 male, 28 female; median age, 64 years; age range, 38–86 years). The same patient population was used in a previously published study. The follow-up data consisted of the clinical data collected thereafter (median 12.6 months, range 6.1–23.2 months), in order to classify the lesions as false negative, false positive, true negative, or true positive. The tumor marker chromogranin A was used in all patients as a laboratory chemical follow-up marker. Furthermore, the follow-up measures consisted of the repetition of the PET/CT imaging, magnetic resonance imaging (MRI), repeated CT and clinical follow-up.
Tracer production
The tracer, 68Ga-DOTATATE, was produced on-site using automated synthesis, as described by Meyer et al. (13). Briefly, 30 μL of 1 mg/mL DOTATATE stock solution (ABX) in metal-free H2O was used for the standard labelling procedure. 68Gallium was eluted from an ITG 68Ge/68Ga-generator (Isotope Technologies Garching).
PET/CT protocol
The radiotracer was applied intravenously. The PET/ceCT scans were acquired 40 minutes after injection. The PET/ceCT scans were performed with a Philips Gemini TF 16 PET/CT scanner (Philips Medical Systems).
First, a native, low-dose, whole-body CT from the base of the skull to the upper thigh was performed for attenuation correction purposes. Second, a late-phase ceCT of the same volume was acquired during a deep inspiration and breath-hold (tube current-time product, 30 mAs; tube voltage, 120 kVp). For image reconstruction, a medium-smooth soft-tissue kernel with a slice thickness of 5 mm and an overlapping increment of 3.5 mm was used. Then, 100 mL of contrast medium (Ultravist 300, Bayer Healthcare) were administered intravenously, followed by a saline chaser bolus (30 mL of 0.9% sodium chloride solution) that was administered at the same flow rate. The CT scan was started with a delay of 70 s using a bolus tracking method with region of interest (ROI) in the descending aorta.
After the CT scan, a PET scan was performed. The data were gathered in list mode for each scan along with the respective timestamps over multiple timepoints. The acquisition time was 1.5 min (bodyweight <100 kg) or 2 min (bodyweight ≥100 kg) per bed position. All sets of data underwent full correction for random incidences and scatter radiation attenuation (14).
Lesion criteria
Lesions with a higher uptake than background intensity were identified as PET-positive lesions. High physiological uptake could be seen in the liver, spleen, pituitary gland, adrenal glands, pancreatic head, thyroid, and urinary tract. We selected different cutoff values for the pulmonary lesions, osseous lesions, and PET-positive lymph nodes (1, 15). These cutoff values were selected based on the upper limit of physiological uptake for each region (bone marrow, 0.4–2.7; lymph nodes, 0.5–2.2; lungs, 0.6–2.3) (16).
For the CT scans, a positive lesion was defined according to the following criteria for each of the abovementioned tissues. Bone lesions were considered suspect unless they bare clear signs of degenerative or benign origins, e.g., osteophytes, osteochondrosis, or sclerotic margins. All other bone lesions with diameters over 0.5 cm were classified as positive.
If two or more of the three malignancy criteria (diameter, hilum sign, and shape) were positive in a lymphatic lesion, this lesion was also considered tumor positive; otherwise the lesion was considered negative. The criterion size was adapted to different locations in the body. While inguinal lymph nodes with a diameter of 18 mm can be physiological, that size is highly suspicious if found elsewhere. We established cutoff values for lymph node sizes in various areas of the body. The following minimal lymph node diameters were defined as negative: cervical <11 mm; axillary <10 mm; ventral mediastinum <6 mm; aortopulmonary window <15 mm; perihilar <10 mm; infracarinal <10 mm; paraaortic < 7 mm; mesenteric <10 mm; iliac <12 mm, and inguinal <18 mm (14). Lymph node diameters greater than these values were classified as positive.
