Abstract
PURPOSE
We have described unidentified bright objects of spleen (UBOS), a hitherto undescribed entity, as hyperdense areas on arterial phase (AP) computed tomography (CT) seen in relation to splenic lacerations and are isodense to the normal parenchyma on portal venous phase with no correlate on digital subtraction angiography (DSA). UBOS mimic splenic vascular injuries like active contrast extravasation and pseudoaneurysm and need to be differentiated from them as it would have implications on patient management. We undertook this study to identify CT features of UBOS that can differentiate them from splenic vascular injuries and to calculate their diagnostic accuracy.
METHODS
This retrospective study was approved by the institutional ethical committee and the need for informed consent was waived. Patients with splenic injury who had undergone dual-phase CT and DSA were included. All the lesions that were hyperdense on AP were evaluated for their outline, their relation to the adjacent/parallel margins of a laceration (margin sign), string of beads appearance, and the presence of adjacent normal parenchyma (adjacent parenchyma sign). The Hounsfield unit (HU) of the lesion and the aorta on the AP were also noted. The diagnostic accuracy of various signs for distinguishing UBOS from splenic vascular injuries was calculated using DSA as the reference standard.
RESULTS
Of 48 patients, 5 were excluded due to suboptimal quality of the examination or a time difference of more than 6 hours between the CT and DSA. A total of 54 hyperdense lesions were detected on AP in 43 patients. These were classified as vascular injuries (pseudoaneurysm, n=11; active contrast extravasation, n=11) and UBOS (n=32) based on DSA. The margin sign, string of beads appearance, and ill-defined outline had high specificity (95%, 86%, and 82%, respectively) but low sensitivity (50%, 65%, and 63%, respectively). The adjacent parenchyma sign had a moderate sensitivity and specificity of 84% and 77%, respectively. ROC analysis showed that a difference of 50 HU between the aorta and the lesion had a high sensitivity and specificity of 88.9% and 90.6%, respectively, with an area under the curve of 0.90.
CONCLUSION
An attenuation difference of over 50 HU between the aorta and the lesion and the presence of normal adjacent parenchyma had the highest diagnostic accuracy, while an ill-defined outline, string of beads appearance, and margin sign had high specificity but low sensitivity for differentiating UBOS from splenic vascular injuries.
Although there is a wide variation in the computed tomography (CT) protocol for the evaluation of blunt abdominal trauma across centers, arterial phase (AP) CT is increasingly being used as part of the evaluation (1–3). AP is usually acquired as a part of whole-body (chest and abdomen) CT angiography followed by a portal venous phase (PVP) acquisition of the abdomen (4–7). AP has been shown to increase the sensitivity of CT for the detection of splenic vascular injuries like pseudoaneurysms (6–9). These appear hyperdense relative to the surrounding parenchyma on AP, leading to better detection rates on AP. However, due to poorly understood mechanisms, the splenic parenchyma shows heterogeneous enhancement in the arterial phase (10–14). This is further exaggerated in the presence of parenchymal injuries like laceration following blunt abdominal trauma leading to the appearance of hyperdense areas on AP which may masquerade as intraparenchymal pseudoaneurysms or active extravasations.
We describe unidentified bright objects of spleen (UBOS) as hyperdense areas seen in relation to splenic lacerations on AP CT which are isodense to the normal parenchyma on PVP with no abnormal correlate on digital subtraction angiography (DSA). As most splenic vascular injuries are hyperdense on AP and some of them isodense on PVP, these UBOS closely mimic splenic vascular injuries (Fig. 1).
Figure 1. a, b.
Illustration depicting the imaging features of unidentified bright objects of spleen (UBOS) and pseudoaneurysm: UBOS (a, asterisk) show ill-defined outline, normal adjacent parenchyma, string of beads appearance due to multiple adjacent lesions, the presence of lesions on adjacent/parallel margins of laceration. Also, UBOS show no communication with the arterial and is less bright than the adjacent arteries (depicting lesser HU). Pseudoaneurysm (b, asterisk) shows a well-defined lesion with no adjacent normal parenchyma in direct communication with an artery. Brown shaded area represents a laceration with intraparenchymal hematoma.
