Abstract
Background En plaque meningiomas are a rare subtype of meningiomas that are frequently encountered in the spheno-orbital region. Characterized by a hyperostotic and dural invasive architecture, these tumors present unique diagnostic and treatment considerations.
Objective The authors conduct a narrative literature review of clinical reports of en plaque meningiomas to summarize the epidemiology, clinical presentation, diagnostic criteria, and treatment considerations in treating en plaque meningiomas. Additionally, the authors present a case from their own experience to illustrate its complexity and unique features.
Methods A literature search was conducted using the MEDLINE database using the following terminology in various combinations: meningioma , meningeal neoplasms, en plaque , skull base , spheno-orbital, and sphenoid wing . Only literature published in English between 1938 and 2018 was reviewed. All case series were specifically reviewed for sufficient data on treatment outcomes, and all literature was analyzed for reports of misdiagnosed cases.
Conclusion En plaque meningiomas may present with a variety of symptoms according to their location and degree of bone invasion, requiring a careful diagnostic and treatment approach. While early and aggressive surgical resection is generally accepted as the optimal goal of treatment, these lesions require an individualized approach, with further investigation needed regarding the role of new therapies.
Keywords: en plaque meningioma, skull base, sphenoid wing, spheno-orbital, recurrent meningioma
Introduction
Meningiomas are the most common type of primary brain tumor with reported age adjusted incidences as high as 7.8 cases per 100,000 persons for World Health Organization (WHO) Grade I meningiomas in the United States. 1 Meningiomas en plaque (MEP) are a rare subtype of meningioma that comprise only 2 to 9% of all meningiomas. 2 3 4 MEP are unique from the more common en masse meningiomas and defined by their characteristic “carpet-like” invasion of adjacent bone, with extensive hyperostosis and dural thickening. 5 MEP are primarily located in the spheno-orbital regions and less frequently along the cerebral convexity, temporal bone, and foramen magnum. 4 6 They present a diagnostic challenge due to their unusual radiologic appearance and are surgically challenging due to their tendency to invade bony structures and infiltrate nearby fissures and foramina. 7 8 9 10 Debate still continues regarding the need for gross total resection with concern for increased surgical morbidity associated with cavernous sinus infiltration, significant skull base reconstruction, and proximity to neurovascular structures. The era of adjuvant radiation has further contributed to the argument against overtly invasive efforts to achieve a gross total resection. To date, a recent updated comprehensive overview on management of MEP of the spheno-orbital region does not exist. One recent review focused entirely on convexity MEP. 4 Current literature available on MEP consists primarily of case reports and case series. The objective of this chapter is to synthesize a comprehensive narrative review of the clinical presentation, diagnostic workup, and treatment considerations for MEP in this modern medical era with new treatment modalities and means to access the skull base.
Methods
A systematic review of the literature was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( Fig. 1 ). The PubMed/MEDLINE, Scopus, and Ovid databases were queried using the following terminology 11 : meningioma , meningeal neoplasms, en plaque , skull base , spheno-orbital, and sphenoid wing . Only literature published in English between 1938 and 2018 was included, yielding 471 abstracts after removal of duplicates. Exclusion criteria for screening abstracts included letters to the editors, cadaveric or postmortem studies, radiological studies, technical case reports, and animal studies. Application of inclusion and exclusion criteria left a total of 231 papers. The full-text articles were then reviewed for their contribution to clinical presentation, diagnosis, and treatment of spheno-orbital tumors of the sphenoid wing. A total of nine case series met full criteria for inclusion, all of which were retrospective in nature.
Fig. 1.
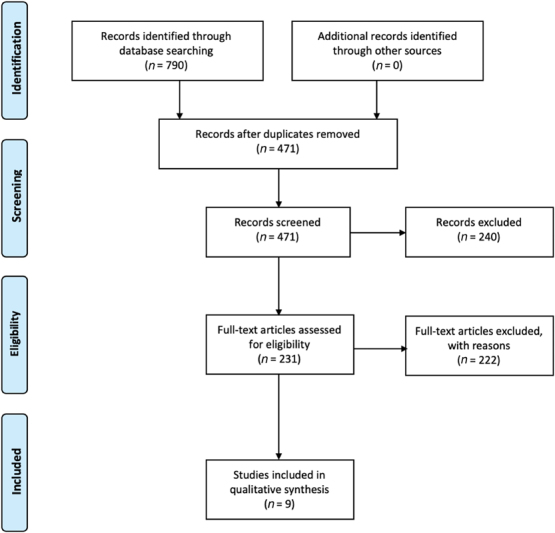
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram for systematic literature reviews.
Results
The majority of the available literature on MEP are in the form of case reports and case series, and all were retrospective studies. There were a limited number of available case series that exclusively describe en plaque lesions and that also provided adequate follow-up information necessary to monitor outcomes, complications, and recurrence rates. 4 A major challenge in analyzing outcomes of these studies is the wide variation in follow-up time. One study specifically stated that their data are significantly influenced by length of follow-up. 8 The average lengths of follow-up time for the studies, described in Table 1 , range from 30 to 136 months, with no standard length of time used for reporting overall survival or mean or median time to tumor recurrence or progression. The results of these studies are summarized in Table 1 .
Table 1. Summary of en plaque meningioma case series data and outcomes collected.
| Author | Year | n | Sex ratio (female: male) | Mean age | Gross total resection achieved | Postoperative radiation received | Second-stage surgery performed | Tumor recurrence | Residual tumor progression | Mean follow-up period (months) | Additional notes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amirjamshidi et al 2 | 2015 | 88 | 1.8:1 | 46 | 36% | 36% | 0% | 14% | n/a | 136 | Also compared lateral supraorbital vs. pterional approach |
| Boari et al 20 | 2013 | 40 | n/a | n/a | 56% | 44% | 5% | 10% | n/a | 73 | – |
| De Jesus a | 2001 | 6 | 6:0 | 51 | 83% | 17% | 33% | 17% | 17% | 48 | Approached using frontotemporal craniectomy with orbital decompression |
| Jiranukool et al 23 | 2016 | 26 | 25:1 | 44 | 4% | 44% | 15% | 0% | 36% | 52 | – |
| Li et al 10 | 2009 | 37 | 1.5:1 | 46 | 16% | 16% | 0% | 16% | 23% | 30 | Postoperative radiation given via gamma knife radiosurgery |
| Mirone et al 5 | 2009 | 71 | 6.9:1 | 53 | 83% | 1.0% | 1% | 5% | 25% | 77 | Average time to tumor recurrence: 43 months |
| Oya et al 15 | 2011 | 39 | 6.8:1 | 48 | 39% | 13% | 0% | 0% | 18% | 41 | Cavernous sinus meningiomas with intraorbital extension were excluded from study |
| Schick et al 8 | 2006 | 67 | 3.8:1 | 54 | 60% | 7.5% | 3% | 35% | 23% | 48 | Reports that tumor recurrence rate reported is significantly influenced by duration of follow-up |
| Simas and Farias 9 | 2013 | 18 | 4.9:1 | 52 | 39% | 17% | 0% | 28% | 18% | 54 | Postoperative radiation via radiosurgery (1 patient) and fractionated stereotactic radiation (2 patients) |
Data not provided.
