Abstract
Objective Surgical resection is widely accepted as a critical component for definitive treatment of sinonasal mucosal melanoma. Systemic immunotherapy, including multiple newer agents, has been used to treat metastatic or unresectable disease. In this study, we examine its efficacy in locoregional control when used in conjunction with surgical resection for primary mucosal lesions.
Design Present study is a retrospective review of all patients at a tertiary academic medical center with primary sinonasal mucosal melanoma and distant metastatic disease.
Results A total of four patients were identified. In all cases, patients were treated with a combination of surgical resection of the primary tumor and systemic immunotherapy. Three patients were initially treated with surgery at the primary site followed by immunotherapy for distant metastases. Response to immunotherapy at the sites of primary and metastatic disease was seen in two patients. All four patients developed progression or recurrence at the primary site following initiation of immunotherapy for which they underwent surgical resection. One patient remains in follow-up without evidence of disease 20 months after initial treatment; three succumbed to the disease at 135, 37, and 16 months after initial treatment.
Conclusion Surgical resection for local control plays a critically important role in the treatment of sinonasal mucosal melanoma regardless of the presence of metastases and whether immunotherapy will be given. This case series suggests that, though immunotherapy may demonstrate efficacy in managing distant disease, surgery should remain the first-line treatment for the primary site.
Keywords: mucosal melanoma, sinonasal malignancy, endoscopic skull base surgery, immunotherapy
Introduction
Sinonasal mucosal melanoma (SNMM) is a rare, highly aggressive malignancy associated with advanced stage at initial presentation, high rates of disease recurrence, propensity for distant metastasis, and poor overall prognosis. 1 2 3 Surgical resection, using endoscopic and/or open approaches, is widely accepted as a critical component for definitive treatment and is considered as the first-line therapy for control at the primary site, and adjuvant radiotherapy and chemotherapy are often given. 4 5 Ipilimumab, pembrolizumab, and nivolumab, which activate antitumor cytotoxic T cells through different mechanisms, are Food and Drug Administration (FDA) approved for use in metastatic or unresectable melanoma. 6 Recent clinical trials have demonstrated disease response to these therapies ranging from 12 to 37% for patients with mucosal melanoma, though the sites of response are unknown. 7 Limited evidence exists on the efficacy of such targeted immunotherapies in locoregional control when used in conjunction with surgical resection for primary mucosal lesions. 8 9
Existing studies have not investigated the role of surgery for the primary site in the setting of existing distant metastasis for mucosal melanomas. For cutaneous melanomas, there is a survival benefit for surgical resection with curative intent in patients with advanced-stage disease with limited numbers and sites of distant metastases. 10 Though it is often assumed that surgical resection of the primary tumor should be avoided if distant metastases are present, 11 we hypothesized that surgery at the primary site may still be an important adjunctive treatment strategy for patients with metastatic disease. In the population of patients receiving systemic targeted immunotherapy, the addition of surgery may be required to control disease at the primary site and reduce the rate of local recurrence. Furthermore, the benefit of surgery may still hold true for patients who have previously undergone resection at the primary site and present with local recurrence. This case series further highlights the low morbidity of surgery.
Methods
After institutional review board's approval, we conducted a retrospective chart review of all patients diagnosed with primary SNMM, with distant metastatic disease, treated with targeted immunotherapy, followed by surgical resection of the primary lesion at the Hospital of the University of Pennsylvania between 2010 and 2017. The study included patients operated on by the senior authors (N.D.A., J.N.P., M.S.G., and B.W.O. Jr.) for resection of sinonasal mucosal melanoma which resulted in four patients who were included for further analysis in this case series ( Table 1 ).