The 2018 revision of the organ injury scale for splenic injuries by the American Association for Surgery in Trauma (AAST) has incorporated CT-diagnosed vascular injuries into the grading system. The grade of injury is upgraded to grade 4/grade 5 if there are associated splenic vascular injuries irrespective of the grade of parenchymal injuries (15–17). Hence, it is imperative to accurately diagnose the splenic vascular injuries on CT and to differentiate UBOS, a previously undescribed entity, from splenic vascular injuries, as it would have implications on the grading of injury and further management.
There are no studies describing such an entity or its imaging features. We undertook this retrospective study to describe CT features of UBOS and to identify features that can differentiate UBOS from pseudoaneurysms or active extravasations and test their diagnostic accuracy.
Methods
This retrospective study was approved by the institutional ethics committee (Ref no. IESC/T-421) and the need for informed consent was waived. A retrospective search of patient records at our level 1 Trauma Center during the period from October 2014 to March 2017 (30 months) was done. All patients who had sustained a splenic injury following blunt abdominal trauma and had undergone a dual-phase CT (AP followed by PVP) and splenic digital subtraction angiography (DSA) as part of their management were included. Exclusion criteria were poor quality of selective splenic angiogram and more than 6 hours between CT scan and DSA.
CT acquisition
All CT examinations were performed on SOMATOM Definition AS (64 detector row scanner, Siemens Medical) or SOMATOM Sensation scanner (40 detector row scanner, Siemens Medical). As a part of our protocol, a dual-phase CT (AP followed by PVP) is performed only in patients over 18 years of age. This was acquired after injecting a bolus of 100 mL of intravenous contrast material (Iohexol, Omnipaque 350, GE Healthcare; containing 350 mg iodine per mL) at a rate of 4 mL/s followed by a saline flush of 20 mL at 4 mL/s using automatic bolus tracking technology. The region of interest (ROI) was placed in the descending aorta just below the level of the diaphragm and above the level of origin of the renal arteries and the threshold for initiation was set at 100 HU. Following this, the PVP images were acquired with a standard delay of 35 seconds after the completion of AP (65–70 s from the start of contrast injection).
Digital subtraction angiography
DSA was performed on the GE Innova (GE Healthcare Technologies) in all patients. Initial celiac artery angiogram was followed by selective splenic angiography which was performed in an anteroposterior projection to look for vascular injury or active contrast extravasation. As a part of our institution protocol, in patients with grade 3 splenic injury with significant hemoperitoneum on CT or with grade 4/5 splenic injury, nonselective proximal splenic artery embolization was performed with endovascular coils even if pseudoaneurysms or active extravasations were not detected on the initial splenic angiography. Selective embolization of the branch artery was done using coaxial microcatheter and micro-coils if pseudoaneurysms or active extravasations were detected on the initial splenic angiography.
Arterial phase analysis
One radiologist (12 years of experience in trauma radiology) who was blinded to all the clinical information and the findings on DSA reviewed the AP of all the patients at our console with multiplanar reconstructions. The AP was reviewed for the presence of any lesion that was hyperdense to the surrounding parenchyma. Both qualitative and quantitative features were noted. Qualitative features included the outline (well defined/ill-defined) of the lesion, its relation to the margins of the laceration, and its relation to surrounding normal parenchyma. Quantitative features included the size of the lesion (three dimensions in orthogonal planes), the attenuation values (HU) of the lesion, and aorta at the same level. Three circular regions of interest (ROIs) were drawn and a mean of the values was used. A minimum area of 1 cm2 was used for the ROIs of the aorta. For the ROIs of the lesion, the area varied depending on the size of the lesion, and care was taken to draw the ROIs to include at least two-thirds of the central portion of the lesion in each ROI.
Comparison with reference standard DSA
A second radiologist (12 years of experience in trauma radiology), who was blinded to all the clinical information and the CT findings, independently reviewed all the DSA images. Selective splenic angiography was used as the reference standard for the diagnosis of vascular injuries. The selective splenic angiograms were reviewed for the presence of vascular injuries and the findings were characterized as normal, pseudoaneurysm, or active extravasation.