Epidemiology
The incidence of en plaque meningiomas is three to six times more common in females than in males, with females comprising between 53 and 100% of study populations for papers discussed in this review. 8 12 13 The mean age of presentation is in the fifth decade of life. Race and ethnic characteristics were not routinely reported in the studies included in this review. One study reported that MEP comprised ∼9% of all cases of intracranial meningiomas treated surgically at their institution. 13
Lack of accurate epidemiological data is likely influenced by the variation in classification schema for MEP, which has undergone significant evolution. MEP were first classified within sphenoid wing meningiomas by Cushing and Eisenhardt and distinguished from globular (clinoidal), middle third (alar), and outer third (pterional) meningiomas. 14 Methods of categorizing sphenoid meningiomas and MEP have undergone numerous iterations. Historically, there is some diagnostic overlap between MEP and of spheno-orbital meningiomas, without a pathological difference. Modern characterization of spheno-orbital meningiomas includes an intraosseous and/or hyperostosing component and intraorbital extension. 15 16 However, most recently Simon and Schramm have argued for a simplified approach broadly distinguishing between globular lateral and medial sphenoid wing meningiomas and hyperostosing en plaque/spheno-orbital meningiomas. Consideration of cavernous sinus involvement, tumor size, boney infiltration, and orbital invasion should be considered for all cases. 17 18 19
Diagnosis
Clinical Presentation
Clinical manifestations of en plaque meningiomas are generally based on tumor location and spread. Due to the high frequency of sphenoid wing location, boney invasion, hyperostosis, and compression of neural structures, the most common presenting symptom is proptosis followed by decreased visual acuity, retrobulbar pressure, visual field defects, headaches, orbital pain, temporal swelling, and periorbital swelling. 9 10 20 Hearing loss is the most frequent symptom for lesions involving the temporal bone, but dizziness, tinnitus, and otorrhea have also been reported. 5 In some rare cases and more often with convexity MEP, subcutaneous palpable masses have been reported. 21 22
Radiographic Diagnosis
Radiographic imaging assists in diagnostic and treatment considerations by assessing the extent of involvement of surrounding structures, although preoperative imaging is known to underestimate extent of tumor infiltration. 9 23 First-line diagnostic imaging studies include both computed tomography (CT) and magnetic resonance imaging (MRI). Some authors prefer thin-slice CT bone window to best delineate bony involvement and hyperostosis, particularly at the skull base. 24 25 MRI will identify dural and intradural involvement, with a typical pattern of contrast enhancement seen on T1 postgadolinium sequences. 26 MRI will also help to characterize tumor invasion into the orbit, cavernous sinus, and clinoidal structures. Orbital invasion can be evaluated on postcontrast fat suppression T1-weighted MRI, which delineates the extent of dural enhancement and soft-tissue involvement. 24 Hyperostoses often are seen in a periosteal pattern with surface irregularity of involved structures and inward bulging of the lesion. These features may be subtle, but may help to differentiate from other types of hyperostotic lesions such as primary intraosseous meningioma. Many of these radiographic features are shared by other hyperostotic and dural-based lesions, leading to numerous cases of misdiagnosis ( Tables 2 and 3 ). 27 Intracranial tuberculoma, lymphoma, sarcoid, histiocytoma, plasmacytoma, and fibrosarcomas have been misdiagnosed as MEP, among other misdiagnoses. 28 29 When there is any suspicion of MEP, MRI should be obtained to best minimize the risk of misdiagnoses. Given the nonsurgical management of some of these lesions, a comprehensive preoperative history, physical, and laboratory testing are essential in addition to a nuanced understanding of the imaging features of MEP.
Table 2. Reports of lesions misdiagnosed as en plaque meningiomas.
| Actual diagnosis | Misdiagnosis | Findings/differentiating features of actual diagnosis from en plaque meningioma | Most recent referenced study | Management approach taken a |
|---|---|---|---|---|
| Diffuse large B cell lymphoma | En plaque meningioma | Radiography: bone lysis histopathology: diffusely infiltrative large cells with little cytoplasm, distinct nucleoli, and frequent mitotic figures Immunohistochemistry revealing CD10 and CD20 expression, high Ki67 |
Brito et al 26 | Corticosteroid therapy + chemotherapy |
| Primary dural lymphoma (low grade B cell) | En plaque meningioma | Histopathology: small basophilic infiltrating cells, with CD20 and CD138 expression and moderate Ki67 Molecular analysis and polymerase chain reaction (PCR): Immunoglobulin (Ig) chain rearrangements |
Kulkarni et al 60 | Partial surgical resection + radiation therapy |
| Plasmacytoma | En plaque meningioma | Histopathology: uniform plasmacytoid cells with nuclear anaplasia and IgG and k monoclonality (and bone biopsy without signs of myeloma) | Vaicys et al 61 | Partial surgical resection + chemotherapy + radiation therapy |
| Meningeal fibrosarcoma | En plaque meningioma | Histopathology: immunohistochemistry positive for vimentin reactivity, and negative for glial fibrillary acidic protein (GFAP) and epithelial membrane antigen (EMA) reactivity, high nuclear proliferation index, areas of necrosis present | Donnet et al 62 | Total surgical resection |
| Benign fibrous histiocytoma | En plaque meningioma | Histopathology: immunohistochemistry positive for vimentin reactivity negative for GFAP and EMA, low mitotic activity, no necrosis present | Deb et al 63 | Total surgical resection |
| Cerebral metastases | En plaque meningioma | Varies based on primary lesion; PET imaging usually recommended | Nowosielski et al 64 | Varied |
| Osteoma | En plaque meningioma | Radiography: arise from outer table, frequent sinus involvement, grow outward; intracranial extension rare Histopathology: sheets of lamellar and/or cancellous bone organized like haversian canals, minimal osteoblastic |
Chitkara et al 65 | Total surgical resection |
| Actinomycosis | En plaque meningioma | Histopathology: gram positive, nonacid-fast branching filamentous bacteria. Radiography: mimics intracranial abscess | Deora et al. 66 | Penicillin, ± amoxicillin or rifampicin |
| Coccidioidomycosis | En plaque meningioma | Histopathology: Spherules containing endospores, may mimic budding yeasts if ruptured. Cerebrospinal fluid (CSF) positive for completement-fixing antibodies Clinical: patients often have lymphadenopathy and pulmonary symptoms |
Komotar and Clatterbuck 67 | High-dose fluconazole, consider surgical debulking |
| Neurosarcoidosis | En plaque meningioma | Intraoperative appearance: dura appearing as red fibrous mass Histopathology: noncaseating and necrotizing granulomas (and no evidence of acid-fast bacilli or fungi) |
Tobias et al 68 | Total surgical resection + corticosteroid therapy |
| Tuberculoma | En plaque meningioma | Histopathology: tubercles of necrotic areas and Langhans giant cells, acid-fast bacilli on seen on Ziehl–-Neelsen staining | Kumar et al 49 | Total surgical resection + antituberculosis therapy |
Indicates management approach described in referenced study.