Table 1. Summary of individual patient case presentations.
| Patient | Clinical History |
|---|---|
| 1 | A 60-year-old female found to have a nasal polyp consistent with malignant melanoma, first treated with subtotal resection and radiation therapy with disease control for 6 years, followed by local recurrence and regional and distant metastases, all of which were treated surgically or radiotherapy. She was then treated with ipilimumab and pembrolizumab. The hepatic metastases responded to treatment, but the patient developed local recurrence unresponsive to continued immunotherapy and eventually required reresection. The patient later developed distant metastases and succumbed to disease 135 months after diagnosis. |
| 2 | A 44-year-old male found to have sinonasal malignant melanoma after expelling a large piece of tissue from his nose. He was treated with a combined open and endoscopic resection but developed local recurrence and regional and distant metastases, so was started on ipilimumab and radiation therapy. This was followed by endoscopic gross-total resection at the primary site due to rapid disease progression in this region. He developed progression of disease and both primary and distant sites despite continued immunotherapy and succumbed to disease 16 months after diagnosis. |
| 3 | An 85-year-old female with worsening nasal congestion and bloody postnasal drip was found to have a nasal mass consistent with malignant melanoma. She was also found to have an adrenal metastasis. She was treated with pembrolizumab, which led to relative stability of the adrenal metastasis but progression at the primary site, so underwent endoscopic resection at the primary site. This was followed by adjuvant radiation therapy. The patient is currently doing well off immunotherapy without evidence of active disease 33 months after diagnosis. |
| 4 | A 70-year-old female found to have sinonasal malignant melanoma and multiple sites of distant metastases. The primary site was treated with surgical resection followed by ipilimumab. Though there was initially a partial response at both the primary and distant sites, there was later progression of disease at the primary site and so she underwent endoscopic resection. She later had progression of distant metastases despite multiple immunotherapy regimens and succumbed to disease 37 months after diagnosis. |
Outcomes included response to immunotherapy, surgical approach, extent of surgical resection, postoperative complications (if applicable), location and degree of recurrence (if applicable), and overall survival from the time of diagnosis.
Results
Case 1
The patient is a 60-year-old female who was found to have stage-III SNMM after routine biopsy of a nasal mass. She was initially treated with an endoscopic subtotal septectomy at an outside institution. The lesion was found to originate from the right nasal septum but gross tumor was unable to be fully resected from the superior and posterior septum. Postoperatively, the patient was asymptomatic. The patient deferred completion of surgical resection of the melanoma so was treated with intensity-modulated radiation therapy to the primary site (6,000 cGy in 30 fractions), resulting in disease control for 6 years. She was then found to have local recurrence at the skull base and underwent a more extensive combined open and endoscopic resection. The mass was found to originate along the left ethmoid skull base. Due to its intradural involvement, the dura was resected with negative margins and the skull base defected reconstructed with a pericranial flap. She was found to have regional recurrence in the right neck, so underwent a selective neck dissection followed by a revision neck dissection for recurrent disease. The following year she developed a recurrence in the sphenoid and underwent endoscopic resection of the mass from the sphenoid rostrum, planum sphenoidale, dorsum sella, and anterior wall of pituitary. Liver metastases were subsequently found and were treated with Cyber Knife radiosurgery. The patient was then started on ipilimumab 3 mg/kg at every 3 weeks, initially demonstrating a response but was followed by local and distant disease progression after 9 months. Immunotherapy was changed to pembrolizumab 2 mg/kg at every 3 weeks. While on pembrolizumab, the patient underwent three additional endoscopic resections for local recurrence in the sphenoid and along the anterior skull base. Apart from intermittent epistaxis, the patient tolerated each procedure well and did not develop postoperative complications. The patient developed leptomeningeal metastases and succumbed to her disease from refractory seizures while on hospice care 135 months after initial diagnosis.