Oval lymph nodes were rated as negative, whereas circular lymph nodes were rated as positive. A fatty hilum was considered benign, but if a fatty hilum was absent, the lesion was rated positive (17). Since small lymph nodes rarely have a fatty hilum, lymph nodes with a diameter of less than 4 mm were counted as positive if there was infiltrative growth and/or increased contrast enhancement compared with other lymph nodes in the area. Any lymph node located retrocrurally, retroclavicularly, aortocavally, or inside the liver hilum or the perirectal fascia was considered malignant due to its location. Pulmonary nodules were counted as positive lesions, unless they showed one or more of the following calcification patterns: popcorn calcification, homogeneous calcification, or central calcification (18).
In addition, any identified lesions were divided into body regions on a patient-by-patient basis for further analysis. Bone lesions were grouped according to the following classifications: 1.1, trunk, including the clavicles, ribs, shoulder blades, breastbone, and pelvis; 1.2, spinal column; and 1.3, bones of the extremities. Lymph nodes were grouped as follows: 2.1, head/neck, including the supraclavicular and axillary regions; 2.2, mediastinum; 2.3, abdomen, including the mesentery, paraaortic, iliac, and inguinal regions.
Review process
In the first step of the review process, the PET and ceCT images were analyzed separately by two experienced nuclear medicine specialists and two experienced radiologists, respectively, for a consensus reading that was blinded to the patients’ identities and histories. The same was done with the PET/ldCT images, which had been used only for attenuation correction in our previous study.
In the second step of the review process, all four reviewers had to reach a consensus reading of the images for each detected lesion, using a five-point grading system adapted from Nakamoto et al. (19). The entire analysis was finished within a period of four weeks. The graded lesions were later divided into positive lesions, if they received a score of 3 or 4, and negative lesions, if they received a score of 1 or 2 (19). These results were considered representative of the combined PET/ceCT readings. A second assessment was performed every time a lesion was detected in only one of the modalities mentioned in step one. During this second assessment, the reviewers were asked to verify their initial evaluation. Thereafter, the results of the ceCT and PET/ceCT reviews were compared with the clinical follow-up data as a reference standard (median, 12.6 months; range, 6.1–23.2 months) to identify lesions as true positive, true negative, false positive, or false negative. This process was separately conducted for the three types of extrahepatic manifestations (bone, lung, and lymph nodes) and for all the lesions grouped together to obtain an overall sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for PET/ldCT and PET/ceCT. The clinical follow-up data included repeated PET/CT imaging, magnetic resonance imaging (MRI), repeated CT-scans, and measurements of the tumor marker chromogranin A (20–22).
Results
In the PET/ceCT images, 139 true-positive bone lesions were detected. By comparison, 140 lesions were detected in the PET/ldCT images, leading to the identification of one additional lesion in the vertebral column. In the PET/ceCT images, 106 true-positive lymph node metastases were detected, compared with 90 metastases detected in the PET/ldCT images. The PET/ceCT images were also used to detect 26 true-positive lung lesions, while the PET/ldCT images were used to detect 6 true-positive lung lesions. The results of the metastasis analysis according to various organs as identified with PET/ldCT are shown in Table 1, and those of ceCT are shown in Table 2.
Table 1.
Findings in various organs with PET/ldCT
| PET/ldCT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Findings in bones | 140 | 14 | 46 | 0 | 100 | 77 | 91 | 100 | 93 |
| Findings in lymph nodes | 90 | 26 | 49 | 23 | 80 | 65 | 78 | 68 | 74 |
| Findings in lungs | 6 | 1 | 15 | 20 | 23 | 94 | 86 | 43 | 50 |
| Findings in total | 236 | 41 | 110 | 43 | 85 | 73 | 85 | 72 | 80 |
PET/ldCT, positron emission tomography/low-dose computed tomography; TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value.
Table 2.