Table 3. Reports of en plaque meningiomas misdiagnosed as other lesions.
| Actual diagnosis | Misdiagnosis | Findings/differentiating features of missed diagnosis from en plaque meningioma | Most recent referenced study | Management approach taken a |
|---|---|---|---|---|
| En plaque meningioma | Paget's disease | Histopathology: extensive osteoclast activity during lytic phase; osteoblast activity predominates in sclerotic phase, with dense bone in mosaic pattern. Hematology: elevated alkaline phosphatase |
Jayaraj et al 69 | Total surgical resection |
| En plaque meningioma | Osteosarcoma | Histopathology: osteoid tumor matrix, spindle-like cell proliferation, multinuclear giant cells | Asil et al 70 | Total surgical resection |
| En plaque meningioma | Fibrous dysplasia | Histopathology: trabeculae of woven bone with surrounding fibrous stroma, but with absence of osteoblastic rimming. immunohistochemistry: negative for relevant malignant markers, i.e., endothelial growth factor receptor (EGFR) and CD117 | Mingo et al 71 | Total surgical resection |
Indicates management approach described in referenced study.
Histopathology
Gross pathology will demonstrate thickening of the calvarium and skull base. Histopathologic diagnosis will demonstrate meningothelial cell invasion and expansion of the haversian canals of the bone. 12 30 Hyperostosis is secondary to both tumor invasion and osteoblastic activity. 31 In addition to osseous invasion, rare instances of muscle and angioinvasion have been reported. 32 Despite the locally invasive nature, the majority of MEP cases still are classified as WHO Grade 1 tumors due to a low proliferative index. 30
Treatment
Historical literature dating back to the 1950s reports that MEP were not routinely resected due to significant surgical morbidity and mortality. 33 34 35 There are few reports discussing the nonoperative management of MEP, most of which are outdated. 14 33 One study performed in 1952 followed nine patients who were managed nonoperatively, and reported that some patients remained stable while others developed increasing exophthalmos and discomfort. 33 The growth rate of MEP appears overall comparable to that of other benign meningiomas; however, their close proximity to the orbit and cavernous sinus is what creates a high likelihood for MEP to become symptomatic, and thus an observation-only approach is rarely recommended. 36 37 Substantial advances in imaging and microsurgical and craniofacial techniques have allowed for improved surgical approaches and better outcomes. 12 17 30 38 39 40 As such, early and aggressive surgical resection is the mainstay of treatment with the goal of maximally safe resection, with the possibility for adjuvant radiation therapy for subtotal resections or recurrence.
Surgical Approach and Adjunctive Strategies
Thorough analysis of preoperative imaging is essential when determining the optimal surgical approach to these lesions, seeking to create a path that allows for wide margins beyond the hyperostotic bone without endangerment of critical neurovascular structures. While many approaches have been explored, including transzygomatic, transcranial–transmalar, and cranio-orbital, the most commonly used for tumors with sphenoid wing involvement remains the pterional craniotomy, with orbital and zygomatic modifications incorporated as needed. 30 33 Preoperative imaging is important to recognize anatomical constraints, to assess for the presence of bony and muscle invasion that may be encountered upon exposure, and to evaluate cranial nerve, optic nerve, optic canal, superior orbital fissure, and orbital compression. 21 Positioning, exposure, and craniotomy approaches are highly individualized based on the tumor location, and certain adjunctive techniques are often employed specific to MEP features. In some cases, large and even bicoronal skin incisions have been utilized to harvest sufficient pericranium for repair of dural defects. 41 Some cases have reported bony and muscular invasion encountered upon exposure. 15 21 Given the widespread carpet-like nature of the tumor and extensive dural infiltration, oftentimes a large craniotomy is necessary due to tumor-infiltration along convexity dura. The squamosal and lateral sphenoid bone may be hypervascular and hyperostotic, and it is recommended to complete bony removal prior to dural opening and tumor resection. 41 If tumor or hyperostosis extends down toward the infratemporal fossa, it is the authors practice to reflect the zygoma inferiorly to facilitate inferior drilling access.
All involved cranial nerve foramina should be appropriately decompressed with clear visualization of underlying neural structures. As such, the osteotomies should be wide and extensive, ensuring maximal symptomatic relief since invaded and expanded osseous elements are the primary cause for most symptoms. Removal of the lateral wall and roof of the orbit can be completed if there is concern for orbital invasion. Alternatively, stripping of periorbital or aggressive coagulation of residual tumor tissue may be performed in instances of intraorbital invasion without periorbital or orbital roof reconstruction. 15 41
Reconstruction
There is a discrepancy in the literature regarding the necessity of reconstructive surgery following resection in preventing cosmetic deformity and pulsatile enophthalmos or exophthalmos. Cosmetic problems were previously believed to be at high risk of developing when more than one orbital wall was removed, and studies advocated for reconstruction in these cases. 15 16 30 37 42 43 However, numerous studies followed in which no reconstruction was performed, without a subsequent increase in functional and cosmetic problems seen, particularly in instances of preserved periorbita. 19 37 42 44 In one study of 34 patients, reconstruction was not performed even in cases where there was complete removal of dorsal and posterolateral orbital walls. Despite this, 78% of patients had resolution of proptosis postoperatively and none suffered from enopthalmos. 15 The authors argued that preservation of the orbital rim (frontal portion of the orbit) due to its attachment to the periorbita is sufficient to prevent postoperative enophthalmos. Furthermore, preservation of the periorbita will also maintain the structure and position of the globe. This is argument is consistent with most current literature recommendations, that is, the decision to perform reconstruction should be driven by the extent of violation of the periorbita rather than of bony removal. 36 42 In cases where periorbita is violated, a porous polyethylene 3-day printed implant used for craniofacial reconstruction may be used to reconstruct the orbit. This technique is frequently utilized in oculoplastic surgery and orbital wall trauma. The cranial side allows for fibrous ingrowth to provide a bioactive structural support. The orbital side of the implant is nonporous to prohibit tissue ingrowth and prevent intraocular muscle entrapment or scarring. 45
Alternative materials have also been reported to be used for reconstruction such as titanium mesh and autologous bone grafting for orbital wall reconstruction. 20 46 Approach and material selected for reconstruction are largely based upon surgeon preference.
