Abstract
Esthesioneuroblastoma (ENB) is a rare olfactory malignancy that can present with locally advanced disease. At our institution, patients with ENB in whom the treating surgeon believes that a margin-negative resection is initially not achievable are selected to undergo induction with chemotherapy with or without radiotherapy prior to surgery. In a retrospective review of 61 patient records, we identified six patients (10%) treated with this approach. Five of six patients (83%) went on to definitive surgery. Prior to surgery, three of five patients (60%) had a partial response after induction therapy, whereas two of five (40%) had stable disease. Microscopically margin-negative resection was achieved in four of five (80%) of the patients who went on to surgery, while one patient had negative margins on frozen section but microscopically positive margins on permanent section. Three of five patients (60%) recurred after surgery; two of these patients died with recurrent/metastatic ENB. In summary, induction therapy may facilitate margin-negative resection in locally advanced ENB. Given the apparent sensitivity of ENB to chemotherapy and radiotherapy, future prospective studies should investigate the optimal multidisciplinary approach to improve long-term survival in this rare disease.
Keywords: esthesioneuroblastoma, induction, therapy, neoadjuvant, outcomes
Introduction
Esthesioneuroblastoma (ENB) is a rare cancer that arises from malignant transformation of the olfactory epithelium. 1 It comprises approximately 6% of all sinonasal malignancies and affects 0.4 patients per million per year. 2 3 The usual management of ENB includes upfront surgery followed by adjuvant radiotherapy. 1 4 5 6 Several studies have shown that ENB is sensitive to platinum-based chemotherapy, 7 8 but the impact of chemotherapy before and after surgery on overall survival (OS) for patients with localized disease is less clear. 1 8 9
Nonspecific symptomatology, such as nasal airway obstruction, anosmia, and epistaxis, frequently delays diagnosis in ENB. 10 Consequently, patients can present with locally advanced disease infiltrating the skull base that may appear unresectable on neuroimaging. Studies in both children and adults have suggested that neoadjuvant chemotherapy, with or without radiotherapy, prior to primary surgery (from here, referred to as “induction therapy”) produces responses and facilitates definitive surgical resection; indeed, this is standard practice at several centers. 11 12 13 14 15 16 17
At our institution, most patients with ENB are treated with upfront surgery followed by radiotherapy with or without chemotherapy. IT is reserved for patients in whom the treating surgeon believes that a margin-negative resection is initially not achievable based on neuroimaging. The effect of IT in this particular setting is unclear in terms of both surgical outcomes (i.e., achievement of margin-negative resection) and OS. The primary objective of the current study was to describe patients with locally advanced ENB who underwent this approach, notably at an institution that favors upfront surgery without prior IT.
Methods
This study was approved by the Mayo Clinic's Institutional Review Board and is in accordance with the Declaration of Helsinki. We conducted a retrospective analysis of all patients ( n = 61) who were treated for ENB at a large tertiary referral center from January 1, 1994 to December 31, 2015. Inclusion criteria required patients to have received chemotherapy or chemoradiotherapy as their initial treatment for locally advanced ENB (i.e., part or all of it defined as IT). Patients were excluded if they had evidence of metastases outside of the cervical region at the time of diagnosis.
Histological confirmation and Hyams grading of tumors was performed by an expert neuropathologist at our institution. 18 Tumor staging was performed using the modified Kadish staging system. 19 Response to IT was characterized on the basis of review of radiologic imaging by the study authors, in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria. 20 The dates of local recurrence, regional recurrence, or distant metastases were obtained from the medical record. Recurrence-free survival was calculated from the date of initiation of chemotherapy to the date of recurrence. OS was calculated as the time of initiation of chemotherapy to death.
Results
Patient Demographics
Of the 61 patients with ENB treated at our institution during the study period, six (10%) underwent IT after presenting with locally advanced disease that appeared unlikely to be resected with negative margins. Five of these six patients (83%) underwent definitive surgery after IT ( Table 1 ).