Findings in various organs with PET/ceCT
| PET/ceCT | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Findings in bones | 139 | 6 | 54 | 1 | 99 | 90 | 96 | 98 | 97 |
| Findings in lymph nodes | 106 | 10 | 64 | 8 | 93 | 86 | 91 | 89 | 90 |
| Findings in lungs | 26 | 5 | 11 | 0 | 100 | 69 | 84 | 100 | 88 |
| Findings in total | 271 | 21 | 129 | 9 | 97 | 86 | 93 | 93 | 93 |
PET/ceCT, positron emission tomography/contrast-enhanced computed tomography; TP, true positive; FP, false positive; TN, true negative; FN, false negative; PPV, positive predictive value; NPV, negative predictive value.
NPV, PPV, sensitivity, and specificity were also calculated for bone, lung, and lymph node metastasis lesions, subdivided into various body regions: intrapulmonary for lung lesions; cervical, mediastinal, and abdominal for lymph node lesions; and trunk, long bones, and vertebrae for osseous lesions (Table 3). An overview of all primary lesions can be seen in Table 4.
Table 3.
Lesion analysis with PET/ldCT and ceCT based on body region
| PET/ld CT | PET/ceCT | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Sensitivity | Specificity | PPV | NPV | Sensitivity | Specificity | PPV | NPV | |
| Bone lesions | ||||||||
| Trunk | 100 | 73 | 89 | 100 | 36 | 27 | 51 | 16 |
| Vertebrae | 100 | 71 | 89 | 100 | 42 | 29 | 58 | 18 |
| Long bones | 100 | 100 | 100 | 100 | 17 | 0 | 33 | 0 |
|
| ||||||||
| Lymph node lesions | ||||||||
| Cervical | 100 | 82 | 75 | 100 | 33 | 18 | 18 | 33 |
| Mediastinal | 81 | 81 | 81 | 81 | 56 | 13 | 39 | 22 |
| Abdominal | 78 | 56 | 77 | 57 | 65 | 40 | 68 | 37 |
| Lung lesions | 23 | 94 | 86 | 43 | 100 | 69 | 84 | 100 |
PET/ldCT, positron emission tomography/low-dose computed tomography; PET/ceCT, positron emission tomography/contrast-enhanced computed tomography; PPV, positive predictive value; NPV, negative predictive value.
Table 4.
Locations of primaries
| n=54 | |
|---|---|
| Pancreas | 9 |
|
| |
| Duodenum | 1 |
|
| |
| Jejunum | 2 |
|
| |
| Ileum | 14 |
|
| |
| Ileocecal valve | 2 |
|
| |
| Cecum | 3 |
|
| |
| Colon | 1 |
|
| |
| Rectum | 4 |
|
| |
| Lung | 4 |
|
| |
| Neobladder | 1 |
|
| |
| Endometrium | 1 |
|
| |
| Paraganglioma | 1 |
|
| |
| Cancer of unknown primary (CUP) overall | 11 |
|
| |
| Detected organ sites in follow-up of CUP | n=11 |
| Lung | 1 |
| Cervix | 1 |
| Duodenum | 1 |
| Ileum | 1 |
| Thyroid gland | 1 |
| Remaining unknown | 6 |
Discussion
The review of the performance of ceCT in the detection of bone metastases indicated that ceCT is not mandatory for this application, since sensitivity is 100% for PET/ldCT and 99% for PET/ceCT. Bone metastases do not commonly have significant contrast enhancement, and they often have a high tracer uptake, resulting in the satisfactory NPV (100%) of PET/ldCT in the detection of bone metastases (Fig. 1). The superiority of PET/ldCT over ceCT in this application is due to the visibility of somatostatin-expressing metastases and the improved sensitivity of DOTATATE-PET/CT (23). However, high-dose ceCT could be selected for use in follow-up exams of non-DOTATATE-avid NETs, as PET/ldCT loses its superiority in those cases. In our view, the high cost, tracer administration, and long scan times of routine high-dose ceCT are not justified. Furthermore, peptide receptor radionuclide therapy is no longer an option for these patients. However, a PET/ldCT scan must be performed to rate a NET case as non-DOTATATE-avid.
Figure 1. a, b.