In patients with multiple hyperdense lesions on AP, a third radiologist (5 years of experience in trauma radiology) correlated the DSA images with coronal reformatted AP CT images to identify the lesions corresponding to the vascular injuries seen on DSA.
The hyperdense lesions seen on AP with no abnormal correlate on DSA were labeled UBOS. The lesions were classified into two groups for further analysis: UBOS group with a normal angiogram and vascular injury (VI) group with pseudoaneurysms or active extravasations on angiogram.
Statistical analysis
All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 22.0, IBM Corp. For the qualitative features, chi-square test was applied to test the difference between the two groups, and the diagnostic accuracy of the imaging features was also calculated. For quantitative features, unpaired Student’s t-test was used to test for a significant difference between the two groups. The difference in HU of the aorta and the lesion was calculated for both groups. Area under the curve (AUC) for receiver operating characteristic (ROC) curves were calculated to determine the optimal HU difference that could be used for differentiating UBOS from splenic vascular injuries.
Results
A total of 48 patients who had sustained a splenic injury due to blunt abdominal trauma and had undergone a dual-phase CT and a splenic angiography during their stay in the hospital were identified. Five of them were excluded (time difference between the DSA and CT > 6 hours, n=4; poor quality of AP CT due to significant motion-related artifacts impairing proper interpretation, n=1). Hence, a total of 43 patients comprising 34 males (79%) and 9 females (21%) were analyzed (age range, 18–63 years; mean age, 32.9 years). Road traffic accident was the most common mode of injury (n=31, 72.1%), followed by fall from height (n=8, 18.6%) and physical assault (n=4, 9.3%).
Hyperdense AP lesions were seen in 29 of 43 patients (67.4%); 7 patients had 2 lesions each, and 9 patients had 3 lesions each. Thus, a total of 54 AP hyperdense lesions were identified. On DSA, vascular injuries were identified in 22 patients which included 11 pseudoaneurysms and 11 active extravasations. DSA did not reveal any vascular injury corresponding to the other 32 AP hyperdense lesions and hence were labeled as UBOS (Fig. 2).
Figure 2.
Flowchart shows the study groups. CT, computed tomography; DSA, digital subtraction angiography; AP, arterial phase; PA, pseudoaneurysm; AE, active extravasation; UBOS, unidentified bright objects of spleen.
There was no significant difference in demographic factors (age, sex, mode of injury) or clinical characteristics (hemodynamic status, Glasgow coma scale, associated injuries) between patients with UBOS and vascular injuries.
The quantitative and qualitative characteristics of the lesions on AP were compared between the UBOS and VI groups. The results of the qualitative imaging signs in both groups are presented in Table 1; the measures of diagnostic accuracy of each of the signs are presented in Table 2.
Table 1.
Summary of diagnostic performance of the various qualitative imaging features
| Outline | DSA | Total | Presence on adjacent or parallel margins | DSA | Total | ||
|---|---|---|---|---|---|---|---|
| UBOS | VI | UBOS | VI | ||||
| Ill defined | 20 | 4 | 24 | Present | 16 | 1 | 17 |
| Well defined | 12 | 18 | 30 | Absent | 16 | 21 | 37 |
| Total | 32 | 22 | 54 | Total | 32 | 22 | 54 |
| Normal adjacent parenchyma | DSA | Total | String of beads appearance | DSA | Total | ||
| UBOS | VI | UBOS | VI | ||||
| Present | 27 | 5 | 32 | Present | 21 | 3 | 24 |
| Absent | 5 | 17 | 22 | Absent | 11 | 19 | 30 |
| Total | 32 | 22 | 54 | Total | 32 | 22 | 54 |
DSA, digital subtraction angiography; UBOS, unidentified bright objects of spleen group (normal DSA); VI, vascular injury group (pseudoaneurysm or active extravasation on DSA).
Table 2.