Case 2
The patient is a 44-year-old male who blew his nose after a car accident and expelled a large piece of tissue from his right nasal cavity. The tissue was brought to his primary doctor and was sent for pathology, demonstrating stage-III SNMM without regional or distant disease on staging workup. The tumor was found to be originating from the superior nasal septum anterior to the frontal recess. He underwent combined open and endoscopic resection which involved an endoscopic septectomy as well as a lateral rhinotomy for resection of the anterior maxillary face, piriform aperture, middle turbinate, inferior turbinate, ethmoids, sphenoid, nasal dorsum, and anterior skull base from the frontal recess to the sphenoid–septal junction. He deferred adjuvant therapy and developed local recurrence and regional and distant hepatic and pulmonary metastases after 9 months. The patient was treated with a modified radical left neck dissection with 1 of 38 nodes positive. He was started adjuvant radiation therapy (2,400 cGy in three fractions) and ipilimumab 3 mg/kg at every 3 weeks as part of a clinical trial. Endoscopic gross-total reresection of the remaining nasal septum, anterior skull base, and sphenoclival junction was performed due to a lack of treatment response which was well-tolerated without complications. The patient developed metastases to the brain requiring craniotomy for resection followed by Gamma Knife stereotactic radiosurgery. He continued on immunotherapy but succumbed 16 months after diagnosis while on hospice care.
Case 3
The patient is an 85-year-old female who presented with worsening nasal congestion and bloody postnasal drip. She was found to have a nasal mass originating from the left posterior nasal septum and an adrenal metastasis consistent with stage-IV SNMM ( Fig. 1 ). The patient was started on pembrolizumab 2mg/kg at every 3 weeks but demonstrated disease progression at both the primary ( Fig. 2 , left) and distant sites after 3 months of treatment. The patient then underwent endoscopic resection ( Fig. 2 , right) with gross-total resection of disease. Postoperatively, the patient did well and was largely asymptomatic. This was followed by adjuvant radiotherapy at the primary site (4,500 cGy in 18 fractions) and stereotactic body radiation therapy (1,200 cGy in two fractions) to the adrenal metastasis. No further treatment has been given to date. The patient is currently doing well without evidence of active disease 14 months after diagnosis. Recent positron emission tomography (PET) imaging demonstrated stable fludeoxyglucose (FDG) avidity of the adrenal mass and no local disease ( Fig. 3 ).
Fig. 1.
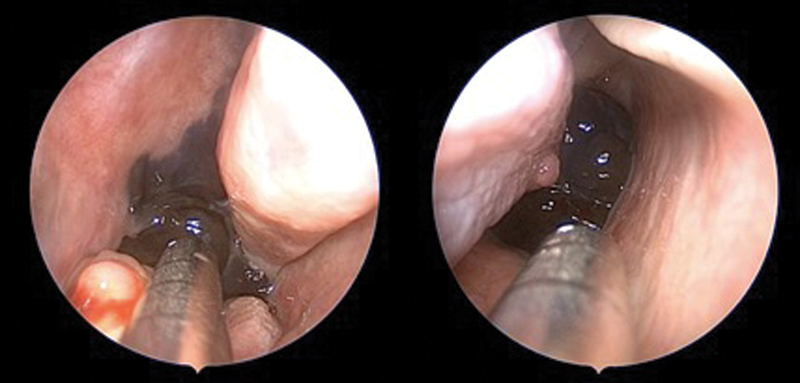
Intraoperative images demonstrating melanoma involving the left posterior nasal septum (left) with extension into the nasopharynx on the contralateral side (right).
Fig. 2.
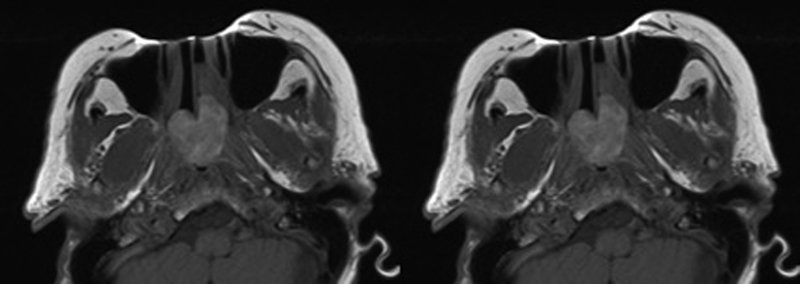
Preoperative T1-weighted post-contrast MRI images demonstrating a large polypoid mass originating from the left posterior nasal cavity protruding into the nasopharynx and filling the right choana (left). Postoperative scans with expected postsurgical changes but no evidence of disease (right). MRI, magnetic resonance imaging.