A patient with extensive bone metastases. Sagittal fusion image (a) of low-dose CT with 68Ga-DOTATATE. Sagittal high-dose contrast-enhanced CT (ce-CT) image (b) with bone tissue windowing. Both images reveal sclerotic bone metastases in multiple vertebrae. No significant value is gained from the high-dose ceCT compared with the low-dose PET/CT alone. The low-dose PET/CT provides a clear differentiation of the bone metastases (exemplified by the red arrows) and the non-enhancing degenerative processes (exemplified by the blue arrows).
For the detection of lymphatic metastases, high-dose ceCT still seems to be necessary, despite the overall satisfactory performance of PET/ldCT. For diagnosing lymph node metastases, PET/ceCT is clearly superior (93% sensitivity and 86% specificity vs. 80% sensitivity and 65% specificity of PET/ldCT, Fig. 2).
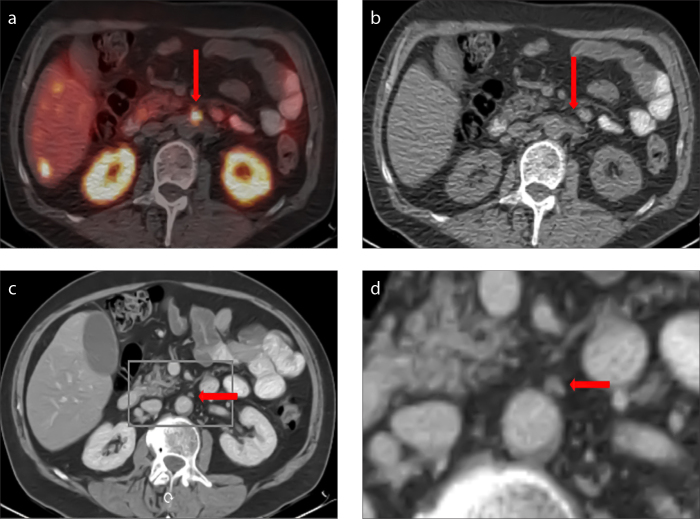
Figure 2. a–d.
The cross-sectional 68Ga-DOTATATE PET/CT image (a) shows a focal tracer uptake (arrow); however, the use of cross-sectional low-dose CT alone does not permit the differentiation of unspecific intestinal activity versus lymph node metastasis (b, arrow). The additional use of contrast-enhanced CT (ceCT) provides a clear delineation of a lymph node as the source of the pathological tracer uptake (c, arrow), as seen in the magnified image section of ceCT (d).
When analyzing lymph node metastases, ldCT yields a high number of false-positive results (24) that cannot be proficiently excluded through PET. Differentiation between a lymph node metastasis and unspecific uptake in the small intestine can be difficult, leading to the high sensitivity and poor specificity of PET/ldCT in the detection of lymph node metastases. These false-positive results can be successfully excluded through high-dose ceCT.
The ldCT used for attenuation correction in PET/CT has a very low sensitivity (23%) and NPV (43%) for lung metastases (Fig. 3). It should be noted that the ldCT lung scans in this study were not acquired during maximum inspiration with a breath-hold; they were instead taken during continuous shallow breathing to optimize co-registration with the PET images. This led to decreased sensitivity for sub-centimeter-sized nodules. Additionally, normal anatomic structures, such as blood vessels, may have appeared ambiguous, indicating abnormalities where there were none (23). The available data for adults suggest that the sensitivity problem with end-tidal expiration in localized ldCT scans for pulmonary nodule diagnosis is not the reduced dose but the lack of a full inspiration (24). In our study, fewer true-positive lung lesions were detected in ldCT than in PET/ceCT (6 vs. 26). This result was due to the scans being acquired during free breathing and is consistent with the findings of other authors who have reported improved volumetric lesion definition during a breath-hold CT (25). Similarly, other studies have reported that a significantly greater number of lung lesions were detected when using the breath-hold technique (26). Since the breath-hold technique is not standard in our institution, and as it often requires manual co-registration, it was not used in this study (27). Furthermore, it can be said that the overall accuracy of PET/ceCT of 93% exceeded that of PET/ldCT (80%) by far. The individual accuracies of the two methods, broken down by organ manifestation, can be studied in detail in Tables 1 and 2.