Table 1. Demographic and clinical characteristics for the six patients presenting with esthesioneuroblastoma that was deemed unlikely to be resected with negative margins prior to induction therapy.
| Patient number | Age (y) | Gender | Kadish stage | Hyams grade | IT regimen (number of cycles) | Response to IT | Radiation before surgery | Surgical approach | Time from start of IT to surgery | Completeness of resection | Postsurgery adjuvant therapy | Disease recurrence | Survival (RFS/OS a ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | C | N/A |
CAV,
2
EP,
2
EP (2 b ) |
PR | 6,480 cGy | Cranio-endoscopic | 7 months | Gross total, (-) microscopic margins | None | L, DM | DOD (29/79) |
| 2 | 22 | Male | D | High | CAV, 6 CAV, 2 CEP 1 | PR, SD c | 6,000 cGy | Bifrontal craniotomy | 12 months | Gross total, (-) microscopic margins |
Chemoradiotherapy to neck
d
(P b ; 6,000 cGy) |
None | AND (26/26) |
| 3 | 25 | Male | C | High | EP 3 | PR | None | Cranio-endoscopic | 3 months | Gross total, (-) microscopic margins | Chemoradiotherapy to primary tumor site (EP b ; 6,000 cGy) |
None | AND (28/28) |
| 4 | 51 | Female | D | N/A | EP, 5 P (2 b ) | SD | 4,500 cGy | Bifrontal craniotomy | 12 months | Gross total, (+) microscopic margins | None | R (delayed LN metastasis in unirradiated neck) | AWD (43/60) |
| 5 | 40 | Female | C | Inter-mediate | CEP 2 | SD | None | Bifrontal craniotomy | 2 months | Gross total, (-) microscopic margins | Radiotherapy to primary tumor site (5,580 cGy) |
L, R (delayed LN metastasis in unirradiated neck) | DOD (33/165) |
| 6 | 53 | Male | D | Low | P (2 b ), EP 3 | CR | 6,020 cGy | N/A | N/A | N/A | N/A | DM | DOD (11/11) |
Abbreviations: AND, alive, no evidence of disease; AWD, alive, with evidence of disease; CAV, cyclophosphamide, anthracycline, vincristine; CEP, cyclophosphamide, etoposide, cisplatin; cGy, centiGray; CR, complete response; DM, distant metastases; DOD, dead of disease; EP, etoposide, cisplatin; IT, induction therapy; L, local recurrence; LN, lymph node; N/A, not applicable; OS, overall survival; P, cisplatin; PR, partial response; R, regional recurrence; RFS, recurrence-free survival; SD, stable disease.
RFS and overall survival are shown in months from the time of the start of induction therapy.
Chemotherapy given concurrently with radiotherapy.
Patient 2 received six cycles of CAV followed by 6,000 cGy of external beam radiotherapy and achieved a PR but then had local progression very soon thereafter. He received two more cycles of CAV, then one cycle of CEP, and had stable disease before being taken to definitive surgery.
Patient 2 was treated with 6,000 cGy of adjuvant radiotherapy to right side of neck after surgery because one cervical lymph node that was surgically removed contained esthesioneuroblastoma. The primary tumor site was not irradiated a second time.
The median age of the entire cohort was 42 (range = 22–51) and included four men and two women. There were three patients (50%) with Kadish stage C disease, and three (50%) with Kadish stage D disease. All three patients with Kadish stage D ENB presented with cervical nodal metastases alone (i.e., none had distant metastatic disease at presentation).
Induction Therapy Regimens
The complete treatment course, including response to IT, is outlined in Fig. 1 . All six patients received chemotherapy as part of IT with etoposide and cisplatin (EP), with two receiving cyclophosphamide in addition to EP (CEP). The median number of cycles of EP +/− cyclophosphamide was three (range = 1–5). Further, two patients (33%) also received IT consisting of cyclophosphamide, anthracycline (either doxorubicin or epirubicin), and vincristine (CAV).
Fig. 1.