Fig. 3.
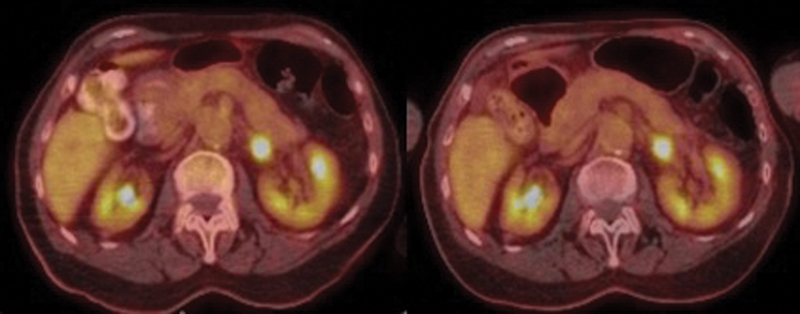
Fused PET/CT images demonstrating stability of adrenal metastasis before (left) and after (right) treatment with immunotherapy. CT, computed tomography; PET, positron emission tomography.
Case 4
The patient is a 70-year-old female who underwent endoscopic sinus surgery at an outside institution and was found to have stage-IV SNMM originating from the right inferior turbinate, as well as multiple metastases, to the right femoral head, lung pleura, peritoneum, and subcutaneous soft tissues of the leg and flank. She underwent near-total resection of the mass, followed by ipilimumab 3 mg/kg for her distant disease. Immunotherapy initially demonstrated a partial response at the primary and distant sites but was transitioned to pembrolizumab 2 mg/kg at every 3 weeks after three doses of ipilimumab due to autoimmune toxicity, with later addition of dabrafenib and trametinib. However, local disease progressed in the right maxillary sinus, so endoscopic reresection was performed in which gross-total resection was achieved. She was then treated with adjuvant radiotherapy with 5,100 cGy in 17 fractions to the primary site and 3,000 cGy in 10 fractions to each distant site in the lower extremities and spine. The patient continued on immunotherapy, including regimens of pembrolizumab, dabrafenib, trametinib, and ipilimumab but had progression of distant metastases in the extremities, subcutaneous soft tissues, and spine. She succumbed to disease 37 months after diagnosis.
In all cases, patients were treated with a combination of surgical resection of the primary lesion and targeted immunotherapy ( Table 2 ). Three patients were initially treated with surgery at the primary site followed by immunotherapy for distant metastases. Response to immunotherapy at the sites of metastatic disease was seen in three patients; however, all four patients developed disease progression or recurrence at the primary site following initiation of immunotherapy for which they underwent surgical resection. Gross-total resection was achieved in three patients. Following treatment with both surgery and immunotherapy, three patients maintained locoregional control. None of the patients developed intraoperative or postoperative cerebrospinal fluid (CSF) leak. Following surgical resection performed after initiation of immunotherapy, only one patient developed recurrence at the primary site. One patient remains in follow-up without evidence of disease 20 months after initial diagnosis; three succumbed to the disease at 135, 16, and 37 months after initial diagnosis.
Table 2. Outcomes following treatment of SNMM with systemic immunotherapy and surgical resection at the primary site.
| Pt. | Initial treatment | Response to immunotherapy | Resection after immunotherapy | Recurrence after immunotherapy and surgery | Survival (mo) | |
|---|---|---|---|---|---|---|
| Primary site | Distant sites | |||||
| 1 | Surgery | Progression | Response (hepatic) | Subtotal | Distant | 135 |
| 2 | Surgery | Progression | Progression (pulmonary, intracranial, and hepatic) | Gross total | Local, distant | 16 |
| 3 | Immunotherapy | Progression | Stability (adrenal) | Gross total | None | 20 a |
| 4 | Surgery | Progression | Response (osseous, soft tissue, and pulmonary) | Gross total | Distant | 37 |
Abbreviations: Pt, patient; SNMM, sinonasal mucosal melanoma.