Figure 3. a, b.
Cross-sectional images of the right lung in lung tissue windowing with ceCT (a) and low-dose CT (b). In image (a), there are three intrapulmonary nodules (circles) that cannot be seen in (b) due to the lower applied radiation dose and the lack of breath-hold, which resulted in image blurring. Note that due to the maximum inspiration and the use of breath-hold during image acquisition, image (a) is far clearer than image (b), which has been acquired during free breathing to match the PET images for later image fusion. Therefore, the large lobe fissure is located farther anterior, and the bronchial bifurcation appears further spread out.
When further subdividing the lesions according to their locations in various body regions and analyzing the respective performance of each method (Table 3), the shortcomings of the methods become quite obvious. Our data strengthen the knowledge that CT alone is not sufficient for the detection of osseous metastases and is known to be inferior to other methods, especially in the detection of neuroendocrine metastases (28). In our case, ceCT alone was inferior to PET/ldCT in each bone region, suggesting that tracer uptake is the key to a correct diagnosis. A value of zero for NPV and specificity may be due to the fact that there are generally few metastases in long tubular bones, and therefore, it is statistically difficult to evaluate the methods, since even small deviations may cause large changes in the results.
Lymph-node-based lesions were judged by their size and location using ceCT; all results were systematically inferior to the results obtained by using PET/ldCT. These ceCT results were, therefore, not satisfactory. In particular, cervical lymph node metastases were often excluded as nonspecific. Enlarged cervical lymph nodes can have multiple causes, of which NET metastasis is a rather unlikely one among many differential diagnoses. Swollen cervical lymph nodes can also mean something completely different than malignancy, which is why tracer imaging provides important information for NET patients.
As already described, high-dose CT of the lungs offers higher spatial resolution and lower movement artifacts caused by breathing compared with low-dose CT in free shallow breathing. This leads to a higher detection rate of even smaller intrapulmonary nodules and further offers the possibility of a more accurate characterization of individual lesions based on their morphology as compared with results from low-dose CT with shallow breathing. In the consensus reading, the high-dose CT was evaluated as very sensitive, and many of the lesions found in the lungs were described and evaluated as potentially malignant, even if the differential diagnosis could also include granulomas or scars. All lesions that did not show a progress in growth or contrast enhancement and/or tracer uptake in follow-up were excluded as benign.
The data in Table 1 clearly depict each method’s shortcomings and benefits for various body regions and organ types. Each method is somewhat insufficient when used as a stand-alone method in staging NET metastases. Their combination, on the other hand, gives the examiner a comprehensive and reliable overview of the disease. Complementary examinations should therefore be used when indicated. The decision to use ceCT for NET detection with 68Ga-DOTATATE should always be made on an individual, case-by-case basis. If, for instance, the abdomen is the primary focus of the diagnosis, a PET/ldCT scan might be sufficient. PET/ldCT might also be considered as the first method of choice when examining younger patients with higher sensitivity to ionizing radiation and for patients with increased risk of contrast-induced allergic reactions, hyperthyroidism, or nephropathy.
The fact remains that PET/CT is more sensitive than CT alone. Very small lesions described in high-dose CT in this study were not visible in ldCT (Fig. 3) due to abovementioned reasons. They do not accumulate tracer due to their small size, and thus elude the added value of PET/CT compared with CT alone.
