Abstract
PURPOSE
This study was planned to assess the application of three-dimensional (3D) cardiac modeling in preoperative evaluation for complex congenital heart surgeries.
METHODS
From July 2015 to September 2019, 18 children diagnosed with complex congenital heart diseases (CHDs) were enrolled in this study (double outlet right ventricle in nine patients, complex types of transposition of the great arteries in six patients, congenitally corrected transposition of the great arteries in two patients, and univentricular heart in one patient). The patients’ age ranged from 7 months to 19 years (median age, 14 months). Before the operation, 3D patient-specific cardiac models were created based on computed tomography (CT) data. Using each patient’s data, a virtual computer model (3D mesh) and stereolithographic (SLA) file that would be printed as a 3D model were generated. These 3D cardiac models were used to gather additional data about cardiac anatomy for presurgical decision-making.
RESULTS
All 18 patients successfully underwent surgeries, and there were no mortalities. The 3D patient-specific cardiac models led to a change from the initial surgical plans in 6 of 18 cases (33%), and biventricular repair was considered feasible. Moreover, the models helped to modify the planned biventricular repair in five cases, for left ventricular outflow tract obstruction removal and ventricular septal defect enlargement. 3D cardiac models enable pediatric cardiologists to better understand the spatial relationships between the ventricular septal defect and great vessels, and they help surgeons identify risk structures more clearly for detailed planning of surgery. There was a strong correlation between the models of the patients and the anatomy encountered during the operation.
CONCLUSION
3D cardiac models accurately reveal the patient’s anatomy in detail and are therefore beneficial for planning surgery in patients with complex intracardiac anatomy.
Congenital heart diseases (CHDs), especially complex ventricular–arterial (VA) relationships (double outlet right ventricle [DORV], complex types of transposition of the great arteries [TGA], and congenitally corrected TGA [c-TGA]) are a heterogeneous and complex group of cardiac malformations. The planning of an optimal surgical repair of some of these pathologies requires a clear and complete understanding of spatial relationships; hence, they sometimes require advanced diagnostic imaging (1). It is important to reveal the anatomy and three-dimensional (3D) spatial relationships of cardiac structures before the ultimate decision is reached on whether to perform a single ventricular or biventricular repair.
Before surgical procedures, the primary noninvasive and widely used diagnostic tool is echocardiography (2–4). While most decisions for treatment can be made with echocardiography (5), it may not be sufficient for decision-making in some complex CHDs, especially with complex VA relationships. In particular, the spatial relationship of great vessels and ventricular septal defects (VSD) is difficult to determine with echocardiography (6). Computed tomography angiography (CTA) has been widely used for the diagnosis of CHDs, and in some instances, it may eliminate the anatomical shortcomings of echocardiography (7). However, even CTA may not provide sufficient data on intracardiac anatomy, particularly regarding the relationship of VSD with great arteries (6). This, in turn, has resulted in an increased need for advanced diagnostic imaging and additional engineering techniques to achieve adequate presurgical planning, particularly before biventricular surgical repair.
3D cardiac modeling (i.e., 3D virtual intracardiac modeling and printing techniques) is an innovative technology that involves computer-aided processing of 3D imaging data for physical outputs of virtual objects (8–10). More advanced 3D imaging can provide significant information on complex VA relationships and help to select the appropriate surgical procedure considering the complexity of the spatial planes in complex CHDs (11). The relationship between great vessels, VSDs, and semilunar valves can be clearly identified and the suitability of a left ventricular (LV)–aortic tunnel can be confirmed with 3D cardiac modeling (12). Numerous authors have reported the benefits of 3D cardiac modeling, and this approach has been a helpful diagnostic tool for presurgical decision-making in many centers worldwide (1, 13, 14). However, to our knowledge, there have been no studies conducted in our country examining the use of 3D cardiac model techniques (i.e., 3D virtual intracardiac modeling and printing techniques) for presurgical decision-making with complex CHDs. In this retrospective study, we share our experience with surgical planning based on 3D cardiac modeling for complex CHDs and introduce 3D cardiac modeling as a valuable tool in presurgical decision-making in complex CHDs to be adopted throughout the country.
Methods
After approval from the hospital ethical board (2018–40), all CHD patients who had 3D cardiac modeling prior to surgical intervention between July 2015 and September 2019 were retrospectively evaluated. All patients signed an informed consent form. Enrolled in this study were 18 children diagnosed with complex CHDs who underwent surgical treatment. Demographic, clinical, and imaging data were collected from the database of the hospital. All patients had previously undergone extensive clinical evaluation and cardiovascular imaging and were then discussed and reviewed by the cardiac team (congenital heart surgeons, pediatric cardiologists, and radiologists) for the planning of surgical options. The decisions were reached after the complete review of all echocardiography, CTA, and/or cardiac catheterization data.