Measures of diagnostic accuracy of the imaging features
| AP features | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| Outline | 62.5 (43.7–78.9) | 81.8 (59.7–94.8) | 59.2 (45.0–72.4) | 83.3 (66.5–92.7) | 70.4 |
| Normal adjacent parenchyma | 84.4 (67.2–94.7) | 77.3 (54.6–92.2) | 84.4 (71.1–92.2) | 77.3 (59.6–88.7) | 81.5 |
| Presence on adjacent or parallel margins | 50.0 (31.8–68.1) | 95.5 (77.1–99.9) | 94.1 (69.6–99.1) | 56.7 (47.8–65.3) | 68.5 |
| String of beads appearance | 65.6 (46.8–81.4) | 86.4 (65.1–97.1) | 87.5 (70.4–95.4) | 63.3 (51.0–74.1) | 74.1 |
| HU of aorta - HU of lesion (cutoff 50 HU) | 88.9 (74.9–98.0) | 90.6 (70.8–98.9) | 93.6 (79.4–98.2) | 86.9 (86.9–95.2) | 90.4 |
AP, arterial phase CT; PPV, positive predictive value; NPV, negative predictive value; HU, Hounsfield units.
An ill-defined outline was present in 20 of 32 lesions (63%) in the UBOS group, while it was seen in only 4 of 22 lesions (18%) in the VI group, resulting in a low sensitivity and high specificity (63% and 82%, respectively) (Fig. 3).
Figure 3. a–c.
Typical features of UBOS in 3 different patients (a–c): normal adjacent parenchyma (thin arrows), string of beads appearance (arrowhead), the presence of lesions on adjacent/parallel margins of a laceration (open arrows). All these lesions were isodense on portal venous phase and splenic angiography did not show any vascular injury (not shown).
Normal adjacent parenchyma was seen in 27 of 32 lesions (84%) in the UBOS group and 5 of 22 lesions (22.7%) in the VI group, leading to a sensitivity of 84% and specificity of 77%. Location on the adjacent or parallel margins of a laceration was present in 16 lesions (50%) in the UBOS group and only 1 lesion (4.5%) in the VI group, leading to a high specificity of 95%, but a low sensitivity of 50%. Similarly, the string of beads appearance was seen in 21 lesions (65.6%) in the UBOS and only 3 lesions in the VI group leading to a high specificity of 86% and a moderate sensitivity of 65%.
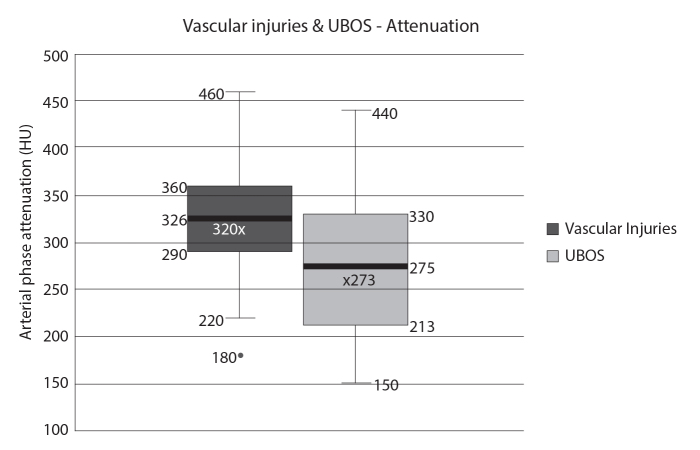
Among the quantitative features, the mean size was smaller and attenuation on AP was less in the UBOS group. However, there was significant overlap in these values, with no significant difference between the two groups (mean size, 14 mm in VI group vs. 6 mm in UBOS group, p = 0.34; mean attenuation, 320 HU in VI group vs. 273 HU in UBOS group, p = 0.1). This is demonstrated in the box and whisker plot for attenuation values of the lesions in the two groups (Fig. 4).
Figure 4.
Box and whisker plot of the attenuation values of the vascular injuries and the UBOS shows a significant overlap of the values between the 2 groups although the mean attenuation was lower in the UBOS group.