Survival to date.
Discussion
Sinonasal mucosal melanoma is a rare entity associated with extremely poor prognosis. 12 It accounts for less than 4% of melanomas of the head and neck and less than 1% of all melanomas. 13 The most common presenting symptoms include epistaxis and nasal obstruction. 14 A review of the literature demonstrated an approximate 5-year overall survival of 25 to 40%. 2 3 15 16 17 18 Its poor prognosis is often attributed to its late stage of presentation and aggressive tumor biology. 19 The most common cause of treatment failure for SNMM is distant metastases (35%), followed by local (18%) and regional (11%) recurrence. 16 Whenever feasible, surgical excision, whether open or endoscopic (or a combined approach), is the standard treatment for SNMM. 20 21 Adjuvant radiotherapy is often given, which has been suggested to improve locoregional control, 22 though has not been demonstrated to improve overall survival. 4 23 The addition of postoperative chemotherapy has similarly not been shown to improve overall survival and is typically reserved for patients with metastatic disease. However, multimodality therapy has been demonstrated in several studies to improve overall survival. 4 18
Limited data exists on the effect of immunotherapy for SNMM, partly due to the relatively low prevalence of SNMM and the recent introduction of immunotherapy agents. Ipilimumab, nivolumab, and pembrolizumab are currently FDA-approved for treatment of melanoma, though the vast majority of data on these immunotherapies is limited to cutaneous melanomas. Ipilimumab is an anti-CTLA4 antibody that increases cytotoxic T-cell activity, while nivolumab and pembrolizumab are anti-PD-1 antibodies that allow bypassing of immune checkpoints. 14 A retrospective analysis of 12 patients treated with ipilimumab with locally advanced or metastatic SNMM demonstrated a partial response in one patient and stable or progressive disease in the remaining 11. 24 In a series of 71 patients with mucosal melanoma, of which 15 were sinonasal, treatment with ipilimumab led to an overall response rate of 12% in all patients. 25 An analysis of patients with mucosal melanomas demonstrated improved overall response rate and progression-free survival in patients receiving combined anti-CTLA4 and anti-PD-1 therapy compared with either treatment alone; however, the primary site was not included in this analysis. 26
While these newer therapies may play a complementary role for patients with metastatic disease, our results suggest that local control with surgical resection remains critical in the treatment strategy for SNMM. In this study, we describe four cases of SNMM treated with a combination of surgery and targeted immunotherapy. It is widely accepted that surgical resection is the mainstay of treatment for SNMM and may confer a survival advantage. 7 27 Large national cancer database analyses investigating sinonasal malignancies as a whole have suggested that adequate local control improves overall survival which was seen in patients with surgically accessible tumors or negative surgical margins. 12 16 28 Prior studies have demonstrated that failure to achieve local control for SNMM may increase the risk of distant metastasis and death. 29 30 With recent FDA approval and increasing use of several targeted immunotherapy agents for metastatic melanoma, treatment paradigms for sinonasal mucosal melanoma have begun to shift, with several clinical trials investigating their effectiveness in this disease entity. This is the first study in the literature to specifically examine the role of targeted immunotherapy in SNMM, giving attention to local and distant response to treatment.
In this series, no postoperative complications occurred; in particular, none of our patients developed postoperative CSF leak. Three of the four patients underwent multiple resections for recurrent disease at the primary site, all of which were well-tolerated and uncomplicated. Resection of sinonasal masses has also been demonstrated to improve quality of life in multiple domains. 31 32 This also held true for patients with sinonasal malignancies. 33 34 Other studies have demonstrated similar improvements in symptoms reduction following surgical resection for SNMM regardless of approach, though endoscopic surgery was associated with lower morbidity and comparable disease control. 27 35 This low-complication rate may be due in part to a more conservative approach to highly invasive disease at the skull base. The impact of near-total versus gross-total resection did not seem to impact the rate of local recurrence in our series, though others have suggested improved survival in patients with negative surgical margins.