Our results complement those of Mayerhoefer et al. (29) by surveying the whole body for extrahepatic metastases. The study by Mayerhoefer et al. (29) found only a limited increase in sensitivity and specificity for PET/ceCT compared with PET/ldCT, but their analysis was limited to the abdomen. Moreover, 67% of the detected lesions in their study were located in the liver. By contrast, liver lesions were excluded from our study. There are already effective methods of detecting liver lesions in precise detail, e.g., with MRI and ceCT of the liver or contrast-enhanced ultrasound. As most patients present with liver metastases at the time of their diagnosis, and as distant metastases are directly related to a poorer five-year survival rate, this study focused on extrahepatic lesions (30). If only lymph node metastases are considered, the study by Mayerhoefer et al. (29) demonstrated that contrast medium has a strong impact on diagnostic performance (a 50% reduction in false positives and 100% reduction in false negatives for experienced investigators), similar to our results. Thus, particularly if lymph node metastases are suspected in NET patients, PET with ceCT should be considered. As described above, it is often difficult to differentiate lymph node metastases from unspecific uptake in the small bowel. In these instances, ceCT is capable of delineating even small lymph node metastases.
Other studies that have analyzed the diagnostic value of PET combined with ceCT using fluorodeoxyglucose (FDG) have reported divergent results. In a retrospective analysis of oncology patients, Nanni et al. (31) found that PET/ceCT increased diagnostic quality, especially with regard to nodal metastases and small distant lesions. In addition, ceCT was shown to be of value in providing fully diagnostic morphologic data, thus complementing the PET imaging (32). In contrast, van Hamersveld et al. (33) demonstrated that in patients with newly diagnosed FDG-avid lymphoma, unenhanced low-dose FDG-PET/CT alone was the primary imaging modality of choice for staging. This confirmed an earlier study by Schaefer et al. (34), who found that PET/ldCT was more sensitive and specific than ceCT in patients with Hodgkin disease and high-grade non-Hodgkin lymphoma. That study, however, did not compare PET/ldCT with PET/ceCT. Shen et al. (35) point out the importance of glucose metabolism when imaging cancer cells from a molecular imaging point of view. Other studies also suggest that 68Ga-DOTATATE PET/CT has among the highest detection rate in the diagnosis of SDHA-related metastatic pheochromocytoma and paraganglioma (36). Jha et al. (37) also recommend 68Ga-DOTATATE PET/CT as a means of detecting pheochromocytoma and paraganglioma in children. In line with that study, our results further strengthen the assumption that ceCT aids and supplements 68Ga-DOTATATE PET/CT in the detection of extrahepatic metastases associated with NETs.
As a limitation of this study, the retrospective design should be mentioned. Furthermore, a comparison with other imaging methods such as MRI or scintigraphy would have been interesting but has not been done. This will be subject to further investigation.
In conclusion, it can be said that an individualized approach should be taken when staging NETs. Our results showed that ldCT imaging was inferior to that of ceCT, especially for identifying lymph node metastases (though not so much for bone lesions). Therefore, ceCT should not be omitted from clinical screening. A ceCT scan can also be used for follow-up to reduce radiation exposure when a patient has already undergone a PET/ceCT scan and suffers from non-DOTATATE-avid NET. These results need to be confirmed in a prospective trial.
Main points
Low-dose CT is inferior to contrast-enhanced CT in the detection of extrahepatic metastases.
Contrast-enhanced CT should not be ommitted from clinical screening.
Lung scans in shallow breathing are inferior to lung scans in inspirational breath-hold in the detection of intrapulmonary nodules.