Outcomes
Gross total resection is often difficult due to anatomical constraints and tumor involvement of cranial nerves or cavernous sinus. Common reasons for subtotal resections include intraorbital tumor, cavernous sinus invasion, tumor extending beyond the tentorial notch, and invasion of the superior orbital fissure. 5 9 23 46 Areas such as the cavernous sinus and superior orbital fissure are considered surgical limits due to high postoperative morbidity risks; however, these limits pose challenging decisions for the surgeon intraoperatively as any residual tumor increases the risk of tumor progression. If gross total resection is achieved, some authors found the risk for recurrence of MEP to be comparable to those of similar Simpson grade following resection, with rate of regrowth occurring at a rate of 2 to 24 mm per year, and with more calcified tumors showing less growth. 47 However, recurrence rates are significantly higher in tumors involving the orbit and cavernous sinus, with reports of 2 to 3 years to recurrence for infraorbital tumors and 2 to 5 years for tumors involving the cavernous sinus. 10 27 One study estimated the 10-year risk of recurrence to be as high as 54% in tumors extending into the orbit. 37 Of note, this study also reported recurrence rates of 9 to 15% in completely resected tumors, and risk factors contributing to these recurrences are still not entirely understood. Overall, patients' presenting symptoms have shown marked improvement postoperatively, with one study reporting improvement in proptosis in 100% of patients and complete resolution in 75% of these postoperatively. 9 10 48
Postoperative Complications
The most frequently reported postsurgical complications include cranial nerve deficits—particularly oculomotor and abducens nerve palsies, ophthalmoplegia, and trigeminal hypesthesia, among other deficits specific to the tumor's location. Cerebrospinal fluid leak and seizures have also been frequently reported. 9 10 49 The surgical approach utilized must be considered when analyzing surgical outcomes, as this significantly impacts the structures at greatest risk for damage. For instance, one series reported six cases of temporalis muscle atrophy, but a lateral orbitotomy approach was exclusively utilized in this series, involving significant temporalis muscle manipulation intraoperatively. 2 There is an overall inconsistency in the reporting of surgical complications, with some studies only reporting permanent neurologic deficits, or failing to distinguish whether the deficit is new from preoperative presentation. Another series found that cranial nerve impairment occurred in 14 patients (20%), whose tumors involved oculomotor, trochlear, and/or abducens nerves. 23 Only three of these patients, however, had lasting nerve injuries. Thus, it is important to consider the timing and context of the complications reported. A summary of complications encountered is described in Table 4 .
Table 4. Summary of complications, as reported.
| Complication (new/worsened from preoperative) | Amirjamshidi et al 2 ( n = 88) | Jiranukool et al 23 ( n = 26) | Li et al 10 ( n = 37) | Mirone et al 5 ( n = 77) | Oya et al 15 ( n = 39) | Schick et al 8 ( n = 67) | Simas and Farias 9 ( n = 18) | Combined ( n = 352) |
|---|---|---|---|---|---|---|---|---|
| CN deficits (any) | 28 (31.8%) | 4 (3.8%) | 6 (16.2%) | 26 (33.8%) | 12 (30.8%) | 5 (7.5%) | 4 (22.2%) | 85 (24.1%) |
| Abducens nerve deficit | 21 (23.9%) | 2 (7.7%) | 6 (16.2%) | 3 (3.9%) | – | 1 (1.5%) | – | 33 (9.4%) |
| Trigeminal nerve deficit | – | 12 (15.6%) | 9 (23.1%) | 3 (4.5%) | 1 (5.6%) | 25 (7.1%) | ||
| Ophthalmoplegia | 5 (5.7%) | 8 (21.6%) | 14 (18.2%, transient) | – | – | 22 (6.3%) | ||
| Oculomotor nerve deficit | – | 1 (3.8%) | – | 9 (11.7%) | 3 (7.7%) | 1 (1.5%) | 3 (16.7%) | 17 (4.8%) |
| Cerebrospinal fluid leak/fistula | – | 2 (7.7%) | 1 (2.7%) | – | – | 8 (11.9%) | 1 (5.6%) | 12 (3.4%) |
| Ptosis | – | 7 (18.9%) | – | – | 4 (6.0%) | – | 11 (3.1%) | |
| Visual acuity deficit | 6 (6.8%) | 1 (3.8%) | – | 3 (3.9%) | – | – | 10 (2.8%) | |
| Seizure | 1 (1.1%) | 2 (7.7%) | – | – | 2 (5.1%) | 3 (4.5%) | – | 8 (2.3%) |
| Exophthalmos | 2 (7.7%) | – | 2 (2.6%) | 3 (7.7%) | 0 (0%) | – | 7 (2.0%) | |
| Temporalis muscle atrophy | 6 (6.8%) | – | – | – | – | – | – | 6 (1.7%) |
| Visual field deficit | – | 3 (11.5%) | – | – | – | – | 2 (11.1%) | 5 (1.4%) |
| Enophthalmos | 4 (4.5%) | – | – | – | – | – | – | 4 (1.1%) |
| Epidural hematoma/abscess | – | 1 (3.8%) | – | – | – | 2 (3.0%) | – | 3 (0.9%) |
| Trochlear nerve deficit | – | – | – | 2 (2.6%) | – | – | – | 2 (0.6%) |
| Facial nerve deficit | – | 1 (3.8%) | – | – | – | – | – | 1 (0.3%) |
Abbreviation: CN, cranial nerve.
Radiation Therapy
There has been ongoing investigation regarding the use of radiation therapy both as isolated and adjuvant treatment for these tumors, with no clear consensus regarding its effect on outcomes. The majority of authors support the use of radiation as an adjuvant therapy in cases of residual tumor or signs of regrowth following resection. 23 37 In instances of superior orbital fissure and/or cavernous sinus invasion, adjuvant radiosurgery may help to reduce surgical morbidity, and most authors agree that the optic nerves and surrounding neurovasculature can safely be radiated at 10 Gy or less. 20 50 51 52 Multiple studies that administered radiation therapy for residual tumor documented little to no growth of the residual tumor on follow-up studies. 23 24 53 However, the length of patient follow-up reported in these studies was highly variable, and it is recommended that patients be followed over a longer duration following radiation therapy. While most MEP are WHO Grade I tumors, some authors have advocated for postoperative radiosurgery in cases of residual atypical or malignant meningiomas, but the available data are limited and future studies should explore this further. 10 13 54 In general, recurrence rates are often underreported in literature due to insufficient time to follow-up, and more consistent reporting of recurrence rates would improve the quality of future studies.