The disease's high propensity for recurrence at the primary site despite systemic therapy, which occurred in all four patients, may indicate that targeted immunotherapy alone may not be sufficient to treat disease at the primary site. Among patients who demonstrated a response to immunotherapy, only a brief partial response was seen at the primary site and was always followed by primary site progression or recurrence. This is the first paper in the literature to investigate the outcomes of patients with SNMM treated with both surgery at the primary site and targeted immmunotherapy. Won et al investigated outcomes after treatment of SNMM and found that postoperative systemic therapy, which included both targeted and nontargeted immunotherapies, did not affect the rate of local recurrence. 17 A recent review of the National Cancer Database on patients with SNMM suggested that immunotherapy was associated with improved survival in patients with metastatic disease. 28 Our work is the first to suggest that, more specifically, targeted immunotherapy may show limited effectiveness in managing disease at the primary site. This case series suggests that, though immunotherapy may demonstrate some efficacy in managing distant disease, surgery may still be the more effective means of treatment for the primary site compared with systemic therapies. Due to the rarity of this disease entity, additional data are needed to establish a treatment regimen with proven survival benefit over other modalities. From our experience, disease in the sinonasal cavity that did not exhibit a durable response to targeted immunotherapy and required surgical resection for local control. Future analyses of immunotherapy in this rare disease process would benefit from comparison with patient cohorts that have not received surgical treatment. Because of the limited size of this series, further studies are required to evaluate the impact of targeted immunotherapy and surgery on disease-free and overall survival.
Conclusion
Sinonasal mucosal melanoma is a rare aggressive malignancy with a high propensity for both local recurrence and distant metastasis. Surgical resection has long been the mainstay of treatment of the primary site, but novel targeted immunotherapies have started to change the landscape of melanoma treatment, especially in patients with metastatic disease. Here, we present a case series of four patients with SNMM treated with surgery and immunotherapy. All patients tolerated surgical resection and reresection well without complications, with improvement in preoperative symptoms of nasal obstruction, and epistaxis postoperatively. After receiving targeted immunotherapy, all patients developed disease progression or recurrence at the primary site, regardless of whether they demonstrated partial transient response at distant sites. These results may suggest that in the sinonasal cavity, systemic immunotherapy may have limited value at the primary site in maintaining local disease control. Surgical resection should remain as the treatment of choice for managing local disease and be considered for curative or palliative intent even in patients receiving therapy for regional or distant metastases.
Funding Statement
Funding None.
Conflict of Interest B.W.O. Jr. reports other from Olympus, outside the submitted work; In addition, B.W.O. Jr. has a patent FKWO Retractor (Olympus) with royalties paid.
Note
This article was originally presented as a poster presentation at the North American Skull Base Society Annual Meeting in Coronado, California on February 16, 2018.