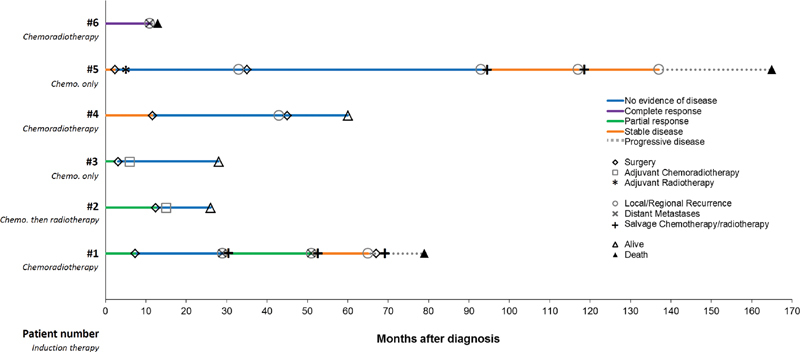
Swimmer plot demonstrating the clinical course of the six patients in our cohort from the time of diagnosis. Patient number and IT are denoted in the y-axis. Best response to IT before surgery is denoted with the color of the line (i.e., stable disease, partial response, or complete response). Time of surgery is denoted for both primary and salvage surgery. Postsurgery adjuvant chemotherapy and/or radiotherapy are indicated. Salvage chemotherapy/radiotherapy is indicated, with best responses shown. Time of local/regional recurrence is indicated with circles; time of distant metastases is indicated with Xs. Patients alive at the time of last follow-up are indicated with an open triangle; filled triangles denote the time of death. IT, induction therapy.
Four patients (66%) received external beam radiotherapy as part of IT: three with concurrent chemotherapy (i.e., chemoradiotherapy) whereas one received radiotherapy alone (after chemotherapy was completed). In the three patients treated with chemoradiotherapy, single-agent cisplatin (P) was administered concurrently in two patients; the other patient received concurrent EP.
Only one patient (patient 2) received radiotherapy twice: once to the primary tumor site as part of IT (6,000 cGy), and once as an adjuvant therapy after surgery (6,000 cGy). The latter radiotherapy course was to the right side of neck after surgery to prevent recurrence because one surgically removed cervical lymph node demonstrated metastatic esthesioneuroblastoma.
Responses to Induction Therapy
Responses to IT were as follows: three patients (50%) achieved a partial response (PR), two (33%) had stable disease (SD) and one (17%) had a complete response (CR). Three of four patients receiving chemotherapy and radiotherapy as IT achieved a complete or PR. One of two patients receiving only chemotherapy as IT achieved a PR.
Representative neuroimaging of two of the patients (patients 3 and 1) who achieved PR after IT are shown in Figs. 2 and 3 , respectively: patient 3 was treated with CAV, EP, and then EP with 6,480 cGy of concurrent radiotherapy; patient 1 was treated with EP without radiotherapy.
Fig. 2.
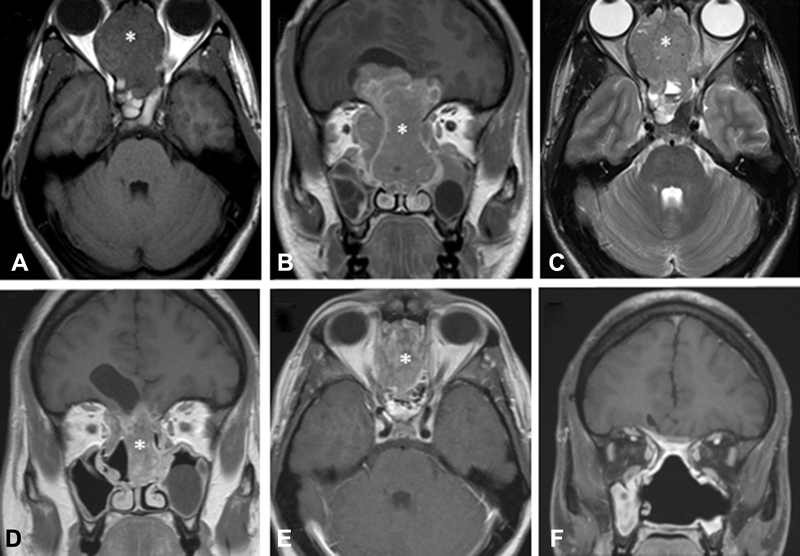
Magnetic resonance imaging of a 25-year-old male with esthesioneuroblastoma initially deemed unresectable prior to induction therapy (patient 3). ( A ) Axial T1-weighted pregadolinium image demonstrating a large locally destructive sinonasal mass (*). ( B ) Corresponding coronal T1-weighted gadolinium-enhanced image. ( C ) Corresponding axial T2-weighted image. ( D ) Coronal T1-weighted postgadolinium image status postinduction chemotherapy with three cycles of etoposide and cisplatin, demonstrating a partial response to induction chemotherapy (without radiotherapy). He was subsequently taken to surgery, which achieved a gross total resection with negative microscopic margins. ( E ) Corresponding axial T1-weighted gadolinium-enhanced image. ( F ) 2-year postoperative coronal T1-weighted gadolinium enhanced imaging demonstrating no evidence for residual or recurrent disease after postsurgery chemoradiotherapy.