There was a significant difference between the two groups when the difference in HU between the aorta and the lesion was measured (mean attenuation difference, 40 HU in the VI group vs. 117 HU in the UBOS group; p = 0.001). ROC analysis showed that the optimal cutoff for differentiating between the two groups was 50 HU (Fig. 5). An attenuation difference of over 50 HU between the aorta and the lesion (Youden index) showed high sensitivity and specificity (88.9% and 90.6%, respectively) for detecting UBOS, with AUC of 0.896 (95% confidence interval, 0.776–0.964).
Figure 5.
Receiver operating characteristic curve for difference in attenuation between aorta and lesion.
Discussion
UBOS is a hitherto undescribed radiological entity that mimics splenic vascular injury on AP CT. There is no histopathological correlate described thus far. Hence, we consider it to be a radiological pitfall of AP CT in the CT evaluation of splenic trauma.
Microscopically, a splenic laceration shows hemorrhage and neutrophilic infiltrate at the site of laceration with congestion of the surrounding red pulp (18). These focal areas of congestion of the red pulp likely exacerbate the heterogeneous splenic parenchymal enhancement on AP CT leading to focal hyperdense areas along the edges of a laceration. We postulated that UBOS represent these areas of congestion of the red pulp adjacent to a laceration. Thus, we postulated that UBOS are more likely to be ill-defined, present adjacent to one another, along the edges of a laceration, unlike a pseudoaneurysm which is usually well defined and usually surrounded by a hematoma with no normal surrounding parenchyma.
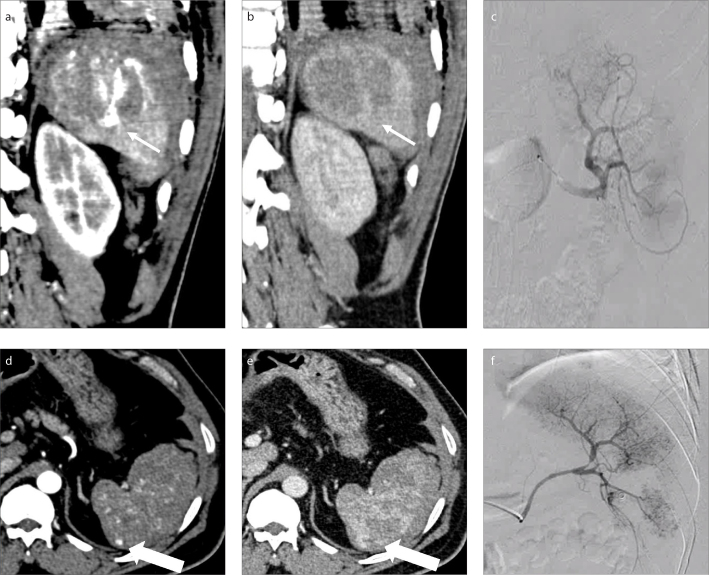
Among the qualitative features, the presence of ill-defined outline and the presence in relation to the margins of a laceration had the lowest diagnostic accuracy. However, the latter showed a very high specificity (95.5%) and positive predictive value (94.1%). Hence, the presence of a lesion in relation to the margins of a laceration would help in diagnosing UBOS with greater certainty (Fig. 6).
Figure 6. a–f.
Typical UBOS in 2 different patients: patient 1 (a–c, thin arrows); patient 2 (d–f, thick arrows). An ill-defined AP hyperdense lesion on the arterial phase (a, d) in relation to the margin of a laceration, which was isodense on the portal venous phase (b, e). Selective splenic angiogram (c, f) showed no vascular injury in the patients.
Among the quantitative features, there was a significant overlap in the size and the absolute attenuation of UBOS and VI groups. The former could be attributed to the wide range of size of pseudoaneurysms that can be seen in the trauma setting (2–18 mm in this study), while the latter could be attributed to the variation in the hemodynamic status of the patients. Although all patients were imaged with the same protocol, there could be variations in the heart rate, blood pressure, and degree of peripheral and splanchnic vasoconstriction, thus altering the contrast dynamics.
