Abstract
Objective Endovascular treatment of carotid-cavernous fistulas (CCFs) has been consistently shown to give excellent results and is currently the mainstay of treatment of these complex vascular pathologies. Onyx is currently the most widely used agent, but there has been concern over high rates of cranial nerve (CN) deficits seen in patients with CCF treated with Onyx and paucity of data on long-term outcomes.
Methods This is a retrospective analysis of patients who underwent transvenous Onyx embolization between 2011 and 2018. The data collected included demographics, comorbidities, presenting symptoms, CCF morphology, degree of obliteration, procedure-related complications, clinical outcomes, and follow-up.
Results A total of seven patients (five females) were included. The median age was 66 years (range: 15–79 years). Median duration of symptoms before treatment was 4 weeks (range: 1–24 weeks). There were three direct and four indirect CCFs. Barrow classification is as follows: A-3; B-3; C-0; and D-1. Immediate complete occlusion was achieved in all cases. There was also one case of immediate postoperative change in CN function (new partial CN VI deficit) that resolved completely at 1-month follow-up. The mean length of stay was 3 days (±2). The preoperative extraocular movement CN deficits had the following outcomes: three resolved; two improved; and one persisted. Proptosis, chemosis, conjunctival injection, and tinnitus were resolved in all patients. The median follow-up was 34 months (range: 10–91 months).
Conclusion Transvenous Onyx embolization is a safe and effective treatment of CCFs when technical aspects to reduce complications are performed diligently. Our technique demonstrates safety of the Onyx as a stand-alone embolization for the treatment of CCF.
Keywords: carotid-cavernous fistula, endovascular, transvenous embolization, ethylene vinyl alcohol, dimethyl sulphoxide
Introduction
Carotid-cavernous fistulas (CCFs) are abnormal arteriovenous communications between the carotid arterial system and the cavernous sinus. CCFs have a wide range of clinical presentation from a grotesque-appearing red pulsating eye to a subtle conjunctival congestion. Various classification systems exist based on etiology (traumatic vs. spontaneous), hemodynamics (high vs. low flow), and angiographic anatomy (direct vs. indirect, Barrow classification system). 1 2 The treatment of CCFs has had a paradigm shift in the last few decades. Current treatment of CCFs is mostly done through a variety endovascular techniques and embolization materials, which achieve high success rate with minimal mortality and morbidity. 3 4 5 6
The use of ethylene vinyl alcohol copolymer (branded in the United States as Onyx, Medtronic Neurovascular, Irvine, California, United States) for embolization of cerebral arteriovenous malformations was first approved by the Food and Drug Administration in 2005. Its applications have expanded to include CCFs, dural arteriovenous fistulas, and other intracranial vascular pathologies. The distinct advantages of Onyx are that it is nonadhesive and is relatively radio opaque due to the added tantalum. Its initial use in CCFs showed good results for early occlusion of the fistula, but was associated with significant procedural-related cranial neuropathies (25%). 7 This has led neurointervention surgeons to a more guarded approach in using Onyx as a stand-alone embolization agent in CCFs and it is usually used in combination with other embolization materials or devices. In addition, there is a dearth of literature on mid- and long-term outcomes of Onyx embolization for CCFs. 5 6 In this study, we report our experience and cranial nerve (CN) deficit outcomes with transvenous embolization using Onyx as the sole embolic agent for the management of CCFs.
Methods
This is an Institutional Review Board approved, single institution, retrospective study of patients who underwent transvenous Onyx embolization for CCFs, from 2011 to 2018. The information collected from patient's electronic medical records included: demographics, presenting symptoms, neuro-ophthalmological exam, CCF morphology, operative notes, degree of obliteration, postoperative course, clinical follow-up, and radiological imaging. Each patient's length of hospital stay and complications (directly or indirectly) during the postoperative course were noted. Neuro-ophthalmological assessment included visual acuity, perimetry, and ocular movement, with or without diplopia at maximal gaze in all directions. The extraocular movement (EOM) deficits were differentiated into CN III (oculomotor), CN IV (trochlear), and CN VI (abducens) deficits. The preoperative neuro-ophthalmological exam was compared with the first postoperative outpatient visit (between 3 and 6 weeks) and last follow-up exams. The modified grading system for binocular vision (ocular motility) as described by Akutsu et al 8 was used to compare outcomes for the patients with EOM CN deficits.