References
- 1.Moreno M A, Roberts D B, Kupferman M E. Mucosal melanoma of the nose and paranasal sinuses, a contemporary experience from the M. D. Anderson Cancer Center. Cancer. 2010;116(09):2215–2223. doi: 10.1002/cncr.24976. [DOI] [PubMed] [Google Scholar]
- 2.Roth T N, Gengler C, Huber G F, Holzmann D. Outcome of sinonasal melanoma: clinical experience and review of the literature. Head Neck. 2010;32(10):1385–1392. doi: 10.1002/hed.21340. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi D, Bottazzoli M, Turri-Zanoni M.Sinonasal mucosal melanoma: A 12-year experience of 58 cases Head Neck 201638(S1, Suppl 1):E1737–E1745. [DOI] [PubMed] [Google Scholar]
- 4.Gore M R, Zanation A M. Survival in sinonasal melanoma: a meta-analysis. J Neurol Surg B Skull Base. 2012;73(03):157–162. doi: 10.1055/s-0032-1301400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayed Z, Migliacci J C, Cracchiolo J R. Association of Surgical approach and margin status with oncologic outcomes following gross total resection for sinonasal melanoma. JAMA Otolaryngol Head Neck Surg. 2017;143(12):1220–1227. doi: 10.1001/jamaoto.2017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashat L, Le C H, Chiu A G. The role of targeted therapy in the management of sinonasal malignancies. Otolaryngol Clin North Am. 2017;50(02):443–455. doi: 10.1016/j.otc.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Amit M, Na'ara S, Hanna E Y. Contemporary treatment approaches to sinonasal mucosal melanoma. Curr Oncol Rep. 2018;20(02):10. doi: 10.1007/s11912-018-0660-7. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer T, Satzger I, Gutzmer R. Clinics, prognosis and new therapeutic options in patients with mucosal melanoma: a retrospective analysis of 75 patients. Medicine (Baltimore) 2017;96(01):e5753. doi: 10.1097/MD.0000000000005753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crippen M M, Kılıç S, Eloy J A. Updates in the management of sinonasal mucosal melanoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26(01):52–57. doi: 10.1097/MOO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 10.Essner R, Lee J H, Wanek L A, Itakura H, Morton D L. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139(09):961–966. doi: 10.1001/archsurg.139.9.961. [DOI] [PubMed] [Google Scholar]
- 11.Lee G, Baek C-H, Choi N Y, Chung M K. The prognostic role of the surgical approach and adjuvant therapy in operable mucosal melanoma of the head and neck. Clin Exp Otorhinolaryngol. 2017;10(01):97–103. doi: 10.21053/ceo.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robin T P, Jones B L, Gordon O M. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer. 2017;123(16):3040–3049. doi: 10.1002/cncr.30686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Ch'ng S, Shannon K F, Ashford B. 2013. pp. 2432–2438. [Google Scholar]
- 14.Yde S S, Sjoegren P, Heje M, Stolle L B. Mucosal melanoma: a literature review. Curr Oncol Rep. 2018;20(03):28. doi: 10.1007/s11912-018-0675-0. [DOI] [PubMed] [Google Scholar]
- 15.Gal T J, Silver N, Huang B. Demographics and treatment trends in sinonasal mucosal melanoma. Laryngoscope. 2011;121(09):2026–2033. doi: 10.1002/lary.21925. [DOI] [PubMed] [Google Scholar]
- 16.Amit M, Tam S, Abdelmeguid A S. Patterns of treatment failure in patients with sinonasal mucosal melanoma. Ann Surg Oncol. 2018;25(06):1723–1729. doi: 10.1245/s10434-018-6465-y. [DOI] [PubMed] [Google Scholar]
- 17.Won T-B, Choi K Y, Rhee C-S. Treatment outcomes of sinonasal malignant melanoma: a Korean multicenter study. Int Forum Allergy Rhinol. 2015;5(10):950–959. doi: 10.1002/alr.21558. [DOI] [PubMed] [Google Scholar]
- 18.Sun C-Z, Li Q-L, Hu Z-D, Jiang Y-E, Song M, Yang A-K. Treatment and prognosis in sinonasal mucosal melanoma: a retrospective analysis of 65 patients from a single cancer center. Head Neck. 2014;36(05):675–681. doi: 10.1002/hed.23355. [DOI] [PubMed] [Google Scholar]