Multidetector CTA examinations were performed with the use of a 320-row multidetector scanner (Aquilion ONE, Toshiba Medical Systems) with a gantry rotation time of 350 ms and temporal resolution of 175 ms. The voltage and tube current were adjusted to the patient’s weight (80 kV dosage was used for patients weighing <20 kg, and 100 kV for those weighing 20 to 80 kg; tube current was 10 mA/kg for patients weighing <9 kg, and 5 mA for each additional kg). The imaging data were obtained during intravenous injection of 1 to 1.5 mL/kg of the contrast agent iohexol at a rate of 1 to 3 mL/s for children and manual intravenous injection of the drug in newborns and children under 1 year old (Iohexol, Omnipaque 300 mg/mL, GE Healthcare). The contrast agent was removed with 4–15 mL saline according to the patient’s weight. Images were reconstructed to 1 mm in thickness and to a reconstruction interval with a 25f kernel filter; processed on a separate workstation (Vitrea, Vital Images) with multiplanar reformatting, maximum-intensity projection, and volume rendering.
Advanced 3D examinations were planned for patients with different possible surgical options and when the cardiac team was uncertain about the initial surgical decisions. 3D cardiac models were performed to gather extra insight into the cardiovascular anatomy and particularly to define the locations of the VSDs and their relationship with the great vessels and also to avoid postponing the decision to the surgical theatre.
The 3D cardiac models were reconstructed together with Koç University Faculty of Engineering using DICOM (Digital Imaging and Communications in Medicine) images of patients. Virtual 3D cardiac models were created and 3D physical models were printed for 18 patients, all of whom underwent operations. The cardiac team examined the virtual and printed 3D cardiac models both before and after surgery, reviewing them for usefulness in identifying the intracardiac anatomy and for planning potential strategies.
The workflow of the 3D cardiac modeling design based on the patient’s anatomy is shown in Fig. 1. The CTA images were segmented by image processing tools (Mimics Innovation Suite 17, Materialise). After this process, the 3D cardiac model of the blood pool was obtained based on this segmentation, and a mold of the blood pool was generated. The hollow structure in the heart was obtained by subtracting the blood pool model from the mold model using Boolean algorithms in a computer-aided design tool (CAD) (15). The images obtained with the paraseptal and sagittal incisions were determined to be the most suitable in terms of relevant anatomical structures, ventricles, VSDs, and TGA and were easily seen in all of the models. The physical models were created from the virtual model using a 3D printer (Projet 260C, 3D Systems). To represent the true heart anatomy of the patient, myocardial tissue was formed with a separate segmentation and combined with the blood pool model.
Figure 1.
The workflow of the routine procedure.
Although the hollow models to be created from 3D blood pool models, which were confirmed to be consistent with the echo report measurements and that were reconstructed from contrast-dyed sections, can be obtained in the same image processing program by subtracting the blood pool mask from the expanded version, this method was not preferred. With this method, there is a possibility that information about small details and the originality of the patient-specific image data will be lost. Therefore, an offset was added to keep the volume of the blood pool model empty after transferring it to the CAD program in Standard Triangle Language (STL) form. This offset is created with a sensitivity that will not allow for loss of existing details, provided that it is not smaller than 1 mm.
The volumes of the models were compared with two different image segmentation tools for validation and then exported in the STL format, which is the most suitable file type for 3D printers.
Unlike fused deposition modeling (FDM) and stereolithographic (SLA) printers, which are preferred in limited studies on 3D heart anatomy, the Envisiontech Ultra 3SP (Scan Spin and Selectively Photocure) printer was preferred for this study. The lack of stair-stepping on inner and outer surfaces and layerless technology of this printer, which has a smooth voxel resolution of 100 μm, provided smooth results on complex surfaces. By using ABS flex white material, even small details on complex surfaces, both durable and partially flexible, could be created. Problems such as tearing of the hollow structures and fractures during the curing process encountered with SLA printers in the elevator of the platform, the support materials being placed in the hollow spaces, and the unwanted layer lines found in FDM printers were eliminated with the selected printer and material.
The measurements from the radiological data have been compared with the corresponding dimensions of the 3D reconstructed and printed models for the validation. Measurements on the 3D model were obtained by orientation to the same position as the measurements on the radiological data or the echo viewing angle. To determine consistency of the model, different sections in the same segment were located on the reconstructed 3D model and their average diameter was taken into consideration.
Although the cost is expensive compared to FDM printers, it is the same as an SLA printer, and results that better served the purpose in terms of production speed, smoothness, and accuracy were obtained with minimal material waste.
Results
Eighteen 3D cardiac models were created in total, with the diagnoses as follows: DORV in nine patients, TGA in six patients, and c-TGA in two patients. The remaining patient had a functional single ventricle (double inlet left ventricle with VA discordance and restrictive VSD). Decisions were made after the evaluation of the 3D cardiac models, and all of the patients were operated on. The median age of the patients at the time of the operation was 14 months (range, 7 months to 19 years), and the median weight was 9.85 kg (range, 5.5–45 kg) (Table 1). No mortality was observed during the median follow-up period of 1.5 years (0.2–4 years).
Table.