Results
Clinical Features
A total of seven patients (two males and five females) were included. The median age was 66 years (range: 15–79 years). The presenting symptoms were CN deficit in six patients (86%), proptosis in five patients (71%), chemosis in three patients (43%), conjunctival injection in two patients (29%), and tinnitus in one patient (14%). With regard to the preoperative EOM function, four patients had only CN III deficit and two patients had only CN VI deficit. The median time to onset of symptoms at presentation was 9.3 weeks (range: 1–24 weeks).
CCF Morphology
There were six spontaneous and one traumatic CCFs. There were three direct and four indirect CCFs. Our series included three cases of Barrow type A, three cases of Barrow type B, and one case of Barrow type D.
The locations (segments/branches) of the internal carotid artery (ICA) fistula in the Barrow type A CCFs were: two at the ascending segment of the cavernous ICA and one at the horizontal segment of the cavernous ICA. All the three Barrow type B CCFs had communication from the meningohypophiseal trunk at the posterior bend of the cavernous ICA. The Barrow type D CCF had the ICA component of the fistula at the meningohypophiseal trunk and the ECA component coming from the meningeal branches of the internal maxillary artery.
A venous drainage component through the superior orbital vein was present in all cases. In addition, there were venous drainage through the inferior petrosal sinus (IPS) in three cases, pterygoid plexus in one case, and frontal anastomotic vein in one case.
Operative Technique
All procedures are performed in a single session under general anesthesia and continuous electrophysiological monitoring of electroencephalography (EEG), motor evoked potentials (MEPs), and somatosensory evoked potentials (SSEP).
We first obtained 6F arterial and venous femoral access. Subsequently, we navigated two Envoy (DePuy Synthes, Raynham, Massachusetts, United States) 6F guide catheters: one for arterial access to the ICA and the other for venous access to the transverse and sigmoid sinus. A transform balloon microcatheter (Stryker Neurovascular, Fremont, California, USA) was advanced in the arterial side of the CCF for ICA protection when necessary during embolization.
An Echelon 10 (Medtronic Neurovascular, Irvine, California, United States) microcatheter was used for access to the cavernous sinus usually through IPS. The embolization was always via venous approach and under constant biplane fluoroscopy using blank roadmap technique. We used Onyx 18 as embolic agent in all procedures.
Onyx injections were performed in a slow and controlled fashion, particularly in the beginning of the embolization when there is extra dimethyl sulphoxide (DMSO) that is used to fill the catheter dead space. For the treatment of indirect CCF, this injection technique with pauses for Onyx cast stability permitted control of the embolic agent flow into the desired target/space.
For the treatment of direct CCF, the balloon protection in the ICA is kept up for at least 1 minute after the Onyx injection is finished. For final Onyx injections, when most of the cast is formed, we would wait up to 5 minutes to deflate the balloon. We found sufficient time for Onyx cast stability.
It is also important to slightly pull back on the Onyx syringe plunger to prevent any forward flow in between embolization injections and during the process of balloon deflation.
Neurophysiology monitoring (SSEP, MEP, and EEG) is crucial in this procedure to assess patency of collateral flow through the circle of Willis and guide timing for balloon deflation. It is worth mentioning that larger direct CCFs have more significant baseline flow steal. Consequently, it increases the chance of patient tolerating ICA balloon occlusion for fistula embolization.
Finally, when performing a transvenous embolization of the direct CCF, there is a theoretical lower risk of Onyx migration into the arterial system since it would have to travel against a high-pressure system.
All the catheters were successfully removed after confirmation of complete occlusion of CCF on digital subtraction angiography. There were no intraoperative changes on neurological monitoring complications in any of the patients
Outcomes of Cranial Nerve Deficits
The six patients with preoperative EOM CN deficits had the following outcomes: three completely resolved symptoms; two improved (residual mild ptosis and no diplopia); and one persisted (CN VI palsy). There was one case of immediate postoperative changes in EOM CN function (new partial CN VI deficit) that resolved completely at 1-month follow-up.
