Abstract
The biological and therapeutic properties of seaweeds have already been well known. Several studies showed that among the various natural marine sources of antioxidants, seaweeds have become a potential source of antioxidants because of their bioactive compounds. Most of the metabolic diseases are caused by oxidative stress. It is very well known that antioxidants have a pivotal role in the treatment of those diseases. Recent researches have revealed the potential activity of seaweeds as complementary medicine, which have therapeutic properties for health and disease management. Among the seaweeds, brown seaweeds (Phaeophyta) and their derived bioactive substances showed excellent antioxidant properties than other seaweeds. This review focuses on brown seaweeds and their derived major bioactive compounds such as sulfated polysaccharide, polyphenol, carotenoid, and sterol antioxidant effects and molecular mechanisms in the case of the oxidative stress-originated disease. Antioxidants have a potential role in the modification of stress-induced signaling pathways along with the activation of the oxidative defensive pathways. This review would help to provide the basis for further studies to researchers on the potential antioxidant role in the field of medical health care and future drug development.
1. Introduction
Brown seaweeds are photosynthetic aquatic algae which belong to the domain of Eukarya, kingdom of Chromista, and class of Phaeophyta [1]. There are 1500 species of brown seaweeds all over the world [2]. In particular, in Asian countries, seaweed has been used as a traditional herbal medicine for the treatment of gastrointestinal problems, cough, boils, ulcers, asthma, cough, and headache as well as vegetables too [3]. Lately, several studies revealed that dietary seaweeds not only are a good source of carbohydrates, dietary fiber, proteins and peptides, vitamins, minerals, and fats but also contain a large concentration of functionally bioactive compounds such as carotenoids, polysaccharides, polyphenols, and sterols, which have potential antioxidant properties as well as antimicrobial, anticoagulant, antithrombotic, anti-inflammatory, antitumor, and antiviral properties for several diseases [4–6]. Therefore, nowadays, seaweeds have been paid attention to for the development of medicine, food, cosmetics, dietary supplements, fertilizer, and bioenergy [7].
Oxidative stress is widely involved in the development of many chronic diseases such as cardiovascular disease, neurodegenerative, cardiovascular, cancer, inflammation, diabetes, obesity, and aging and many of the elderly diseases [8–10]. Antioxidants are the only therapeutic molecules capable of blocking oxidative stress by their excellent reactive oxygen species (ROS) scavenging activity with low or no toxicity [11]. An antioxidant optimizes the human physiological function that helps to protect against disease or disease progression as well as maintains a healthy state [12]. Naturally, endogenous antioxidants are present in our body; the additional exogenous supplement can also be obtained from various natural sources and chemically synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ) [13–15]. However, studies proved chemically synthesized antioxidants to be toxic and carcinogenic, whether the natural antioxidant is safe, more effective, and easily absorbed by the body [15]. The dietary antioxidants such as α-tocopherol, ascorbic acid, carotenoids, amino acids, peptides, proteins, flavonoids, and other phenolic compounds were found effective in the boosting of the antioxidant mechanism [16]. The marine world is a rich source of bioactive molecules. Among the various natural sources of antioxidants, marine seaweeds and their bioactive compounds are gaining worldwide attention [4, 17] in industry and drugs since 1980 [18]. Numerous evidences have shown that the brown seaweed-derived compounds are capable of improving the oxidative stress-induced diseases including neurodegenerative disease [19], cardiovascular-associated disorders [20], and obesity [21], as well as cancer protection [22]. But unfortunately, we do not have much information about the antioxidant effects and molecular mechanisms of brown seaweeds in oxidative stress diseases.