Fig. 3.
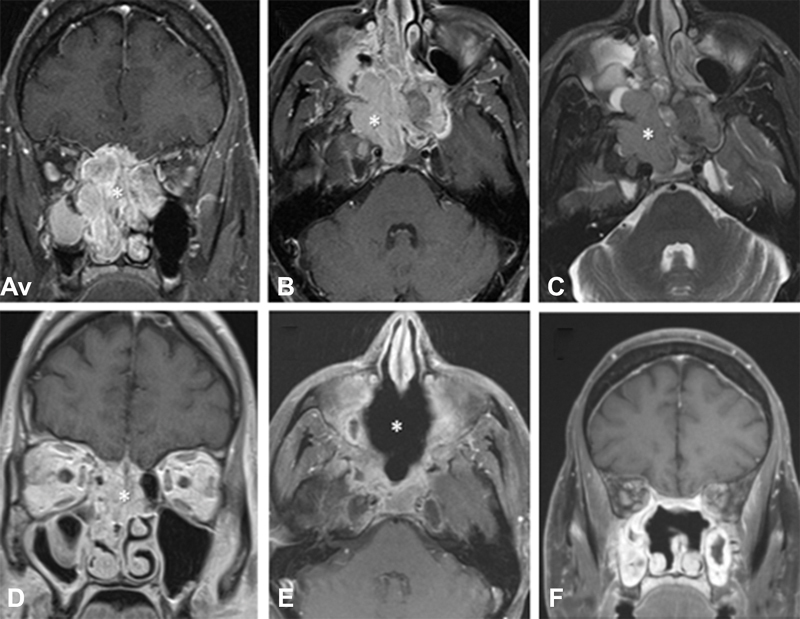
Magnetic resonance imaging of a 44-year-old male presenting with esthesioneuroblastoma initially deemed unresectable prior to induction therapy (patient 1). ( A ) Coronal T1-weighted postgadolinium image demonstrating a large sinonasal mass (*). ( B ) Corresponding axial T1-weighted gadolinium-enhanced image. ( C ) Corresponding axial T2-weighted image. ( D ) Coronal T1-weighted postgadolinium image status postinduction chemotherapy with two cycles of cisplatin, doxorubicin, and vincristine, followed by two cycles of etoposide and cisplatin and then two cycles of etoposide and cisplatin with concurrent 6,480 cGy of external beam radiotherapy in 36 fractions. This image was taken immediately prior to surgery, which was achieved in a gross total fashion with negative microscopic margins. ( E ) 4-month postoperative axial T1-weighted gadolinium enhanced imaging demonstrated no evidence for residual or recurrent disease. ( F ) Corresponding coronal T1-weighted gadolinium-enhanced image.
The third patient achieving PR (patient 2) initially presented with apparently unresectable disease at another institution and was treated with six cycles of CAV followed by 6,000 cGy of external beam radiotherapy, leading to a PR. However, very shortly thereafter he had local progression. He received two more cycles of CAV with no response—then was evaluated at our institution— treated with one cycle of cyclophosphamide, etoposide and cisplatin (CEP) with SD and subsequently taken to surgery.
Of the two patients (33%) with SD after IT (patients 4 and 5), one was treated with five cycles of EP followed by 4,500 cGy of chemoradiotherapy, and one was treated with two cycles of CEP without any radiotherapy prior to surgery.
Patient 6 achieved a CR after IT consisting of chemoradiotherapy but did not go on to surgery—their outcomes are documented in a separate subsection below.
Toxicity from Induction Therapy
Two patients (33%) received granulocyte-colony stimulating factor prophylaxis during IT. One patient (patient 6) had moderate left-sided hearing loss attributed to platinum-based chemotherapy, but no patients in our cohort experienced major adverse events that limited planned IT.