Patients’ demographical data, diagnosis, and surgical outcome
| No | Age / Weight | Diagnosis | Past interventions | Surgical options | Initial options | Recommendation after 3D model | Surgery performed |
|---|---|---|---|---|---|---|---|
| 1 | 7 months/ 5.5 kg | DORV, D-malposed great arteries, severe PS with annular hypoplasia, remote VSD | RVOT stenting | BCPS or biventricular repair, LV-aorta tunnel | BCPS | Biventricular repair | Biventricular repair, LV-aorta tunnel and RV-PA conduit |
| 2 | 3 years/ 12 kg | DORV, anteroposterior great arteries, severe PS with annular hypoplasia, perimembranous-inlet VSD | None | BCPS or biventricular repair, LV-aorta tunnel | BCPS | BCPS, RV hypoplasia | BCPS |
| 3 | 9 months/ 10.5 kg | DORV, D-malposed great arteries, severe PS with mild annular hypoplasia, subaortic VSD | None | BCPS or biventricular repair, LV-aorta tunnel | Biventricular repair | Biventricular repair, LV to aorta baffle and RVOTO procedure | Biventricular repair, LV to aorta baffle and RVOTO repair* |
| 4 | 2 years/ 9.7 kg | TGA, anteroposterior great arteries, severe PS with annular hypoplasia, LPA stenosis, subaortic VSD | PDA stenting | BCPS or biventricular repair, LV-aorta tunnel | Biventricular repair | Biventricular repair, LV to aorta baffle and RV to PA conduit | Biventricular repair, LV to aorta baffle and RV to PA conduit |
| 5 | 19 years/ 45 kg | TGA, situs inversus L-malposed great arteries, severe PS with annular hypoplasia, subaortic VSD, dextrocardia | BCPS | Fontan or biventricular repair, LV-aorta tunnel | Fontan | Fontan surgery, aberrant left coronary artery course across the pulmonic valve | Fontan |
| 6 | 7 years/ 22.5 kg | c-TGA, L-malposed great arteries, pulmonary atresia, inlet VSD | PDA stenting, BDGS and leads implantation for CRT | Fontan or double switch operation | Fontan | Fontan, VSD not suitable for LV to aorta baffle | Fontan |
| 7 | 9.5 months/ 9 kg | TGA, D-malposed great arteries, pulmonary atresia, VSD | Central shunt | BCPS or biventricular repair, LV-aorta tunnel | BCPS | BCPS, VSD not suitable for LV to aorta baffle | BCPS |
| 8 | 11 months/ 6 kg | TGA, D-malposed great arteries, severe PS with mild annular hypoplasia, perimembranous outlet VSD | None | BCPS or biventricular repair, LV-aorta tunnel | BCPS | Biventricular repair | Biventricular repair, LV to aorta baffle and RV to PA conduit |
| 9 | 18 years/ 45 kg | c-TGA, situs inversus D-malposed great arteries, severe PS with annular hypoplasia, inlet large VSD | None | BCPS or double switch operation | BCPS | BCPS, VSD not suitable for LV to aorta tunnel | BCPS |
| 10 | 30 months/ 12 kg | DORV, normally related great arteries, mild LV hypoplasia, subaortic large VSD | Pulmonary banding and aortic arch repair | BCPS or biventricular repair, LV-aorta tunnel | BCPS | BCPS, LV hypoplasia | BCPS |
| 11 | 30 months/ 12 kg | DORV, side-by-side great arteries, subvalvular aortic stenosis, large inlet VSD | PA banding | BCPS or biventricular repair, LV-aorta tunnel | Biventricular repair | Biventricular repair, LV to aorta baffle and LVOTO resection | Biventricular repair, LV to aorta baffle and LVOTO resection, debanding |
| 12 | 13 months/ 7 kg | TGA, D-malposed great arteries, severe PS with moderate annular hypoplasia, subaortic VSD, coronary anomaly (1 RCA-LAD, 2 Cx) | PDA stenting | BCPS or biventricular repair, LV-aorta tunnel | Biventricular repair | Biventricular repair, LV to aorta baffle with VSD enlargement and pulmonary root translocation | Biventricular repair LV to aorta baffle with c, and pulmonary root translocation |
| 13 | 4 years/ 18 kg | DILV, VA discordance, systemic outflow obstruction (restrictive BVF or VSD) | Pulmonary banding, BCPS | BVF enlargement or DKS operation | BVF enlargement | BVF enlargement, RV approach and determination of enlargement location | BVF enlargement and Fontan procedure |
| 14 | 12 months/ 7.6 kg | DORV, normally related great arteries, large perimembranous VSD | Pulmonary banding, aortic coarctation repair | BCPS or biventricular repair, LV -aorta tunnel | BCPS | Biventricular repair, LV to aorta baffle and LVOTO resection | Biventricular repair, LV to aorta tunnel and LVOTO resection, PA debanding |
| 15 | 12 months/ 9 kg | TGA, D-malposed great arteries, pulmonary atresia, inlet VSD | Central shunt, PA plasty | BCPS or biventricular repair, LV-aorta tunnel | BCPS | Biventricular repair, LV to aorta tunnel with VSD enlargement and RV to PA conduit | BCPS, remote VSD, VSD not suitable for LV to aorta tunnel |
| 16 | 12 months/ 10 kg | LAI, DORV, severe PS with mild annular hypoplasia, PLSVC | None | Kawashima procedure or biventricular repair, LV-aorta tunnel | Biventricular repair, LV to aorta tunnel | LV-aorta tunnel with VSD enlargement | Biventricular repair by LV to aorta tunnel with VSD enlargement and RVOTO repair* |
| 17 | 15 months/ 9 kg | DORV, side-by-side great arteries, outlet subpulmonic VSD | Pulmonary banding | BCPS or biventricular repair, LV-aorta tunnel or LV-pulmonary tunnel | BCPS | LV to pulmonary tunnel and ASO | LV to pulmonary artery tunnel with ASO, LVOTO resection, RVOTO repair* |
| 18 | 12 months/ 8 kg | DORV, D-malposed great arteries, severe PS with moderate annular hypoplasia, accessory mitral valve tissue, subaortic VSD | None | BCPS or biventricular repair, LV-aorta tunnel | BCPS, small VSD and accessory mitral valve tissue | LV-aorta tunnel with VSD enlargement, and resection accessory mitral valve tissue | Biventricular repair by LV to aorta tunnel with VSD enlargement, and RVOTO repair*, resection accessory mitral valve tissue |
DORV, double-outlet right ventricle; PS, pulmonary stenosis; VSD, ventricular septal defect; RVOT, right ventricular outflow tract; BCPS, bidirectional cavopulmonary anastomosis; LV, left ventricle; RV, right ventricle; PA, pulmonary artery; RVOTO, right ventricular outflow tract obstruction; TGA, transposition of great arteries; LPA, left pulmonary artery; PDA, patent ductus arteriosus; c-TGA, corrected- transposition of great arteries; LVOTO, left ventricular outflow tract obstruction; RCA, right coronary artery; LAD, left anterior descending artery; Cx, circumflex artery; DILV, double inlet left ventricle; BVF, bulboventricular foramen; DKS, Damus-Kaye-Stansel; LAI, left atrial isomerism; PLSVC, persistent left superior vena cava; ASO, arterial switch operation.