An individualized approach should always be taken when trying to reduce radiation exposure.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ambrosini V, Nanni C, Zompatori M, et al. (68)Ga-DOTA-NOC PET/CT in comparison with CT for the detection of bone metastasis in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2010;37:722–727. doi: 10.1007/s00259-009-1349-9. [DOI] [PubMed] [Google Scholar]
- 2.Putzer D, Gabriel M, Henninger B, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med. 2009;50:1214–1221. doi: 10.2967/jnumed.108.060236. [DOI] [PubMed] [Google Scholar]
- 3.Deroose CM, Hindié E, Kebebew E, et al. Molecular imaging of gastroenteropancreatic neuroendocrine tumors: Current status and future directions. J Nucl Med. 2016 doi: 10.2967/jnumed.116.179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuccurullo V, Faggiano A, Scialpi M, et al. Questions and answers: What can be said by diagnostic imaging in neuroendocrine tumors? Minerva Endocrinol. 2012;37:367–377. [PubMed] [Google Scholar]
- 5.Kwekkeboom DJ, Mueller-Brand J, Paganelli G, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med. 2005;46(Suppl 1):62S–66S. [PubMed] [Google Scholar]
- 6.Zandee WT, de Herder WW. The evolution of neuroendocrine tumor treatment reflected by ENETS guidelines. Neuroendocrinology. 2018;106:357–365. doi: 10.1159/000486096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe JR, Cardona K, Fraker DL, et al. The surgical management of small bowel neuroendocrine tumors: Consensus guidelines of the North American Neuroendocrine Tumor Society. Pancreas. 2017;46:715–731. doi: 10.1097/MPA.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707–714. doi: 10.1097/MPA.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruf J, Schiefer J, Furth C, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: Spotlight on the CT phases of a triple-phase protocol. J Nucl Med. 2011;52:697–704. doi: 10.2967/jnumed.110.083741. [DOI] [PubMed] [Google Scholar]
- 10.Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: Estimation of radiation dose and cancer risk. Radiology. 2009;251:166–174. doi: 10.1148/radiol.2511081300. [DOI] [PubMed] [Google Scholar]
- 11.Persson M, El Ali HH, Binderup T, et al. Dosimetry of 64Cu-DOTA-AE105, a PET tracer for uPAR imaging. Nucl Med Biol. 2014;41:290–295. doi: 10.1016/j.nucmedbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.van der Molen AJ, Reimer P, Dekkers IA, et al. Post-contrast acute kidney injury - part 1: Definition, clinical features, incidence, role of contrast medium and risk factors : Recommendations for updated ESUR Contrast Medium Safety Committee Guidelines. Eur Radiol. 2018;28:2845–2855. doi: 10.1007/s00330-017-5246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer GJ, Mäcke H, Schuhmacher J, Knapp WH, Hofmann M. 68Ga-labelled DOTA-derivatised peptide ligands. Eur J Nucl Med Mol Imaging. 2004;31:1097–1104. doi: 10.1007/s00259-004-1486-0. [DOI] [PubMed] [Google Scholar]
- 14.Albanus DR, Apitzsch J, Erdem Z, et al. Clinical value of 68Ga-DOTATATE-PET/CT compared to stand-alone contrast enhanced CT for the detection of extra-hepatic metastases in patients with neuroendocrine tumours (NET) Eur J Radiol. 2015;84:1866–1872. doi: 10.1016/j.ejrad.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Virgolini I, Ambrosini V, Bomanji JB, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging. 2010;37:2004–2010. doi: 10.1007/s00259-010-1512-3. [DOI] [PubMed] [Google Scholar]
- 16.Shastry M, Kayani I, Wild D, et al. Distribution pattern of 68Ga-DOTATATE in disease-free patients. Nucl Med Commun. 2010;31:1025–1032. doi: 10.1097/MNM.0b013e32833f635e. [DOI] [PubMed] [Google Scholar]
- 17.Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992;158:961–969. doi: 10.2214/ajr.158.5.1566697. [DOI] [PubMed] [Google Scholar]
- 18.Debray MP, Geoffroy O, Laissy JP, et al. Imaging appearances of metastases from neuroendocrine tumours of the pancreas. Br J Radiol. 2001;74:1065–1070. doi: 10.1259/bjr.74.887.741065. [DOI] [PubMed] [Google Scholar]