When the ocular motility grading system was applied to all the seven patients who underwent treatment for the CCFs, we found that preoperatively one patient (without EOM CN deficit as presenting symptom) had “Excellent” function, one patient had “Good” function, two patients had “Fair” function, and three patients had “Poor” function. At the last postoperative follow-up, we found that four patients had “Excellent” function, two patients had “Good” function, and one patient maintained a “Fair” function ( Table 1 ).
Table 1. Postprocedural cranial nerve outcomes in patients undergoing Onyx embolization using the ocular motility scale.
| Postoperative function | Preoperative function | ||||
|---|---|---|---|---|---|
| Excellent ( n = 1) | Good ( n = 1) | Fair ( n = 3) | Poor ( n = 2) | NA ( n = 0) | |
| Excellent ( n = 4) | 1 | 1 | 2 | 0 | 0 |
| Good ( n = 2) | 0 | 0 | 0 | 2 | 0 |
| Fair ( n = 1) | 0 | 0 | 1 | 0 | 0 |
| Poor ( n = 0) | 0 | 0 | 0 | 0 | 0 |
| NA ( n = 0) | 0 | 0 | 0 | 0 | 0 |
Abbreviation: NA, not applicable.
Postoperative Course and Follow-up
Complete obliteration of the CCFs only with Onyx liquid embolic agent was achieved in all cases. The median length of hospital stay was 4 days (range: 1–5 days), with a mean stay of 3.14 days. There was no perioperative complication directly related to the surgery. Mid- and long-term follow-up records were available for all patients and the last follow-up was defined as the date of last imaging. Ideally, we obtain both brain magnetic resonance angiography (MRA) and diagnostic cerebral angiogram to ensure obliteration of the CCF (five patients). In two patients, brain MRA was the only imaging performed postprocedure. The median follow-up was 34 months (range: 10–91 months). Radiological follow-up revealed no recurrences in any of the seven patients.
Proptosis, chemosis, conjunctival injection, and tinnitus symptoms were resolved in all patients. There was one patient who developed an upper extremity deep venous thrombosis 2 weeks after procedure. Interestingly, the patient was young with no comorbidities, but he was the only patient with traumatic CCF in our series.
Case Illustration
A 15-year-old boy with no significant medical history sustained a high-velocity motor vehicle accident 3 months before admission. Following the accident, he noticed proptosis, redness of his right eye, and diplopia. Clinical examination revealed pulsatile proptosis and ecchymosis of right eye. Computed tomography angiogram done initially revealed a right-sided CCF. He underwent angiogram followed by embolization of the fistula under general anesthesia and neuromonitoring. He was extubated immediately after the procedure. There were no pulsations over his eye in the postprocedure period and complete resolution of his ecchymosis. His postprocedural recovery was unremarkable, and he was discharged on postprocedure day 1. Figs. 1 to 3 illustrate the various stages of embolization.
Fig. 1.
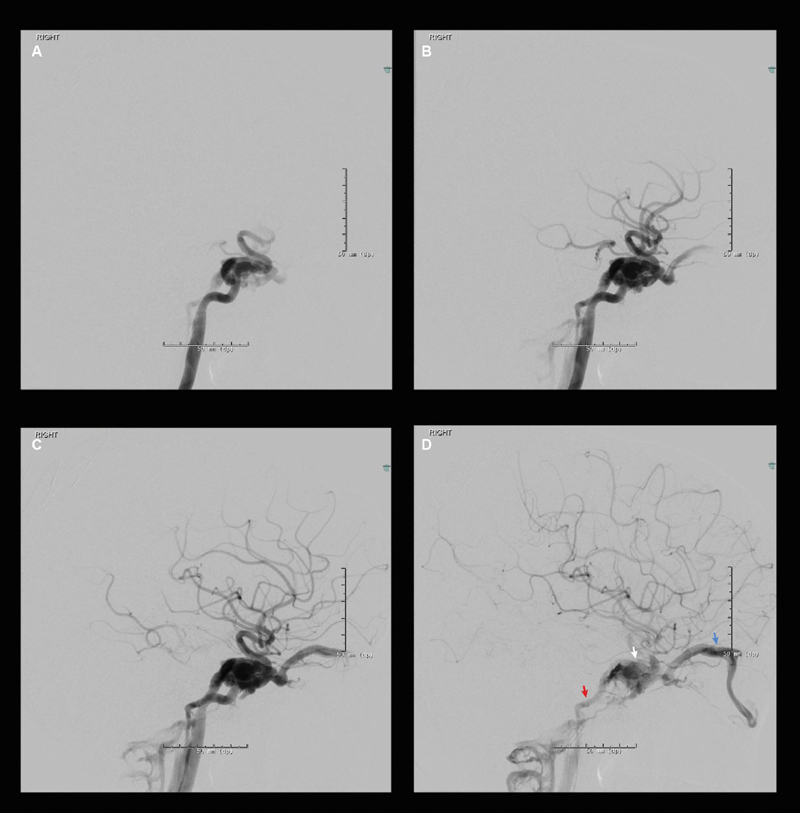
Pre-embolization images of right internal carotid angiogram showing. ( A ) Early arterial phase, ( B ) mid-arterial phase, ( C ) late arterial phase, ( D ) early venous phase showing fistulous drainage into right cavernous sinus (white/middle arrow), right superior ophthalmic vein (blue/far right arrow) and right inferior petrosal sinus (red/far left arrow).