- 19.Tas F, Keskin S, Karadeniz A.Noncutaneous melanoma have distinct features from each other and cutaneous melanoma Oncology 201181(5,6):353–358. [DOI] [PubMed] [Google Scholar]
- 20.Miglani A, Patel S H, Kosiorek H E, Hinni M L, Hayden R E, Lal D. Endoscopic resection of sinonasal mucosal melanoma has comparable outcomes to open approaches. Am J Rhinol Allergy. 2017;31(03):200–204. doi: 10.2500/ajra.2017.31.4435. [DOI] [PubMed] [Google Scholar]
- 21.Cao W, Guan B, Yu A. Treatment and outcomes of endoscopic surgery and traditional open resection in sinonasal mucosal melanoma. Acta Otolaryngol. 2017;137(08):862–867. doi: 10.1080/00016489.2017.1300939. [DOI] [PubMed] [Google Scholar]
- 22.Caspers C JI, Dronkers E AC, Monserez D, Wieringa M H, Baatenburg de Jong R J, Hardillo J AU. Adjuvant radiotherapy in sinonasal mucosal melanoma: A retrospective analysis. Clin Otolaryngol. 2018;43(02):617–623. doi: 10.1111/coa.13033. [DOI] [PubMed] [Google Scholar]
- 23.Amit M, Tam S, Abdelmeguid A S. Role of adjuvant treatment in sinonasal mucosal melanoma. J Neurol Surg B Skull Base. 2017;78(06):512–518. doi: 10.1055/s-0037-1604350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Postow M A, Luke J J, Bluth M J. Ipilimumab for patients with advanced mucosal melanoma. Oncologist. 2013;18(06):726–732. doi: 10.1634/theoncologist.2012-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Vecchio M, Di Guardo L, Ascierto P A. Efficacy and safety of ipilimumab 3mg/kg in patients with pretreated, metastatic, mucosal melanoma. Eur J Cancer. 2014;50(01):121–127. doi: 10.1016/j.ejca.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 26.D'Angelo S P, Larkin J, Sosman J A. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(02):226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund V J, Chisholm E J, Howard D J, Wei W I. Sinonasal malignant melanoma: an analysis of 115 cases assessing outcomes of surgery, postoperative radiotherapy and endoscopic resection. Rhinology. 2012;50(02):203–210. doi: 10.4193/Rhino.11.267. [DOI] [PubMed] [Google Scholar]
- 28.Ganti A, Raman A, Shay A.Treatment modalities in sinonasal mucosal melanoma: A national cancer database analysisLaryngoscope2019 [DOI] [PubMed]
- 29.Penel N, Mallet Y, Mirabel X, Van J T, Lefebvre J-L. Primary mucosal melanoma of head and neck: prognostic value of clear margins. Laryngoscope. 2006;116(06):993–995. doi: 10.1097/01.mlg.0000217236.06585.a9. [DOI] [PubMed] [Google Scholar]
- 30.Manjunath L, Derousseau T, Batra P S. Prognostic value of surgical margins during endoscopic resection of paranasal sinus malignancy. Int Forum Allergy Rhinol. 2015;5(05):454–459. doi: 10.1002/alr.21463. [DOI] [PubMed] [Google Scholar]
- 31.Harrow B R, Batra P S. Sinonasal quality of life outcomes after minimally invasive resection of sinonasal and skull-base tumors. Int Forum Allergy Rhinol. 2013;3(12):1013–1020. doi: 10.1002/alr.21200. [DOI] [PubMed] [Google Scholar]
- 32.Ransom E R, Doghramji L, Palmer J N, Chiu A G. Global and disease-specific health-related quality of life after complete endoscopic resection of anterior skull base neoplasms. Am J Rhinol Allergy. 2012;26(01):76–79. doi: 10.2500/ajra.2012.26.3713. [DOI] [PubMed] [Google Scholar]
- 33.Glicksman J T, Parasher A K, Brooks S G. Sinonasal quality of life after endoscopic resection of malignant sinonasal and skull base tumors. Laryngoscope. 2018;128(04):789–793. doi: 10.1002/lary.26833. [DOI] [PubMed] [Google Scholar]
- 34.Derousseau T, Manjunath L, Harrow B, Zhang S, Batra P S. Long-term changes in quality of life after endoscopic resection of sinonasal and skull-base tumors. Int Forum Allergy Rhinol. 2015;5(12):1129–1135. doi: 10.1002/alr.21608. [DOI] [PubMed] [Google Scholar]
- 35.Ledderose G J, Leunig A. Surgical management of recurrent sinonasal mucosal melanoma: endoscopic or transfacial resection. Eur Arch Otorhinolaryngol. 2015;272(02):351–356. doi: 10.1007/s00405-014-3119-y. [DOI] [PubMed] [Google Scholar]