Hence, we used the attenuation of the aorta as an internal reference standard and the difference in attenuation between the aorta and the lesion was calculated. The optimal cutoff based on ROC curve analysis was estimated as 50 HU. A pseudoaneurysm is in direct continuity with arterial vascular tree and hence its attenuation value on CT would match that of the aorta (19). However, UBOS, which are likely areas of congestion with no direct continuity with the vascular tree are expected to have lower HU than aorta on CT.
There are a few limitations to this study. First, DSA done within 6 hours was used as a reference standard. Severe vasospasm of the affected vessel, intermittent contrast extravasation, or spontaneous thrombosis of the vascular injury (small vessels) during the time interval between the CT and the DSA can lead to a false negative DSA (20, 21). Second, this was a retrospective analysis. Hence, a direct effect on patient management could not be assessed. Third, as an institution protocol, dual-phase CT is performed only in patients over the age of 18. Thus, the presence or absence of UBOS in the pediatric population cannot be ascertained.
In conclusion, we described a new entity, unidentified bright objects of spleen, seen in the setting of blunt abdominal trauma on AP CT, which lacks a described histopathological or angiography correlate. We also described features to differentiate these from vascular injury and presented their diagnostic performance. A difference of over 50 HU between the aorta and the lesion and the presence of normal adjacent parenchyma had the highest diagnostic accuracy, while an ill-defined outline, string of beads appearance and the margin sign had high specificity but low sensitivity for differentiating UBOS from splenic vascular injuries. Radiologists should be aware of this radiological pitfall while analyzing splenic injuries in the trauma setting. Differentiating UBOS from true splenic vascular injuries is possible with the above-described features with reasonable accuracy.
Main points
In this retrospective analysis, we have described a new entity “unidentified bright objects of spleen” (UBOS) which are seen in the setting of blunt abdominal trauma on the arterial phase CT and lack a described histopathological or angiography correlate.
UBOS closely mimic splenic vascular injuries like active contrast extravasation and pseudoaneurysms.
A difference of over 50 HU between the aorta and the lesion and the presence of normal adjacent parenchyma have the highest diagnostic accuracy for differentiating UBOS from vascular injuries.
Radiologists should be aware of this potential radiological pitfall while analyzing splenic injuries and note that these can be differentiated from splenic vascular injuries with the above-described signs with reasonable accuracy.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hinzpeter R, Boehm T, Boll D, et al. Imaging algorithms and CT protocols in trauma patients: survey of Swiss emergency centers. Eur Radiol. 2017;27:1922–1928. doi: 10.1007/s00330-016-4574-1. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Teoh WC, Chin FWK, Tirukonda PS, Cheong SCW, Yiin RSZ. CT of blunt splenic injuries: what the trauma team wants to know from the radiologist. Clin Radiol. 2019;74:903–911. doi: 10.1016/j.crad.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill SB, Hamid S, Nicolaou S, Qamar SR. Changes in approach to solid organ injury: what the radiologist needs to know. Can Assoc Radiol J. 2020;71:352–361. doi: 10.1177/0846537120908069. [DOI] [PubMed] [Google Scholar]
- 4.Iacobellis F, Ierardi AM, Mazzei MA, et al. Dual-phase CT for the assessment of acute vascular injuries in high-energy blunt trauma: the imaging findings and management implications. Br J Radiol. 2016;89:20150952. doi: 10.1259/bjr.20150952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton JD, Kumaravel M, Censullo ML, Cohen AM, Kievlan DS, West OC. Multidetector CT evaluation of active extravasation in blunt abdominal and pelvic trauma patients. Radiographics. 2008;28:1603–1616. doi: 10.1148/rg.286085522. [DOI] [PubMed] [Google Scholar]