Surgical Technique and Outcomes
Of the five patients who underwent surgery, median time from diagnosis to primary surgery was 7.2 months (range = 2.3–12.2). All five patients underwent bifrontal craniotomies; two of which were combined cranioendoscopic resections. Microscopically margin-negative resection was achieved in four of five cases (80%); one patient underwent gross total resection with negative margins intraoperatively on frozen section but was found to have microscopically positive margins on permanent section. This was the undesired result of the tumor location abutting the dura along the tuberculum sella adjacent to the optic nerve. All five patients had viable ENB cells upon review of the resected tissue by expert pathologists, that is, there were no pathologic complete responses. Of note, although not systematically reported on all five tumors, one patient (patient 2) had an estimated pathologic response of 20% based on morphologic evidence of tumor cell necrosis and apoptosis. Neuroimaging after surgery are shown in Figs. 2 and 3 .
Complications after Surgical Resection
By and large, surgical complications were avoided by meticulous multilayer closure of the large defects. In five patients who underwent surgical resection, no postoperative cerebrospinal fluid leaks occurred. In addition, none of the patients developed epidural abscesses.
Survival of Patients who Underwent Surgery after Induction Therapy
Of the five patients who underwent definitive surgery, three of five (60%) went on to receive adjuvant external beam radiotherapy after surgery. Two of these were treated with adjuvant radiotherapy to the primary tumor site (neither received radiotherapy as IT prior to surgery), whereas one patient had adjuvant radiotherapy to the neck (they were treated with 6,000 cGy to the primary tumor prior to surgery as IT). Two of these patients received chemotherapy concurrent with radiotherapy. At last follow-up, three of five patients experienced recurrence. One patient (patient 5) developed regional nodal recurrence at 33 months following therapy initiation with delayed cervical lymph node metastases in a previously unirradiated neck. Another patient (patient 1) developed both local recurrence and distant metastases at 29 months following the start of therapy. These two patients died with metastatic disease at 165 and 79 months, respectively. Patient 4 also developed regional nodal recurrence at 43 months following therapy initiation with delayed cervical lymph node metastases in a previously unirradiated neck but had salvage surgery and remained alive with 17 additional months of follow-up. In total, three of the five patients were alive at last follow-up.
Outcomes of Patient Treated with Induction Therapy Only
Patient 6 was a 53-year-old male diagnosed with Kadish stage D, low Hyams grade ENB, and was treated with 6,020 cGy of radiotherapy concurrently with cisplatin—followed by three cycles of EP—resulting in a CR. Surgery was deferred until he recovered from chemoradiotherapy. In the interim, he developed encephalopathy and was found to have distant metastatic disease throughout abdomen and pelvis on computed tomography scan. He died a few days later due to multiorgan failure, 11 months from the time of diagnosis.
Discussion
Our study demonstrates that induction therapy (IT) facilitated margin-negative resection in a highly selected group of patients, that is , those with locally advanced ENB in whom margin-negative resection was initially felt to be unattainable by the treating surgeon, especially when both chemotherapy and radiotherapy were used as IT. These findings augment reports from other centers where IT has been employed as an upfront therapy in all ENB patients prior to surgery regardless of stage. 11 12 13 14 15 16 17 Of note, three of five patients (60%) in our study were treated with chemoradiotherapy prior to surgery, which is the approach at some centers for all patients with advanced (e.g., Kadish stage C) disease. 16 IT regimens varied significantly in our study, but notably four of six patients (66%) had an objective response to IT; of whom three went on to definitive surgical resection, all with negative microscopic margins. The response rate was numerically higher in patients receiving both chemotherapy and radiotherapy as IT.
Adjuvant therapy after surgery in our study was quite heterogeneous. While it is now established that surgery plus adjuvant radiotherapy is associated with superior survival in ENB, 2 5 6 there is still a question regarding the optimal use of chemotherapy after surgery. 7 8 9 21 Given that ENB can recur years after surgery, there is great interest in determining the optimal consolidation treatment that balances toxicity with efficacy to eradicate minimal residual disease. Further, there are biological characteristics of ENB, including tyrosine kinase mutations and BCL-2 dependency—which could be leveraged to develop more effective chemotherapy strategies in the future. 22 Investigation of these targets could be accomplished in a window-of-opportunity trial schema. 23 In the meantime, targeting more intensive pre and postsurgical therapy to high-risk patients—such as those with dural invasion or high Hyams grade histology—seems reasonable. 5 6 24 Of note, two patients had delayed cervical lymph node metastases several years after surgery in previously unirradiated necks.