Transannular patch repair.
Out of 18 patients, 12 (66.6%) had previously undergone palliative procedures before corrective surgery. Of these patients, two (11%) had a central shunt, two (11%) had patent ductus arteriosus (PDA) stenting, one (2.9%) had right ventricular outflow tract (RVOT) stenting, three (16%) had pulmonary banding, two (11%) had an aortic arch repair and pulmonary banding, and two (11%) had a bidirectional Glenn (BDG) shunt. After 3D cardiac modeling, univentricular heart palliation was performed in six patients (33%; three BDG, three Fontan procedure) and biventricular repair was conducted in 12 patients (67%). All cases survived with good clinical outcomes and no major complications.
The CTA was performed in all patients, and all imaging studies had adequate contrast for image segmentation, 3D volume rendering, and 3D printing (Fig. 2). As an important point, in patients with a BDG shunt, a CTA scan should be evaluated in the venous phase for biventricular filling. Clinical details and anatomical diagnoses are summarized in Table 1. The cardiac team and an imaging engineer reviewed all images of cases prior to surgery. For surgical planning, all members of the cardiac team expressed that all individual 3D cardiac models provided a better understanding of the pathological features of CHDs and were useful for making a decision. The team acknowledged that this might be a routine tool for an optimal surgical approach with CHD.
Figure 2. a–f.
The 3D printing protocol of the institute in the steps of dataset selection (a), thresholding (b), segmentation (c), restoration (d), editing with design tools (e), 3D printing (f).
For five patients (Cases 1, 8, 14, 17, and 18) in whom univentricular palliation was initially planned due to the size and location of the VSD, the 3D cardiac model evaluation resulted in a biventricular repair being considered feasible, and the 3D cardiac models helped the surgeon better understand the location of the VSD. The biventricular repair was subsequently chosen and successfully performed in these five cases. In Case 17, a multidisciplinary review of the 3D cardiac model suggested that an arterial switch type of operation would be suitable due to the favorable position of the VSD. In this case, the 3D cardiac model additionally helped to modify the planned biventricular repair for an arterial switch operation with baffling of the VSD to the native pulmonic valve (neo-aortic valve).
In another case (Case 13), the inspection of the 3D cardiac model modified the Fontan operation by not only implantation of the extracardiac tube but also demonstrating the need to enlarge the restrictive VSD. The surgical plan was to perform a Fontan by extracardiac non-fenestrated tube. The 3D cardiac model revealed that the VSD was restrictive and identified the relationship of the VSD and outlet septum. It also helped with performing a subaortic obstruction removal without any complications. In five cases (Cases 11, 12, 14, 17, and 18), the 3D cardiac model helped to modify the planned biventricular repair for left ventricular outflow tract obstruction (LVOTO) removal and VSD enlargement. Specifically, in three of these patients’ anatomies, LVOTO had not been clearly demonstrated with two-dimensional (2D) images before the 3D cardiac modeling.
The surgical plans made with the help of the 3D models were successfully implemented in all cases except one (Case 14). In this case (Case 14), the surgical plan was changed intraoperatively. Case 14 was a 12-month-old male with TGA, perimembranous VSD with inlet extension pulmonary stenosis. The aorta was located to the right and anterior of the pulmonary artery. The patient was initially palliated with a central shunt, left pulmonary artery reconstruction, and PDA closure. The review of CTA, echocardiography, and cardiac catheterization had suggested that anatomy was too complicated to achieve a successful biventricular repair. But after the 3D cardiac model evaluation, the biventricular repair was considered feasible with VSD enlargement. However, intraoperatively, the surgical risk was found to be too high as to preclude biventricular surgery due to the size and location of the VSD, and the surgical team decided to perform BDG shunt after visualization of the VSD during the operation. Four of the cases (Cases 1, 5, 9, and 10) with interesting features are presented in Figs. 3–6. After the operation, surgeons verified that there was a strong correlation between the 3D cardiac models of these patients and the anatomy detected during the operation. The correlation is the relationship between the aorta or pulmonary artery, VSD, and left ventricle which is essential in determining if a straightforward baffle from the left ventricle to one of the great arteries may be created (with or without an arterial switch procedure or VSD enlargement). We did not use any device or patch related to the 3D model in the operation. Because of this, we did not need any quantitative measurement to demonstrate the accuracy of the 3D printed cardiac model.