- 6.Uyeda JW, LeBedis CA, Penn DR, Soto JA, Anderson SW. Active hemorrhage and vascular injuries in splenic trauma: utility of the arterial phase in multidetector CT. Radiology. 2014;270:99–106. doi: 10.1148/radiol.13121242. [DOI] [PubMed] [Google Scholar]
- 7.Boscak AR, Shanmuganathan K, Mirvis SE, et al. Optimizing trauma multidetector CT protocol for blunt splenic injury: need for arterial and portal venous phase scans. Radiology. 2013;268:79–88. doi: 10.1148/radiol.13121370. [DOI] [PubMed] [Google Scholar]
- 8.Melikian R, Goldberg S, Strife BJ, Halvorsen RA. Comparison of MDCT protocols in trauma patients with suspected splenic injury: superior results with protocol that includes arterial and portal venous phase imaging. Diagn Interv Radiol. 2016;22:395–399. doi: 10.5152/dir.2016.15232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64-detector row CT. Radiology. 2008;246:410–419. doi: 10.1148/radiol.2462070082. [DOI] [PubMed] [Google Scholar]
- 10.Sauter AW, Spira D, Schulze M, Horger MS. Explanations for the heterogeneity of splenic enhancement derived from blood flow kinetic measurements using dynamic contrast-enhanced CT (DCE-CT) Acta Radiol. 2014;55:645–653. doi: 10.1177/0284185113503322. [DOI] [PubMed] [Google Scholar]
- 11.Miles KA, McPherson SJ, Hayball MP. Transient splenic inhomogeneity with contrast-enhanced CT: mechanism and effect of liver disease. Radiology. 1995;194:91–95. doi: 10.1148/radiology.194.1.7997588. [DOI] [PubMed] [Google Scholar]
- 12.Kühn J-P, Hegenscheid K, Siegmund W, Froehlich C-P, Hosten N, Puls R. Normal dynamic MRI enhancement patterns of the upper abdominal organs: gadoxetic acid compared with gadobutrol. AJR Am J Roentgenol. 2009;193:1318–1323. doi: 10.2214/AJR.09.2412. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly LF, Foss JN, Frush DP, Bisset GS. Heterogeneous splenic enhancement patterns on spiral CT images in children: minimizing misinterpretation. Radiology. 1999;210:493–497. doi: 10.1148/radiology.210.2.r99fe16493. [DOI] [PubMed] [Google Scholar]
- 14.McCormick PA, Malone DE, Docherty JR, Kiat C, Christopher BT, Chin JL. Patterns of splenic arterial enhancement on computed tomography are related to changes in portal venous pressure. Eur J Gastroenterol Hepatol. 2019;31:352–356. doi: 10.1097/MEG.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 15.Kozar RA, Crandall M, Shanmuganathan K, et al. Organ injury scaling 2018 update: Spleen, liver, and kidney. J Trauma Acute Care Surg. 2018;85:1119–1122. doi: 10.1097/TA.0000000000002058. [DOI] [PubMed] [Google Scholar]
- 16.Moore EE, Shackford SR, Pachter HL, et al. Organ injury scaling: spleen, liver, and kidney. J Trauma. 1989;29:1664–1666. doi: 10.1097/00005373-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision) J Trauma. 1995;38:323–324. doi: 10.1097/00005373-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Al-Kindi H, Devi L, George M. Splenic pathology in traumatic rupture of the spleen: a five year study. Oman Med J. 2009;24:81–83. doi: 10.5001/omj.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atluri S, Richard HM, Shanmuganathan K. Optimizing multidetector CT for visualization of splenic vascular injury. Validation by splenic arteriography in blunt abdominal trauma patients. Emerg Radiol. 2011;18:307–312. doi: 10.1007/s10140-011-0961-8. [DOI] [PubMed] [Google Scholar]
- 20.Chastang L, Bège T, Prudhomme M, et al. Is non-operative management of severe blunt splenic injury safer than embolization or surgery? Results from a French prospective multicenter study. J Visc Surg. 2015;152:85–91. doi: 10.1016/j.jviscsurg.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Muroya T, Ogura H, Shimizu K, et al. Delayed formation of splenic pseudoaneurysm following nonoperative management in blunt splenic injury: multi-institutional study in Osaka, Japan. J Trauma Acute Care Surg. 2013;75:417–420. doi: 10.1097/TA.0b013e31829fda77. [DOI] [PubMed] [Google Scholar]