Chemotherapy and Molecular Therapy
Given the rate of meningioma recurrence despite maximal resection, various chemo- and molecular therapy strategies have recently emerged as clinical trials. If successful, these therapeutic strategies would be particularly applicable to MEP cases that involve surgically inaccessible regions. Historically, traditional chemotherapies and other targeted therapies have not shown any clinical benefit in meningioma treatment. 55 The National Comprehensive Cancer Network guidelines do mention systemic chemotherapy as an option in their recommendations for surgically inaccessible high-grade meningiomas in patients who cannot receive radiation therapy. 56 However, data on this treatment is extremely limited, and there is no survival benefit seen in patients who received chemotherapy compared with those receiving radiotherapy for surgically inaccessible lesions, and thus chemotherapy is only given consideration when radiotherapy is not possible.
With recent advancements in genetic sequencing, there is increasing knowledge regarding specific oncogenic pathways involved in various meningioma subtypes, allowing for the design of new targeted therapies based on tumor's molecular profile. 55 Skull base meningiomas in particular have been found to have high rates of mutation in the AKT (Protein kinase B) pathway, which is critically involved in tumor growth and chemotherapy resistance. A clinical trial began in 2015 that is investigating the role of an AKT1 inhibitor (Afuresertib), which is one of the first meningioma trials initiated that targets mutations identified with next-generation sequencing. Mammalian target of rapamycin (mTOR) has also been found to be highly active in skull base meningiomas, leading to multiple clinical trials involving administration of an mTOR pathway inhibitor (Everolimus), either alone or in combination with a somatostatin analogue (Octreotide). Additional trials are in place which target epidermal-derived growth factor receptor, platelet-derived growth factor receptor, and vascular endothelial derived growth factor receptor. 57 58 59 These trials are still in early stages, but offer great promise in changing the paradigm of meningioma treatment and survival.
Illustrative Case
Clinical and Radiographic History
A 54-year-old female presented with mild right-sided pressure headaches and mild exophthalmos, and on workup she was found to have an en plaque meningioma with significant sphenoid hyperostosis and compression of the optic canal. MRI with contrast demonstrated a 3-cm dural-based temporal tip lesion with minimal brain edema with evidence of significant osseous expansion, and CT head confirmed these anatomic findings ( Fig. 2 ). On clinical exam, the patient demonstrated mild restriction of right lateral gaze concerning for a mild compressive abducens nerve palsy.
Fig. 2.
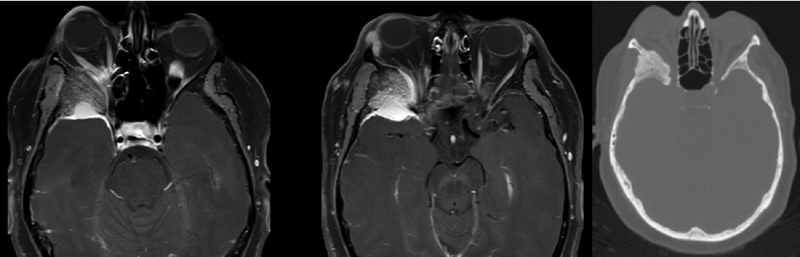
Left: Axial postcontrast T1-weighted magnetic resonance imaging demonstrating a soft tissue mass at the right temporal tip with significant osseous expansion. Right: Axial computed tomography bone window.
Diagnosis and Treatment
Preoperative planning was done with 3-day printed patient-specific skull base models that printed templates for the intended craniotomy, a model for the expected osseous defect, and a 3-day printed polyethylene implant that would ultimately fill in the expected defect. The size of the defect was overestimated to allow for intraoperative customization of the implant. A nonporous shield was available during the procedure in case of periorbita violation.
The patient was taken to the operating room for a modified pterional craniotomy with a zygomatic osteotomy. Temporalis muscle attachments to the zygoma were preserved and reflected inferiorly with the zygomatic cut. The orbital rim was preserved. Extradural drilling with a 3 mm burr and with a diamond drill in areas of hypervascular bone was performed to drill down the sphenoid ridge. Intraoperative navigation with CT guide imaging was used to gauge the extent of drilling. Cautious drilling was performed close to the optic canal and lateral orbital wall to maintain the integrity of the periorbita. Dura was then reflected forward to visualize the sphenoid ridge meningioma. The meningioma was resected and the dura coagulated. Residual meningioma was left in areas where it was significantly adherent to the sylvian veins. A dural matrix was used to repair the dural defect and the custom implant was then applied over the cranial defect, drilled down to fit the defect appropriately, and then fixed with screws to the patient's cranium.
Postoperative Course
Postoperatively, the patient did well with no concern for postoperative enophthalmos. CT demonstrated good decompression of the optic canal and MRI demonstrated a near complete resection of the meningioma ( Fig. 3 ). The patient will be monitored for serial MRIs, and if concern for regrowth arises, the patient would likely undergo radiosurgery.
Fig. 3.
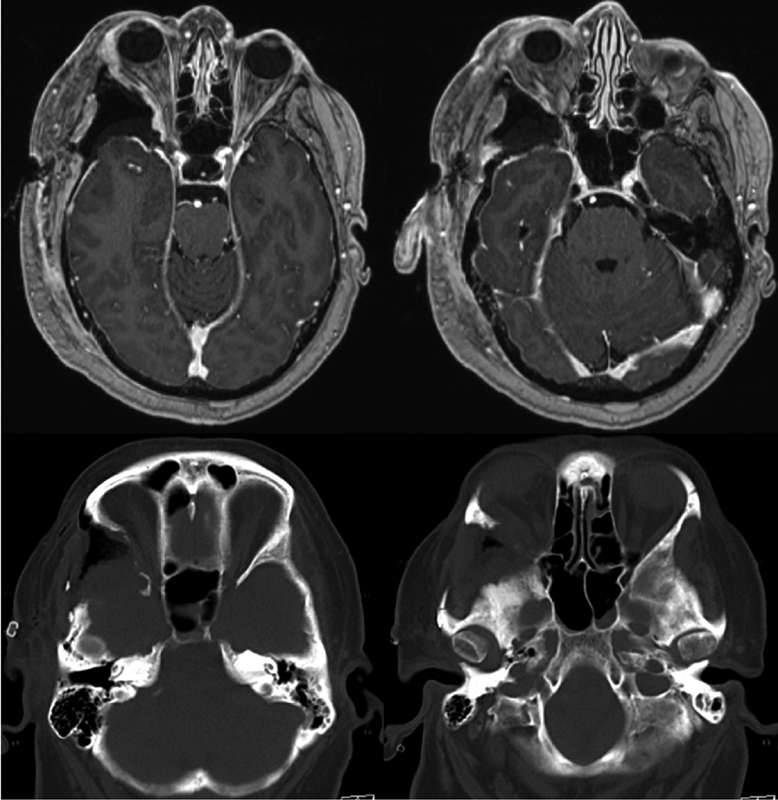
Top: Axial T1-weighted magnetic resonance imagings, postoperative. Bottom: Axial computed tomography, postoperative.