Figure 3. a–d.
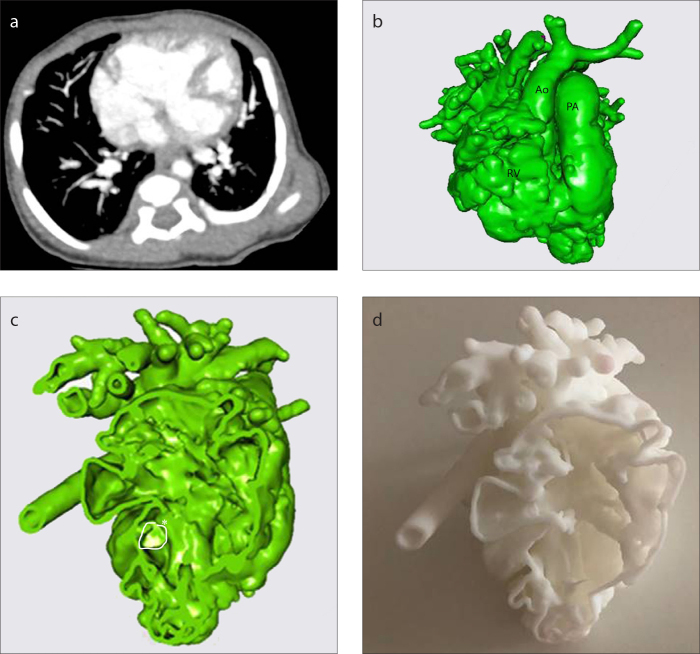
CT scan of Patient 10 (a), 3D virtual heart model (b), intracardiac cross-section (c), 3D replica of the heart (d). See Table for case definition. The LV is hypoplastic and VSD is remote-inlet (c, asterisk), biventricular repair is not feasible. Ao, ascending aorta; PA, pulmonary artery; RV, right ventricle.
Figure 4. a–d.
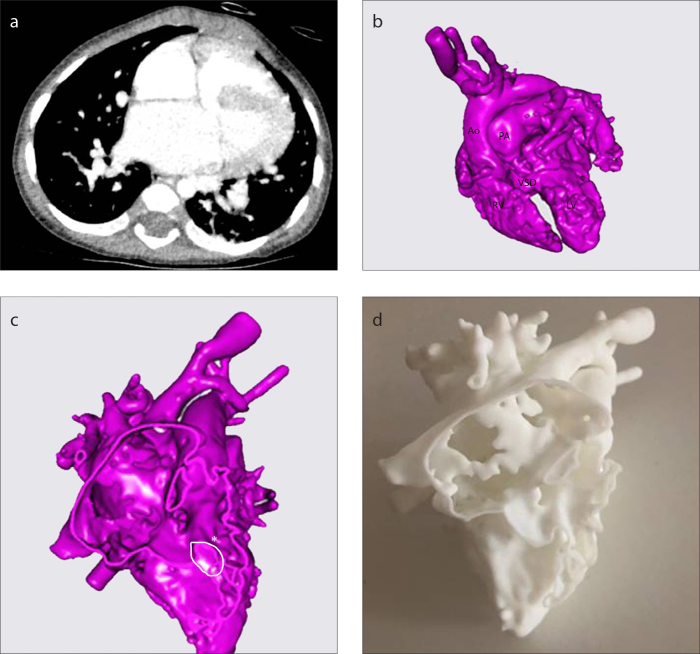
CT scan of Patient 12 (a), 3D virtual heart model (b), intracardiac cross-section (c), 3D replica of the heart (d). See Table for case definition. The 3D model has been extremely helpful to plan biventricular repair with VSD enlargement (c, asterisk). Ao, ascending aorta; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect.
Figure 5. a–d.
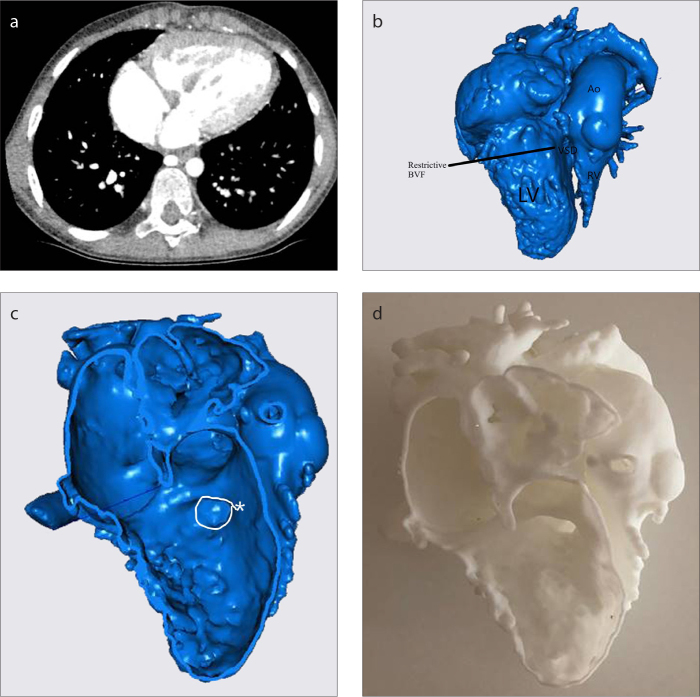
CT scan of Patient 13 (a), 3D virtual heart model (b), intracardiac cross-section (c), 3D replica of the heart (d). See Table for case definition. The 3D model has been extremely helpful to plan transventricular subaortic obstruction (c, asterisk) removal without the AV block and/or injury of structures. Ao, ascending aorta; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect; BVF, bulboventricular foramen.