Fig. 3.
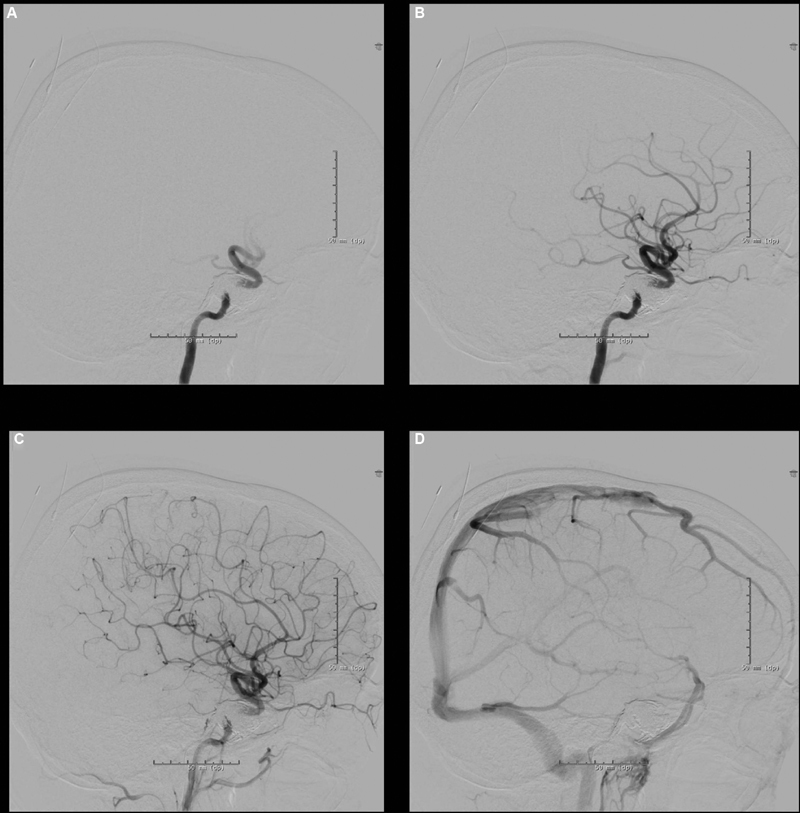
Postembolization angiogram images of right internal carotid artery injection showing complete occlusion of right carotid-cavernous fistula and Onyx cast. ( A ) Early arterial, ( B ) mid-arterial, ( C ) late arterial, ( D ) venous phase.
Fig. 2.