IT does not come without toxicities that could delay surgery; hence, its judicious application at our institution for locally advanced disease that appears unresectable to the treating surgeon. Importantly, no patients experienced toxicities that delayed surgery in our cohort. However, very soon after IT with resultant CR, one patient developed distant metastases and never underwent surgical resection—highlighting the heterogeneous and at times aggressive natural history of ENB.
Induction therapy has been used in other head and neck cancers, such as sinonasal undifferentiated carcinoma, as a prognostic tool guiding later therapy (i.e., to delineate which patients are more likely to respond to definitive chemoradiotherapy vs. surgery). 25 In fact, an approach using response to IT to guide therapy in ENB was described by Fitzek et al at Massachusetts General Hospital. 11 However, given the variable efficacy of current chemotherapy regimens and lack of long-term follow-up, surgery remains the cornerstone of therapy for locally advanced disease at our institution when feasible.
Our study was not without important limitations, including selection bias given that there were no explicit criteria to determine the apparent respectability of tumors besides the individual experience of treating surgeons upon review of neuroimaging. There was undoubtedly also a referral bias; however, this is largely unavoidable in studies of ENB given the rarity of the tumor. With these in mind, we are cautious about the broad application of our findings, and feel strongly that future multi-institutional prospective studies investigating the optimal approach to enhance definitive resection with pre and postsurgical interventions are necessary.
Funding Statement
Funding None.
Conflict of Interest A.V.C. reports funding for clinical trials from Merck, AstraZeneca/MedImmune, Kura Oncology, Inovio, Eisai (all institutional). The other authors report no relevant conflict of interest in submitting this article for publication.
Previous Presentation
This work was presented on February 15, 2019 as an oral abstract at the North American Skull Base Society 29th Annual Meeting in Orlando, Florida.
References
- 1.Dulguerov P, Allal A S, Calcaterra T C. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2(11):683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 2.Broich G, Pagliari A, Ottaviani F.Esthesioneuroblastoma: a general review of the cases published since the discovery of the tumour in 1924 Anticancer Res 199717(4A):2683–2706. [PubMed] [Google Scholar]
- 3.Theilgaard S A, Buchwald C, Ingeholm P, Kornum Larsen S, Eriksen J G, Sand Hansen H. Esthesioneuroblastoma: a Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003;123(03):433–439. doi: 10.1080/00016480310001295. [DOI] [PubMed] [Google Scholar]
- 4.Diaz E M, Jr, Johnigan R H, III, Pero C. Olfactory neuroblastoma: the 22-year experience at one comprehensive cancer center. Head Neck. 2005;27(02):138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 5.Tajudeen B A, Arshi A, Suh J D. Esthesioneuroblastoma: an update on the UCLA experience, 2002-2013. J Neurol Surg B Skull Base. 2015;76(01):43–49. doi: 10.1055/s-0034-1390011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Gompel J J, Giannini C, Olsen K D. Long-term outcome of esthesioneuroblastoma: hyams grade predicts patient survival. J Neurol Surg B Skull Base. 2012;73(05):331–336. doi: 10.1055/s-0032-1321512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElroy E A, Jr, Buckner J C, Lewis J E. Chemotherapy for advanced esthesioneuroblastoma: the Mayo Clinic experience. Neurosurgery. 1998;42(05):1023–1027. doi: 10.1097/00006123-199805000-00040. [DOI] [PubMed] [Google Scholar]
- 8.Porter A B, Bernold D M, Giannini C. Retrospective review of adjuvant chemotherapy for esthesioneuroblastoma. J Neurooncol. 2008;90(02):201–204. doi: 10.1007/s11060-008-9645-y. [DOI] [PubMed] [Google Scholar]