Figure 6. a–d.
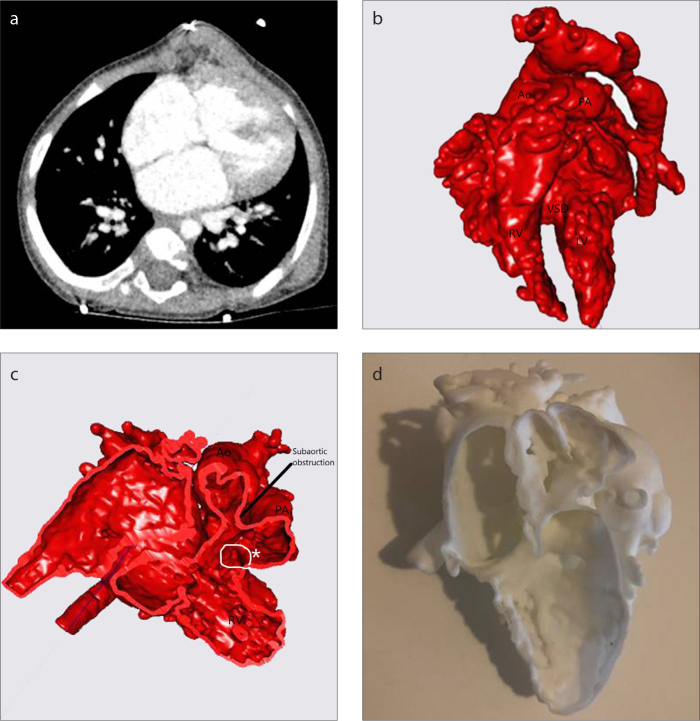
CT scan of Patient 14 (a), 3D virtual heart model (b), intracardiac cross-section (c), 3D replica of the heart (d). See Table for case definition. The 3D model has been extremely helpful to plan biventricular repair with subaortic obstruction removal. Ao, ascending aorta; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; VSD, ventricular septal defect (asterisk).
Discussion
In this study, we shared our experiences related to 3D cardiac modeling (3D virtual intracardiac modeling and printing techniques) for complex CHDs, particularly patients with complex VA relationships, in planning an optimal surgical technique for univentricular or biventricular repair. In addition, we wanted to pioneer the use of this technique for presurgical decision-making in our country and to popularize it with concrete data. To the best of our knowledge, this is the first study in a pediatric patient cohort from Turkey. The first step is to introduce the process, followed by standardization of scanning techniques and post-processing of data sets. Finally, a decision should be made about the appropriate patient selection.
Currently, diagnostic evaluation and preoperative planning of CHD is based on 2D and multisection imaging of volumetric data such as echocardiography, magnetic resonance imaging (MRI), and CTA. The use of these imaging techniques may clarify complex intra-extra cardiac anatomy in most cases, but they may be insufficient to delineate the relationship between VSD and large vessels, particularly for preoperative planning of remote VSD cases (13, 16–18). Due to these known limitations of standard 2D techniques, patient-specific 3D cardiac model for surgical planning has been increasingly used in recent years (19). In light of these data, we started the 3D cardiac modeling with Koç University Faculty of Engineering for complex CHD management in July 2015. During this period, 18 3D cardiac models were created, and all of them were used in operations.
There have been many studies regarding the benefits of 3D cardiac modeling and printing in CHD, in terms of improving medical understanding, teaching, surgical training, and patient information (6, 13, 14). Moreover, it has already been established that these techniques play an irrefutable role in confirming or altering the planned approach and providing data for orientation of a surgically planned interventricular baffle in pediatric cardiac surgery (6, 18, 20). In a recent multicenter study, Valverde et al. (13) reported that 3D models clearly delineated cardiac and vascular anatomy and improved the understanding of complex CHDs. In 50% of the cases in which it had no impact on decision-making, it helped in optimizing surgical planning.