Conclusion
Based on our comprehensive review and analysis of outcomes, we offer multiple points of guidance to surgeons performing en plaque meningioma resection. When there is any suspicion of MEP when performing diagnostic workup, MRI should be obtained to best minimize the risk of misdiagnosis. Once the diagnosis is made, surgical resection should be performed to lower the risk for cosmetic deformity as well as lower the risk of obtaining incomplete resection and related recurrence risks. Intraoperatively, the dura should be extensively removed to prevent risk of recurrence, and all involved cranial nerve foramina sufficiently decompressed. Lastly, if there is any tumor invasion into the cavernous sinus or any signs of recurrence on follow-up imaging, consideration should be given to postoperative radiation therapy.
With ongoing advances in medical imaging, surgical technical capabilities, and novel therapeutic interventions, more treatment options are available for en plaque meningiomas than ever before. Further investigation is needed to assist neurosurgeons in determining optimal treatment strategies when faced with an en plaque meningioma, according to the individual tumor characteristics and patient presentation.
Footnotes
Conflict of Interest None declared.
References
- 1.Ostrom Q T, Gittleman H, Fulop J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-oncol. 2015;17 04:iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amirjamshidi A, Abbasioun K, Amiri R S, Ardalan A, Hashemi S M. Lateral orbitotomy approach for removing hyperostosing en plaque sphenoid wing meningiomas. Description of surgical strategy and analysis of findings in a series of 88 patients with long-term follow up. Surg Neurol Int. 2015;6:79. doi: 10.4103/2152-7806.157074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akutsu H, Sugita K, Sonobe M, Matsumura A. Parasagittal meningioma en plaque with extracranial extension presenting diffuse massive hyperostosis of the skull. Surg Neurol. 2004;61(02):165–169. doi: 10.1016/s0090-3019(03)00521-4. [DOI] [PubMed] [Google Scholar]
- 4.Yao A, Sarkiss C A, Lee J, Zarzour H K, Shrivastava R K. Surgical limitations in convexity meningiomas en-plaque: is radical resection necessary? J Clin Neurosci. 2016;27:28–33. doi: 10.1016/j.jocn.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Mirone G, Chibbaro S, Schiabello L, Tola S, George B.En plaque sphenoid wing meningiomas: recurrence factors and surgical strategy in a series of 71 patients Neurosurgery 200965(6, Suppl):100–108. [DOI] [PubMed] [Google Scholar]
- 6.Mohindra S, Savardekar A, Tripathi M, Rane S. En plaque foramen magnum meningiomas: rare presentations. Br J Neurosurg. 2012;26(06):899–901. doi: 10.3109/02688697.2012.685781. [DOI] [PubMed] [Google Scholar]
- 7.Vrionis F D, Robertson J H, Gardner G, Heilman C B. Temporal bone meningiomas. Skull Base Surg. 1999;9(02):127–139. doi: 10.1055/s-2008-1058159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schick U, Bleyen J, Bani A, Hassler W. Management of meningiomas en plaque of the sphenoid wing. J Neurosurg. 2006;104(02):208–214. doi: 10.3171/jns.2006.104.2.208. [DOI] [PubMed] [Google Scholar]
- 9.Simas N M, Farias J P. Sphenoid wing en plaque meningiomas: surgical results and recurrence rates. Surg Neurol Int. 2013;4:86. doi: 10.4103/2152-7806.114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Shi J T, An Y Z. Sphenoid wing meningioma en plaque: report of 37 cases. Chin Med J (Engl) 2009;122(20):2423–2427. [PubMed] [Google Scholar]
- 11.Moher D, Shamseer L, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Jesús O, Toledo M M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001;55(05):265–269. doi: 10.1016/s0090-3019(01)00440-2. [DOI] [PubMed] [Google Scholar]
- 13.Pompili A, Derome P J, Visot A, Guiot G. Hyperostosing meningiomas of the sphenoid ridge--clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol. 1982;17(06):411–416. doi: 10.1016/s0090-3019(82)80006-2. [DOI] [PubMed] [Google Scholar]
- 14.Cushing H EL. Springfield, IL: Charles C Thomas; 1938. Meningiomas of the sphenoid wing; pp. 298–387. [Google Scholar]
- 15.Oya S, Sade B, Lee J H. Sphenoorbital meningioma: surgical technique and outcome. J Neurosurg. 2011;114(05):1241–1249. doi: 10.3171/2010.10.JNS101128. [DOI] [PubMed] [Google Scholar]
- 16.Shrivastava R K, Sen C, Costantino P D, Della Rocca R. Sphenoorbital meningiomas: surgical limitations and lessons learned in their long-term management. J Neurosurg. 2005;103(03):491–497. doi: 10.3171/jns.2005.103.3.0491. [DOI] [PubMed] [Google Scholar]
- 17.Bonnal J, Thibaut A, Brotchi J, Born J. Invading meningiomas of the sphenoid ridge. J Neurosurg. 1980;53(05):587–599. doi: 10.3171/jns.1980.53.5.0587. [DOI] [PubMed] [Google Scholar]
- 18.Brotchi J, Pirotte B. New York, NY: Thieme; 2006. Sphenoid wing meningiomas; pp. 623–632. [Google Scholar]
- 19.Simon M SJ. New York, NY: Thieme; 2011. Lateral and middle sphenoid wing meningiomas. [Google Scholar]
- 20.Boari N, Gagliardi F, Spina A, Bailo M, Franzin A, Mortini P. Management of spheno-orbital en plaque meningiomas: clinical outcome in a consecutive series of 40 patients. Br J Neurosurg. 2013;27(01):84–90. doi: 10.3109/02688697.2012.709557. [DOI] [PubMed] [Google Scholar]