- 19.Nakamoto Y, Cohade C, Tatsumi M, Hammoud D, Wahl RL. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology. 2005;237:627–634. doi: 10.1148/radiol.2372031994. [DOI] [PubMed] [Google Scholar]
- 20.Chou WC, Hung YS, Hsu JT, et al. Chromogranin a is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology. 2012;95:344–350. doi: 10.1159/000333853. [DOI] [PubMed] [Google Scholar]
- 21.Oberg K. Biochemical diagnosis of neuroendocrine GEP tumor. Yale J Biol Med. 1997;70:501–508. [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz Pérez J, Currás Freixes M. Chromogranin A and neuroendocrine tumors. Endocrinol Nutr. 2013;60:386–395. doi: 10.1016/j.endonu.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Allen-Auerbach M, Yeom K, Park J, Phelps M, Czernin J. Standard PET/CT of the chest during shallow breathing is inadequate for comprehensive staging of lung cancer. J Nucl Med. 2006;47:298–301. [PubMed] [Google Scholar]
- 24.Goerres GW, Burger C, Kamel E, et al. Respiration-induced attenuation artifact at PET/CT: Technical considerations. Radiology. 2003;226:906–910. doi: 10.1148/radiol.2263011732. [DOI] [PubMed] [Google Scholar]
- 25.Bärwolf R, Zirnsak M, Freesmeyer M. Breath-hold and free-breathing F-18-FDG-PET/CT in malignant melanoma-detection of additional tumoral foci and effects on quantitative parameters. Medicine (Baltimore) 2017;96:e5882. doi: 10.1097/MD.0000000000005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balamoutoff N, Serrano B, Hugonnet F, Garnier N, Paulmier B, Faraggi M. Added value of a single fast 20-second deep-inspiration breath-hold acquisition in FDG PET/CT in the assessment of lung nodules. Radiology. 2018;286:260–270. doi: 10.1148/radiol.2017160534. [DOI] [PubMed] [Google Scholar]
- 27.Kandel S, Meyer H, Hein P, Lembcke A, Rueckert JC, Rogalla P. Comparison of free breathing versus breath-hold in perfusion imaging using dynamic volume CT. Insights Imaging. 2012;3:323–328. doi: 10.1007/s13244-012-0169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schraml C, Schwenzer NF, Sperling O, et al. Staging of neuroendocrine tumours: Comparison of [68Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imaging. 2013;13:63–72. doi: 10.1102/1470-7330.2013.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayerhoefer ME, Schuetz M, Magnaldi S, Weber M, Trattnig S, Karanikas G. Are contrast media required for (68)Ga-DOTATOC PET/CT in patients with neuroendocrine tumours of the abdomen? Eur Radiol. 2012;22:938–946. doi: 10.1007/s00330-011-2328-7. [DOI] [PubMed] [Google Scholar]
- 30.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: Comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005;12:1083–1092. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 31.Nanni C, Zompatori M, Ambrosini V, et al. The additional diagnostic value of contemporary evaluation of FDG PET/CT scan and contrast enhanced CT imaging both acquired by a last generation PET/CT system in oncologic patients. Biomed Pharmacother. 2013;67:172–178. doi: 10.1016/j.biopha.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Antoch G, Freudenberg LS, Beyer T, Bockisch A, Debatin JF. To enhance or not to enhance? 18F-FDG and CT contrast agents in dual-modality 18F-FDG PET/CT. J Nucl Med. 2004;45(Suppl 1):56S–65S. [PubMed] [Google Scholar]
- 33.van Hamersvelt HP, Kwee TC, Fijnheer R, Beek FJ, de Klerk JM, Nievelstein RA. Can full-dose contrast-enhanced CT be omitted from an FDG-PET/CT staging examination in newly diagnosed FDG-avid lymphoma? J Comput Assist Tomogr. 2014;38:620–625. doi: 10.1097/RCT.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer NG, Hany TF, Taverna C, et al. Non-hodgkin lymphoma and hodgkin disease: Coregistered FDG PET and CT at staging and restaging--do we need contrast-enhanced CT? Radiology. 2004;232:823–829. doi: 10.1148/radiol.2323030985. [DOI] [PubMed] [Google Scholar]