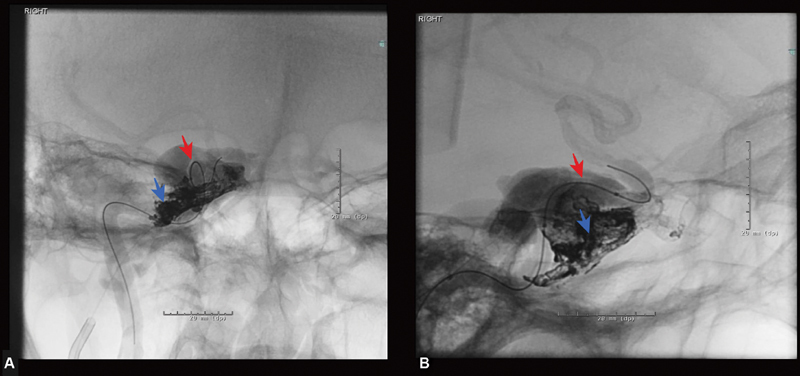
Periprocedural images of Onyx embolization of right carotid-cavernous fistula. ( A ) Anteroposterior view of right cavernous sinus angiogram showing the balloon in the right cavernous internal carotid artery (red arrow) and an Apollo catheter in the right cavernous sinus through the inferior petrosal vein (blue arrow). ( B ) Lateral view of the right cavernous sinus showing a deflated balloon catheter in the right carotid-cavernous artery and Onyx cast in the right cavernous sinus.
Discussion
The treatment of CCFs with open surgery was often challenging with high morbidity. Improved rates of success and less complications started to be reported 9 after the development of endovascular devices and techniques to repair CCF. The initial endovascular approach involved the use of transarterial embolization of the cavernous sinus with detachable balloons. 10 This approach was reasonably successful but associated with high rates of balloon deflation and/or migration overtime. Subsequently, detachable platinum coils started to be used with success for treatment in direct fistulas with single arterial connections, which is often difficult and time consuming in complex, multiple, or indirect fistulas. 11 12 Transvenous route was initially popularized by Debrun et al, who introduced detachable balloons using this route. 13 Though they reported high occlusion rates combing transvenous with transarterial or surgical approaches, they had 20% incidence of new CN III palsy that were transient for the majority of cases. After the introduction of Onyx liquid embolic agent in the early part of this decade, it has quickly become the main stay of treatment, either used as stand-alone agent or in combination with coiling using the transvenous route. Coils can compartmentalize the cavernous sinus and result in incomplete obliteration, while Onyx gradually permeates the sinus interstices.
The first report of successful embolization of CCF using Onyx was reported by Arat et al in 2004. 14 Since then, various reports of successful treatment using Onyx, either exclusively or in combination with coils, have been reported. 15 In 2008, Suzuki et al 15 reported their experience in treating six patients with indirect CCF by using a combination of Onyx and coils. Complete cure was achieved in four patients following a single session and in two patients after an additional session. Transient headache was observed in all patients following embolization and resolved within few days. Two patients developed transient CN VI palsies following transvenous embolization, and one patient developed a transient facial palsy following transarterial embolization of ECA branches prior to definitive treatment via a transvenous route. Lv et al 16 reported the results of transarterial Onyx 18–based embolization in 31 patients with dural arteriovenous fistulas. Of the five CCFs, complete angiographic obliteration was achieved in three. Two patients experienced complications: one hemifacial hypesthesia and one trigeminocarotid reflex, hemifacial hypesthesia, and facial palsy. In 2010, Elhammady et al 7 reported a series of 12 patients treated with Onyx only. Eight patients were treated using a transarterial route and four patients with transvenous route. They reported 100% occlusion of fistulas in 2 months follow-up. Three patients (25%) developed CN palsies, including one patient who developed a complete CN VII palsy that has not been resolved. Two patients developed transient neuropathies—one with a Horner's syndrome and partial CN VI palsy, and one with a complete CN III and partial CN V palsy.
It is evident that transvenous embolization of CCFs is highly effective but is not without inherent risks. Cranial neuropathies following embolization may occur as a result of several postulated mechanisms such as ischemia, mass effect, and direct toxicity. 7 The embolization technique used is not based on volume. The goal is to disconnect the fistulous communication with the minimum Onyx volume. The fluid nature of Onyx permits filling of the cavernous sinus in a more conformational manner reducing the chance of increased pressure, as opposed to coils that need to fill the whole compartment promoting thrombosis and potentially causing acute increase mass effect. The conformability in the space and efficiency as a sealant are the key components of Onyx over coil embolization.