In our study, 3D cardiac models changed the initial surgical plan in 6 out of 18 (33%) cases, and biventricular repair was considered feasible. Five of them were subsequently performed successfully, with only one case (Case 14) where the surgical plan was changed intraoperatively to revert to the initial, pre-3D-model, plan. In addition to these, the 3D models helped to modify planned biventricular repairs in five cases regarding LVOTO removal and VSD enlargement. All members of the cardiac team agreed that the 3D cardiac models provided a better delineation of the VSD anatomy and relationship with the aorta and pulmonary artery for surgical planning. After the operation, the surgeons expressed that there was a strong correlation between the models of these patients and the anatomy observed during the operation. Although it is hard to provide statistical data that 3D-printed models improve operative performance, we think that the use of 3D models in complex CHDs before decision-making aid the pediatric cardiac team and allow for much better mental preparation before surgery (20). Furthermore, it was claimed that the use of 3D models may provide shorter operating and hospitalization times (14). However, we did not evaluate operating time and length of hospitalization for our patients due to the design of this study. Nonetheless, 3D presurgical cardiac models allowed for the examination of internal structure in the desired planes without causing any loss of time in the operating room. Finally, all members of the cardiac team agreed that the 3D cardiac models were helpful for the education of fellows and interdisciplinary communication.
3D modeling is a time-consuming and complex process and is too expensive for practical daily use (21, 22). Imaging involves and integrates the skills of image interpretation and technology with the collaboration of more than one branch (cardiologists, radiologists, CAD software specialists, and 3D printer professionals). Additionally, the efficiency of 3D cardiac models for operative planning may be lower with much simpler CHDs (13). For these reasons, 3D modeling should only be considered for complex CHDs, when needed, after a medical multidisciplinary team meeting. All the cases modeled in our study had definite anatomical complexities that were considered by the pediatric cardiac team to be difficulties for making an optimum surgical decision.
Segmenting the blood pool, intracardiac, and cross-section 3D models created from CTA image datasets took only half a day for each patient in the created workflow plan. Obtaining a 3D intracardiac cross-section replica for each patient from the STL form takes a maximum of six hours. Although FDM printers are preferred in most studies in terms of cost, problems such as inconsistency between the inner and outer surfaces, the appearance of layer lines, and filling of gaps with support material affect the accuracy of the studies. For this reason, it is best to choose a method that can display the speed and the internal cavities of the models in the most accurate way and can be independent of situations such as tearing, breaking, and lack of smoothness.
We think the virtual 3D cardiac model can finely delineate the intracardiac anatomy. It can give enough information to assist with decision-making. Unfortunately, it is not enough for cardiac team communication and education. The main beneficial applications of 3D printing are surgical simulation and teaching. This participation as surgical simulation is another exciting area that can lead to potentially improved surgical outcomes and, therefore, better patient care (23). Even post-surgery, the printed models continue to be used as morphology models to help clinicians understand the complex CHD examples in the training sessions, in case there is no easy access to real anatomic samples. These can also be used as excellent tools for communication with patients and their families and for preoperative counseling.
Our study is limited by the retrospective nature, single-center analysis, and lack of a statistically meaningful endpoint. Other limitations may be related to case selection and randomization. Furthermore, there are no data about cardiopulmonary bypass (CPB), cross-clamping time, length of stay in the hospital, and comparison of surgical repair with and without the 3D model.
There are also many limitations in these 3D cardiac modeling techniques. Some thin and especially mobile structures, such as atrioventricular valves, are difficult to replicate. This is particularly important for a straddling AV valve that may preclude biventricular repair in patients with complex CHDs. For this reason, it should be used as a complement to echocardiography for adequately describing the atrioventricular valves (19). The process of modeling depends on many factors affecting quality, but most important is the quality of the source data provided. At any stage of creating a model, there is a risk of misrepresenting the anatomy. All imaging modalities should be reviewed in order to fully and correctly understand morphological variations (24, 25).
In conclusion, this study demonstrates that 3D cardiac modeling can accurately replicate a patient’s cardiac anatomy and may be beneficial for the preoperative planning of surgery for children with complex CHDs. With patient-specific 3D cardiac models, a patient-based therapeutic strategy can be implemented, and the surgical option can be confirmed, particularly when the VSD position is crucial for biventricular repair. In our experience, it supports the alignment of pediatric cardiologists and surgeons by increasing the strength of interdisciplinary teamwork. These 3D models may also be beneficial for young doctors and surgeons in training. Without a doubt, 3D cardiac modeling is a promising tool for future clinical practice.
Main points
3D cardiac modeling is an innovative production process that uses computer-aided processing of 3D imaging data to create physical outputs of virtual objects for complex congenital heart diseases.
Complex congenital heart diseases provide a unique opportunity to implement 3D modeling technology to eliminate gaps in anatomical detailing, communication, and surgical planning.
In patients with complex ventricular-arterial relationships, 3D cardiac models may help physicians understand the intracardiac anatomy and help with decision-making for surgical planning.