- 21.Jang S Y, Kim C H, Cheong J H, Kim J M. Extracranial extension of intracranial atypical meningioma en plaque with osteoblastic change of the skull. J Korean Neurosurg Soc. 2014;55(04):205–207. doi: 10.3340/jkns.2014.55.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsutsumi S, Izumi H, Yasumoto Y, Ito M. Convexity en plaque meningioma manifesting as subcutaneous mass: case report. Neurol Med Chir (Tokyo) 2013;53(10):727–729. doi: 10.2176/nmc.cr2012-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiranukool J, Iampreechakul P, Dhanachai M, Tirakotai W. Outcomes of surgical treatment and radiation therapy in en plaque sphenoid wing meningioma. J Med Assoc Thai. 2016;99 03:S54–S61. [PubMed] [Google Scholar]
- 24.Nagy M, Eissa S. Outcome of sphenoid wing en plaque meningioma surgery. Egyptian J Neurosurg. 2015;30:259–264. [Google Scholar]
- 25.Tirtaprawita N, Wiradharma W, July J.The key role of MRI modalities in en plaque meningiomaMedicinus 2018;6; doi: 10.19166/med.v6i2.1145
- 26.Brito A B, Reis F, de Souza C A, Vassallo J, Lima C S. Intracranial primary dural diffuse large B-cell lymphoma successfully treated with chemotherapy. Int J Clin Exp Med. 2014;7(02):456–460. [PMC free article] [PubMed] [Google Scholar]
- 27.Lyndon D, Lansley J A, Evanson J, Krishnan A S. Dural masses: meningiomas and their mimics. Insights Imaging. 2019;10(01):11. doi: 10.1186/s13244-019-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramachandran R, Muniyandi M, Iyer V, Sripriya T, Priya B, Govindarajan T G. Dilemmas in the diagnosis and treatment of intracranial tuberculomas. J Neurol Sci. 2017;381:256–264. doi: 10.1016/j.jns.2017.08.3258. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal A, Patra D P, Gupta K, Sodhi H B. Dural tuberculoma mimicking meningioma: a clinicoradiologic review of dural en-plaque lesions. World Neurosurg. 2016;88:6860–6.86E9. doi: 10.1016/j.wneu.2015.10.097. [DOI] [PubMed] [Google Scholar]
- 30.Honeybul S, Neil-Dwyer G, Lang D A, Evans B T, Ellison D W. Sphenoid wing meningioma en plaque: a clinical review. Acta Neurochir (Wien) 2001;143(08):749–757. doi: 10.1007/s007010170028. [DOI] [PubMed] [Google Scholar]
- 31.Brem H., Sr . Philadelphia PA: Saunders; 2004. Youman's Neurological Surgery. Vol 1. Fifth edition. [Google Scholar]
- 32.Basu K, Majumdar K, Chatterjee U, Ganguli M, Chatterjee S. En plaque meningioma with angioinvasion. Indian J Pathol Microbiol. 2010;53(02):319–321. doi: 10.4103/0377-4929.64306. [DOI] [PubMed] [Google Scholar]
- 33.Castellano F, Guidetti B, Olivecrona H. Pterional meningiomas en plaque. J Neurosurg. 1952;9(02):188–196. doi: 10.3171/jns.1952.9.2.0188. [DOI] [PubMed] [Google Scholar]
- 34.Abbott K H, Glass B. Pterional meningioma en plaque; report of a case of thirty-six years' duration. J Neurosurg. 1955;12(01):50–52. doi: 10.3171/jns.1955.12.1.0050. [DOI] [PubMed] [Google Scholar]
- 35.Poppen J L, Horrax G. The surgical treatment of hyperostosing meningiomas of the sphenoid wing. Surg Gynecol Obstet. 1940;71:222–230. [Google Scholar]
- 36.Saeed P, van Furth W R, Tanck M. Natural history of spheno-orbital meningiomas. Acta Neurochir (Wien) 2011;153(02):395–402. doi: 10.1007/s00701-010-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroon J C, Kennerdell J S, Vidovich D V, Abla A, Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80(02):202–208. doi: 10.3171/jns.1994.80.2.0202. [DOI] [PubMed] [Google Scholar]
- 38.Carrizo A, Basso A. Current surgical treatment for sphenoorbital meningiomas. Surg Neurol. 1998;50(06):574–578. doi: 10.1016/s0090-3019(97)00101-8. [DOI] [PubMed] [Google Scholar]
- 39.Derome P.[Spheno-ethmoidal tumors. Possibilities for exeresis and surgical repair] Neurochirurgie 197218011–164., 1–164 [PubMed] [Google Scholar]
- 40.Sandalcioglu I E, Gasser T, Mohr C, Stolke D, Wiedemayer H. Spheno-orbital meningiomas: interdisciplinary surgical approach, resectability and long-term results. J Craniomaxillofac Surg. 2005;33(04):260–266. doi: 10.1016/j.jcms.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Ringel F, Cedzich C, Schramm J.Microsurgical technique and results of a series of 63 spheno-orbital meningiomas Neurosurgery 200760(04, Suppl 2):214–221. [DOI] [PubMed] [Google Scholar]
- 42.Bowers C A, Sorour M, Patel B C, Couldwell W T. Outcomes after surgical treatment of meningioma-associated proptosis. J Neurosurg. 2016;125(03):544–550. doi: 10.3171/2015.9.JNS15761. [DOI] [PubMed] [Google Scholar]
- 43.Gaillard S, Pellerin P, Dhellemmes P, Pertuzon B, Lejeune J P, Christiaens J L. Strategy of craniofacial reconstruction after resection of spheno-orbital “en plaque” meningiomas. Plast Reconstr Surg. 1997;100(05):1113–1120. doi: 10.1097/00006534-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Gonen L, Nov E, Shimony N, Shofty B, Margalit N. Sphenoorbital meningioma: surgical series and design of an intraoperative management algorithm. Neurosurg Rev. 2018;41(01):291–301. doi: 10.1007/s10143-017-0855-7. [DOI] [PubMed] [Google Scholar]
- 45.Holck D.Custom Shaped Porous Polyethylene-Titanium Mesh Orbital Implants for Internal Orbital Floor/Medial-Wall Fracture RepairAnn Fall Sci Syllab2006
- 46.Brusati R, Biglioli F, Mortini P, Raffaini M, Goisis M. Reconstruction of the orbital walls in surgery of the skull base for benign neoplasms. Int J Oral Maxillofac Surg. 2000;29(05):325–330. [PubMed] [Google Scholar]