The main concern with Onyx regarding cranial neuropathy is not the volume or pressure but the DMSO toxicity. Previous reports have suggested neuroischemic effects of DMSO used in cryopreservation of cells in bone marrow transplant. 17 18 Bakar et al 19 evaluated the possible neurotoxic effects of DMSO on brain tissue and carotid artery when it was slowly infused into the ICAs of rats. They found no evidence that DMSO had any direct or indirect effects on the rat arterial or neural tissue when infused slowly. Applying low concentrations of DMSO and utilizing a slow injection technique have been proposed to minimize the risk of these complications, which could lead to inadvertent reflux of liquified Onyx to the vasculature of CNs. 20 Taking a hint from this, we always used slow injections of Onyx, especially in the beginning when there is the extra DMSO that is used to fill the dead space of the catheter.
Though there are various case reports and series on the treatment of CCF, the data on exclusive Onyx use and mid- and long-term outcomes in patients are sparse. One of the earliest preliminary results was reported by Zenteno et al, 21 who reported results of treatment of five consecutive CCFs using Onyx. There was complete immediate occlusion in four patients and the lesion in the remaining patient was subsequently embolized at 6-month follow-up. Barber et al 5 published mid- and long-term results of treating 24 CCFs in 21 consecutive patients with Onyx or n-butyl cyanoacrylate (n-BCA). None of the 24 CCFs was recanalized, regrew, or required any further treatment subsequent to Onyx or n-BCA embolization throughout a mean 12.4 months of angiographic follow-up (range: 1–36 months). Clinically significant complications were seen in three embolization procedures, including CN palsies ( n = 1), embolic infarct ( n = 1), and intraperitoneal hemorrhage ( n = 1). We had complete immediate occlusion in all our patients, which remained occluded in subsequent follow-up angiograms. None of the patients had any relapse of symptoms in the subsequent clinical follow-up.
The main limitations of our study are its retrospective nature and small patient sample. Further studies performed prospectively and ideally in comparison to other liquid embolics will be necessary to further validate this approach. We hope this study can shed some light on the toxicity concerns of Onyx/DMSO to CNs in the cavernous sinus.
Conclusion
Transvenous Onyx embolization is a safe and effective treatment of CCFs when technical aspects to reduce complications are performed diligently. We obtained excellent mid- and long-term outcomes for CCFs occlusion. We report no evidence of CN toxicity on this small series of cavernous sinus CCF embolization with only Onyx.
Funding Statement
Funding Demetrius Lopes—Medtronic: proctor and advisory board; Stryker: proctor and advisory board.
Footnotes
Conflict of Interest Dr. Lopes reports personal fees from Stryker, personal fees from Medtronic, outside the submitted work. All the other authors report no conflict of interest.
References
- 1.Barrow D L, Spector R H, Braun I F, Landman J A, Tindall S C, Tindall G T. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62(02):248–256. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 2.Ringer A J, Salud L, Tomsick T A.Carotid cavernous fistulas: anatomy, classification, and treatment Neurosurg Clin N Am 20051602279–295., viii [DOI] [PubMed] [Google Scholar]
- 3.Hassan T, Rashad S, Aziz W, Sultan A, Ibrahim T. Endovascular modalities for the treatment of cavernous sinus arteriovenous fistulas: a single-center experience. J Stroke Cerebrovasc Dis. 2015;24(12):2824–2838. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Chen T, Kalani M Y, Ducruet A F, Albuquerque F C, McDougall C G. Development of syndrome of inappropriate antidiuretic hormone secretion (SIADH) after Onyx embolisation of a cavernous carotid fistula. BMJ Case Rep. 2016;2016:bcr2015012104. doi: 10.1136/bcr-2015-012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber S M, Rangel-Castilla L, Zhang Y J, Klucznik R, Diaz O. Mid- and long-term outcomes of carotid-cavernous fistula endovascular management with Onyx and n-BCA: experience of a single tertiary center. J Neurointerv Surg. 2015;7(10):762–769. doi: 10.1136/neurintsurg-2014-011266. [DOI] [PubMed] [Google Scholar]