Acknowledgments
The authors thank the radiology department of İstanbul Mehmet Akif Ersoy Cardiovascular Research and Training Hospital.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Vodiskar J, Kütting M, Steinseifer U, Vazquez-Jimenez JF, Sonntag SJ. Using 3D physical modeling to plan surgical corrections of complex congenital heart defects. Thorac Cardiovasc Surg. 2017;65:31–35. doi: 10.1055/s-0036-1584136. [DOI] [PubMed] [Google Scholar]
- 2.Sasson L, Houri S, Raucher SA, et al. Right ventricular outflow tract strategies for repair of tetralogy of Fallot: Effect of monocusp valve reconstruction. Eur J Cardiothorac Surg. 2013;43:743–751. doi: 10.1093/ejcts/ezs479. [DOI] [PubMed] [Google Scholar]
- 3.Li JJ, Liu Y, Xie SY, et al. Newborn screening for congenital heart disease using echocardiography and follow-up at high altitude in China. Int J Cardiol. 2019;274:106–112. doi: 10.1016/j.ijcard.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 4.Perloff JK, Marelli AJ. Perloff’s clinical recognition of congenital heart disease. Elsevier/Saunders; 2012. [Google Scholar]
- 5.Pang KJ, Meng H, Hu SS, et al. Biventricular repair of double outlet right ventricle: preoperative echocardiography and surgical outcomes. World J Pediatr Congenit Heart Surg. 2017;8:354–360. doi: 10.1177/2150135117692973. [DOI] [PubMed] [Google Scholar]
- 6.Bhatla P, Tretter JT, Ludomirsky A, et al. Utility and scope of rapid prototyping in patients with complex muscular ventricular septal defects or double-outlet right ventricle: does it alter management decisions. Pediatr Cardiol. 2017;38:103–114. doi: 10.1007/s00246-016-1489-1. [DOI] [PubMed] [Google Scholar]
- 7.Giannopoulos AA, Chepelev L, Sheikh A, et al. 3D printed ventricular septal defect patch: a primer for the 2015 Radiological Society of North America ( RSNA ) hands-on course in 3D printing. 3D Print Med. 2015;3:31–20. doi: 10.1186/s41205-015-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tappa K, Jammalamadaka U. Novel biomaterials used in medical 3D printing techniques. J Funct Biomater. 2018;9:17. doi: 10.3390/jfb9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia J, Yang Z, Mongrain R, Leask RL, Lachapelle K. 3D printing materials and their use in medical education: a review of current technology and trends for the future. BMJ Simul Technol Enhanc Learn. 2018;4:27–40. doi: 10.1136/bmjstel-2017-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel T, Rivard A, Jimenez A, Mestres CA, Muller S. Medical three-dimensional printing opens up new opportunities in cardiology and cardiac surgery. Eur Heart J. 2018;15:1246–1254. doi: 10.1093/eurheartj/ehx016. [DOI] [PubMed] [Google Scholar]
- 11.Farooqi KM, Gonzalez-Lengua C, Shenoy R, Sanz J, Nguyen K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J Pediatr Congenit Hear Surg. 2016;3:414–416. doi: 10.1177/2150135115610285. [DOI] [PubMed] [Google Scholar]
- 12.Hibino N. Three dimensional printing. World J Pediatr Congenit Hear Surg. 2016;3:351–352. doi: 10.1177/2150135116644886. [DOI] [PubMed] [Google Scholar]
- 13.Valverde I, Gomez-Ciriza G, Hussain T, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg. 2017;6:1139–1148. doi: 10.1093/ejcts/ezx208. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Zhou S, Fan T, Li B, Liang W, Dong H. Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. J Card Surg. 2018;33:24–27. doi: 10.1111/jocs.13523. [DOI] [PubMed] [Google Scholar]
- 15.Barnett E, Gosselin C. Weak support material techniques for alternative additive manufacturing materials. Additive Manuf. 2015;8:95–104. doi: 10.1016/j.addma.2015.06.002. [DOI] [Google Scholar]
- 16.Farooqi KM, Lengua CG, Weinberg AD, Nielsen JC, Sanz J. Blood Pool Segmentation Results in Superior Virtual Cardiac Models than Myocardial Segmentation. Pediatr Cardiol. 2016;6:1028–1036. doi: 10.1007/s00246-016-1385-8. [DOI] [PubMed] [Google Scholar]
- 17.Yoo SJ, Thabit O, Kim EK, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015;2:3. doi: 10.1186/s41205-016-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garekar S, Bharati A, Chokhandre M, et al. Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J Pediatr Congenit Heart Surg. 2016;7:344–350. doi: 10.1177/2150135116645604. [DOI] [PubMed] [Google Scholar]
- 19.Batteux C, Haidar MA, Bonnet D. 3D-printed models for surgical planning in complex congenital heart diseases: a systematic review. Front Pediatr. 2019;7:23. doi: 10.3389/fped.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shearn AIU, Yeong M, Richard M, et al. Use of 3D models in the surgical decision-making process in a case of double-outlet right ventricle with multiple ventricular septal defects. Front Pediatr. 2019;7:330. doi: 10.3389/fped.2019.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farooqi KM, Uppu SC, Nguyen K, et al. Application of virtual three-dimensional models for simultaneous visualization of intracardiac anatomic relationships in double outlet right ventricle. Pediatr Cardiol. 2016;37:90–98. doi: 10.1007/s00246-015-1244-z. [DOI] [PubMed] [Google Scholar]
- 22.Hoashi T, Ichikawa H, Nakata T, et al. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact Cardiovasc Thorac Surg. 2018;27:749–755. doi: 10.1093/icvts/ivy160. [DOI] [PubMed] [Google Scholar]
- 23.Christensen A, Rybicki FJ. Maintaining safety and efficacy for 3D printing in medicine. 3D Print Med. 2017;3:1. doi: 10.1186/s41205-016-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau I, Sun Z. Three-dimensional printing in congenital heart disease: A systematic review. J Med Radiat Sci. 2018;65:226–236. doi: 10.1002/jmrs.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farooqi KM. Rapid prototyping in cardiac disease. Springer International Publishing; 2017. [DOI] [Google Scholar]