- 47.Fariselli L, Biroli A, Signorelli A, Broggi M, Marchetti M, Biroli F. The cavernous sinus meningiomas' dilemma: surgery or stereotactic radiosurgery? Rep Pract Oncol Radiother. 2016;21(04):379–385. doi: 10.1016/j.rpor.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Güdük M, Özduman K, Pamir M N. Sphenoid wing meningiomas: surgical outcomes in a series of 141 cases and proposal of a scoring system predicting extent of resection. World Neurosurg. 2019;125:e48–e59. doi: 10.1016/j.wneu.2018.12.175. [DOI] [PubMed] [Google Scholar]
- 49.Kumar J, Mallik J, Strickland B A, Harsh V, Kumar A. Intracranial en-plaque tuberculoma impersonating en-plaque meningioma: Case report and brief review of literature. Asian J Neurosurg. 2017;12(03):576–579. doi: 10.4103/1793-5482.175619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miralbell R, Cella L, Weber D, Lomax A. Optimizing radiotherapy of orbital and paraorbital tumors: intensity-modulated X-ray beams vs. intensity-modulated proton beams. Int J Radiat Oncol Biol Phys. 2000;47(04):1111–1119. doi: 10.1016/s0360-3016(00)00494-6. [DOI] [PubMed] [Google Scholar]
- 51.Mirimanoff R O, Dosoretz D E, Linggood R M, Ojemann R G, Martuza R L. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62(01):18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 52.Morita A, Coffey R J, Foote R L, Schiff D, Gorman D. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90(01):42–49. doi: 10.3171/jns.1999.90.1.0042. [DOI] [PubMed] [Google Scholar]
- 53.Peele K A, Kennerdell J S, Maroon J C. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103(11):1761–1766. doi: 10.1016/s0161-6420(96)30430-2. [DOI] [PubMed] [Google Scholar]
- 54.Charbel F T, Hyun H, Misra M, Gueyikian S, Mafee R F. Juxtaorbital en plaque meningiomas. Report of four cases and review of literature. Radiol Clin North Am. 1999;37(01):89–100. doi: 10.1016/s0033-8389(05)70080-4. [DOI] [PubMed] [Google Scholar]
- 55.Nigim F, Wakimoto H, Kasper E M, Ackermans L, Temel Y. Emerging medical treatments for meningioma in the molecular era. Biomedicines. 2018;6(03):E86. doi: 10.3390/biomedicines6030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J, Kim K H, Kim Y Z. The clinical outcome of hydroxyurea chemotherapy after incomplete resection of atypical meningiomas. Brain Tumor Res Treat. 2017;5(02):77–86. doi: 10.14791/btrt.2017.5.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen P Y, Yung W K, Lamborn K R. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08) Neuro-oncol. 2009;11(06):853–860. doi: 10.1215/15228517-2009-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crombet T, Torres O, Rodríguez V. Phase I clinical evaluation of a neutralizing monoclonal antibody against epidermal growth factor receptor in advanced brain tumor patients: preliminary study. Hybridoma. 2001;20(02):131–136. doi: 10.1089/02724570152057634. [DOI] [PubMed] [Google Scholar]
- 59.Raizer J J, Grimm S A, Rademaker A. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol. 2014;117(01):93–101. doi: 10.1007/s11060-014-1358-9. [DOI] [PubMed] [Google Scholar]
- 60.Kulkarni K, Sternau L, Dubovy S, Lam B. Primary dural lymphoma masquerading as a meningioma. Journal of Neuro-Ophthalmology. 2012;32(03):240–242. doi: 10.1097/WNO.0b013e31825103a5. [DOI] [PubMed] [Google Scholar]
- 61.Vaicys C, Schulder M, Wolansky L J, Fromowitz F B. Falcotentorial plasmacytoma. Case report. J Neurosurg. 1999;91(01):132–135. doi: 10.3171/jns.1999.91.1.0132. [DOI] [PubMed] [Google Scholar]
- 62.Donnet A, Figarella-Branger D, Grisoli F. Primary meningeal fibrosarcoma: a particular neuroradiological presentation. J Neurooncol. 1999;42(01):79–83. doi: 10.1023/a:1006100119406. [DOI] [PubMed] [Google Scholar]
- 63.Deb P, Singh V, Dutta V, Bhatoe H S, Chandran V M. Primary intracranial benign fibrous histiocytoma: report of an unusual case. J Cancer Res Ther. 2014;10(01):200–202. doi: 10.4103/0973-1482.131417. [DOI] [PubMed] [Google Scholar]
- 64.Nowosielski M, Galldiks N, Iglseder S. Diagnostic challenges in meningioma. Neuro-oncol. 2017;19(12):1588–1598. doi: 10.1093/neuonc/nox101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chitkara N, Sharma N K, Goyal N. Osteoma mimicking a partly calcified meningioma. Neurol India. 2003;51(02):287–288. [PubMed] [Google Scholar]
- 66.Deora H, Beniwal M, Rao S, Rao K VLN, Vikas V, Somanna S. Wolf in Sheep's clothing: Intracranial actinomycosis masquerading as en-plaque meningioma . Surg Neurol Int. 2018;9:39–39. doi: 10.4103/sni.sni_445_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komotar R J, Clatterbuck R E. Coccidioidomycosis of the brain, mimicking en plaque meningioma. J Neurol Neurosurg Psychiatry. 2003;74(06):806–806. doi: 10.1136/jnnp.74.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tobias S, Prayson R A, Lee J H. Necrotizing neurosarcoidosis of the cranial base resembling an en plaque sphenoid wing meningioma: case report. Neurosurgery. 2002;51(05):1290–1294. doi: 10.1097/00006123-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 69.Jayaraj K, Martinez S, Freeman A, Lyles K. Clinical vignette: intraosseous meningioma—a mimicry of Paget's disease? J Bone Miner Res. 2001;16(16):1154–1156. doi: 10.1359/jbmr.2001.16.6.1154. [DOI] [PubMed] [Google Scholar]
- 70.Asil K, Aksoy Y E, Yaldiz C, Kahyaglu Z. Primary intraosseous meningioma mimicking osteosarcoma: case report. Turk Neurosurg. 2015;25(01):174–176. doi: 10.5137/1019-5149.JTN.9907-13.1. [DOI] [PubMed] [Google Scholar]
- 71.Mingo K, Sweeney A D, Thompson R C, Rivas A. Hyperostotic en plaque meningioma mimicking fibrous dysplasia of the temporal bone. Otol Neurotol. 2016;37(09):e317–e318. doi: 10.1097/MAO.0000000000000860. [DOI] [PubMed] [Google Scholar]