- 6.Santos-Franco J A, Lee A, Zenteno M, Gil-Ortiz C, Vega-Montesinos S. Carotid-cavernous fistula treatment with ethylene vinyl alcohol (onyx) exclusively through anterior venous approach. Vasc Endovascular Surg. 2012;46(04):332–337. doi: 10.1177/1538574412442594. [DOI] [PubMed] [Google Scholar]
- 7.Elhammady M S, Wolfe S Q, Farhat H, Moftakhar R, Aziz-Sultan M A. Onyx embolization of carotid-cavernous fistulas. J Neurosurg. 2010;112(03):589–594. doi: 10.3171/2009.6.JNS09132. [DOI] [PubMed] [Google Scholar]
- 8.Akutsu H, Kreutzer J, Fahlbusch R, Buchfelder M. Transsphenoidal decompression of the sellar floor for cavernous sinus meningiomas: experience with 21 patients. Neurosurgery. 2009;65(01):54–62. doi: 10.1227/01.NEU.0000348016.69726.A6. [DOI] [PubMed] [Google Scholar]
- 9.Zanaty M, Chalouhi N, Tjoumakaris S I, Hasan D, Rosenwasser R H, Jabbour P. Endovascular treatment of carotid-cavernous fistulas. Neurosurg Clin N Am. 2014;25(03):551–563. doi: 10.1016/j.nec.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Lewis A I, Tomsick T A, Tew J M., Jr Management of 100 consecutive direct carotid-cavernous fistulas: results of treatment with detachable balloons. Neurosurgery. 1995;36(02):239–244. doi: 10.1227/00006123-199502000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bink A, Goller K, Lüchtenberg M. Long-term outcome after coil embolization of cavernous sinus arteriovenous fistulas. AJNR Am J Neuroradiol. 2010;31(07):1216–1221. doi: 10.3174/ajnr.A2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi K C, Singh D, Tandon M S. Intrafistula pressure measurement in traumatic carotid cavernous fistulas--key to increasing safety and effectiveness of endovascular coiling. Acta Neurochir (Wien) 2014;156(09):1695–1700. doi: 10.1007/s00701-014-2176-8. [DOI] [PubMed] [Google Scholar]
- 13.Debrun G, Lacour P, Vinuela F, Fox A, Drake C G, Caron J P. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55(05):678–692. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 14.Arat A, Cekirge S, Saatci I, Ozgen B. Transvenous injection of Onyx for casting of the cavernous sinus for the treatment of a carotid-cavernous fistula. Neuroradiology. 2004;46(12):1012–1015. doi: 10.1007/s00234-004-1244-9. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki S, Lee D W, Jahan R, Duckwiler G R, Viñuela F. Transvenous treatment of spontaneous dural carotid-cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27(06):1346–1349. [PMC free article] [PubMed] [Google Scholar]
- 16.Lv X, Jiang C, Li Y, Wu Z. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg. 2008;109(06):1083–1090. doi: 10.3171/JNS.2008.109.12.1083. [DOI] [PubMed] [Google Scholar]
- 17.Windrum P, Morris T C. Severe neurotoxicity because of dimethyl sulphoxide following peripheral blood stem cell transplantation. Bone Marrow Transplant. 2003;31(04):315. doi: 10.1038/sj.bmt.1703848. [DOI] [PubMed] [Google Scholar]
- 18.Hoyt R, Szer J, Grigg A. Neurological events associated with the infusion of cryopreserved bone marrow and/or peripheral blood progenitor cells. Bone Marrow Transplant. 2000;25(12):1285–1287. doi: 10.1038/sj.bmt.1702443. [DOI] [PubMed] [Google Scholar]
- 19.Bakar B, Kose E A, Sonal S, Alhan A, Kilinc K, Keskil I S. Evaluation of the neurotoxicity of DMSO infused into the carotid artery of rat. Injury. 2012;43(03):315–322. doi: 10.1016/j.injury.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Pamuk A G, Saatci I, Cekirge H S, Aypar U. A contribution to the controversy over dimethyl sulfoxide toxicity: anesthesia monitoring results in patients treated with Onyx embolization for intracranial aneurysms. Neuroradiology. 2005;47(05):380–386. doi: 10.1007/s00234-004-1323-y. [DOI] [PubMed] [Google Scholar]
- 21.Zenteno M, Santos-Franco J, Rodríguez-Parra V. Management of direct carotid-cavernous sinus fistulas with the use of ethylene-vinyl alcohol (Onyx) only: preliminary results. J Neurosurg. 2010;112(03):595–602. doi: 10.3171/2009.6.JNS09440. [DOI] [PubMed] [Google Scholar]