- 9.Miller K C, Marinelli J P, Van Gompel J J. Utility of adjuvant chemotherapy in patients receiving surgery and adjuvant radiotherapy for primary treatment of esthesioneuroblastoma. Head Neck. 2019;41(05):1335–1341. doi: 10.1002/hed.25558. [DOI] [PubMed] [Google Scholar]
- 10.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970-1990. Laryngoscope. 1992;102(08):843–849. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Fitzek M M, Thornton A F, Varvares M. Neuroendocrine tumors of the sinonasal tract. Results of a prospective study incorporating chemotherapy, surgery, and combined proton-photon radiotherapy. Cancer. 2002;94(10):2623–2634. doi: 10.1002/cncr.10537. [DOI] [PubMed] [Google Scholar]
- 12.Kim D W, Jo Y H, Kim J H. Neoadjuvant etoposide, ifosfamide, and cisplatin for the treatment of olfactory neuroblastoma. Cancer. 2004;101(10):2257–2260. doi: 10.1002/cncr.20648. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya N, Thornton A F, Joseph M P, Goodman M L, Amrein P C. Successful treatment of esthesioneuroblastoma and neuroendocrine carcinoma with combined chemotherapy and proton radiation. Results in 9 cases. Arch Otolaryngol Head Neck Surg. 1997;123(01):34–40. doi: 10.1001/archotol.1997.01900010038005. [DOI] [PubMed] [Google Scholar]
- 14.Eden B V, Debo R F, Larner J M. Esthesioneuroblastoma. Long-term outcome and patterns of failure--the University of Virginia experience. Cancer. 1994;73(10):2556–2562. doi: 10.1002/1097-0142(19940515)73:10<2556::aid-cncr2820731017>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 15.El Kababri M, Habrand J L, Valteau-Couanet D, Gaspar N, Dufour C, Oberlin O. Esthesioneuroblastoma in children and adolescent: experience on 11 cases with literature review. J Pediatr Hematol Oncol. 2014;36(02):91–95. doi: 10.1097/MPH.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 16.Loy A H, Reibel J F, Read P W. Esthesioneuroblastoma: continued follow-up of a single institution's experience. Arch Otolaryngol Head Neck Surg. 2006;132(02):134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 17.Patil V M, Joshi A, Noronha V. Neoadjuvant chemotherapy in locally advanced and borderline resectable nonsquamous sinonasal tumors (esthesioneuroblastoma and sinonasal tumor with neuroendocrine differentiation) Int J Surg Oncol. 2016;2016:6.92373E6. doi: 10.1155/2016/6923730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyams V J, Batsakis J G, Michaels L. Washington, DC: Armed Forces Institute of Pathology; 1988. Tumors of the upper respiratory tract and ear; p. 343. [Google Scholar]
- 19.Morita A, Ebersold M J, Olsen K D, Foote R L, Lewis J E, Quast L M. Esthesioneuroblastoma: prognosis and management. Neurosurgery. 1993;32(05):706–714. doi: 10.1227/00006123-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Eisenhauer E A, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(02):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Kiyota N, Tahara M, Fujii S. Nonplatinum-based chemotherapy with irinotecan plus docetaxel for advanced or metastatic olfactory neuroblastoma: a retrospective analysis of 12 cases. Cancer. 2008;112(04):885–891. doi: 10.1002/cncr.23246. [DOI] [PubMed] [Google Scholar]
- 22.Czapiewski P, Kunc M, Haybaeck J. Genetic and molecular alterations in olfactory neuroblastoma: implications for pathogenesis, prognosis and treatment. Oncotarget. 2016;7(32):52584–52596. doi: 10.18632/oncotarget.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lok S W, Whittle J R, Vaillant F. A phase Ib dose-escalation and expansion study of the BCL2 inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 2019;9(03):354–369. doi: 10.1158/2159-8290.CD-18-1151. [DOI] [PubMed] [Google Scholar]
- 24.Marinelli J P, Jr, Janus J R, Van Gompel J J. Dural invasion predicts the laterality and development of neck metastases in esthesioneuroblastoma. J Neurol Surg B Skull Base. 2018;79(05):495–500. doi: 10.1055/s-0038-1625977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amit M, Abdelmeguid A S, Watcherporn T. Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J Clin Oncol. 2019;37(06):504–512. doi: 10.1200/JCO.18.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]