It has been reported that brown seaweeds have higher antioxidant properties comparatively than red and green seaweeds [23]. Brown seaweeds contain one of the most abundant pigment carotenoid compounds, fucoxanthin, and are estimated to contain around 10% of total carotenoids found in nature [24, 25]. Fucoxanthin has a great antioxidant activity as well as anti-inflammatory, antidiabetic, antiphotoaging, and neuroprotective properties [26]. Polyphenol compounds in brown algae such as phlorotannins are the unique and most dominant complex group of polymers named phloroglucinol (1,3, 5-trihydroxybenzene). It is mainly formed as secondary metabolites in the acetate-malonate pathway. Phlorotannin bioactive compounds exist as soluble compounds or cell-bound forms, mainly produced by brown seaweeds to protect themselves from herbivores and stress conditions, minimizing the oxidative damage caused by nutrient deprivation and ultraviolet radiation. Phlorotannins isolated from brown algae Ecklonia cava, one of the most abundant sources of polyphenolic compounds, have been revealed to have higher antioxidant activity in vitro and in vivo [6, 7, 20, 27]. Another bioactive compound of brown seaweeds is sulfated polysaccharides (SPs), which generally comprise a high content of major groups of sugar, e.g., fucose, galactose, uronic acid, and sulfate [28]. The antioxidant activities of SPs depend on their major sugar, degree of sulfating, low molecular weight, and glycosidic branching [29–31]. The major SPs found in brown seaweeds are fucoidan [32, 33]. Over the past decade, the antioxidant compound sterols called fucosterol have shown great antioxidant property as well as important contributions to human health and wellness [34].
This present review summarizes and discusses brown seaweeds and their major bioderived compound's role as an antioxidant in oxidative stress and their action in the management of disease-related signaling pathways. In Table 1, we show the antioxidant activity of brown seaweed-derived major compounds from the literature review and research articles.
Table 1.
Antioxidant activity of brown algal-derived compounds.
| General category | Antioxidant compound | Antioxidant activity reported |
|---|---|---|
| Carotenoids | Fucoxanthin | [25, 35–39] |
| Polyphenols | ||
| Phlorotannins | Phlorofucofuroeckol A, dieckol, phloroglucinol, eckol, 7 pholoroeckol, and 2-phloroeckol | [27, 40–43] |
| Sulfated polysaccharide | Fucoidans | [16, 32, 44–47] |
| Sterols | Fucosterol | [48, 49] |
2. Oxidative Stress, Antioxidant Defense, and Signal Transduction Pathway in the Cell
Generally, oxidative stress is the imbalance between the production of ROS and the body's own antioxidant defensive system [50]. ROS, usually generated through various extracellular and intracellular processes, are involved in cellular growth, differentiation, progression, and cell death as well as cell signaling [51, 52]. Excessive production of ROS causes oxidative stress and modifies the structure of the cellular macromolecules such as lipids, proteins, nucleic acids, and DNA. Consequently, the cellular and biological functions are inactivated with the modification of cellular signaling. Generally, under the physiological condition, ROS maintain the body's homeostasis by the regulation of several cell signaling pathways which are involved in cellular processes such as mitogen-activated protein kinase (MAPK), nuclear factor-kappa B (NF-κB), and phosphatidylinositol 3‑kinase (PI3K) (Figure 1) as well as the defensive pathway nuclear factor erythroid 2–related factor 2 (Nrf2) signaling; therefore, ROS is considered as a secondary messenger for the activation of cellular signaling [50, 53–55]. Nrf2 is a redox-sensitive transcription factor that binds to antioxidant response elements (ARE) to regulate the expression of antioxidant enzymes that protect the cell against oxidative damage by inducing the antioxidant enzymes. Moreover, excessive ROS modulates the cellular antioxidant defense system that has eventually altered the normal physiological signal and switched to the apoptosis or cell death signals. Therefore, oxidative stress is to be involved in the development of many diseases including cancer, Parkinson's disease, Alzheimer's disease, atherosclerosis, heart failure, myocardial infarction, schizophrenia, and chronic fatigue syndrome (Figure 2) [56–58].
Figure 1.
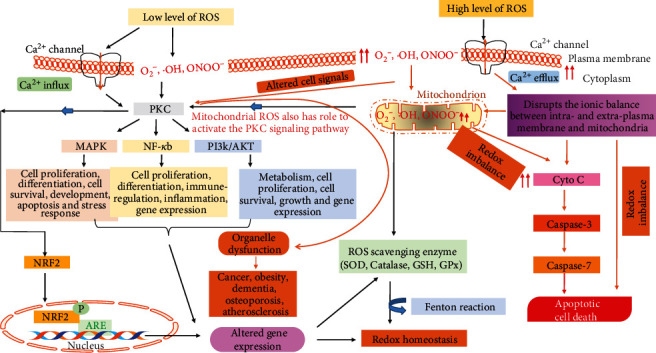
Redox homeostasis with cell signaling pathway under lower and higher levels of ROS.
Figure 2.
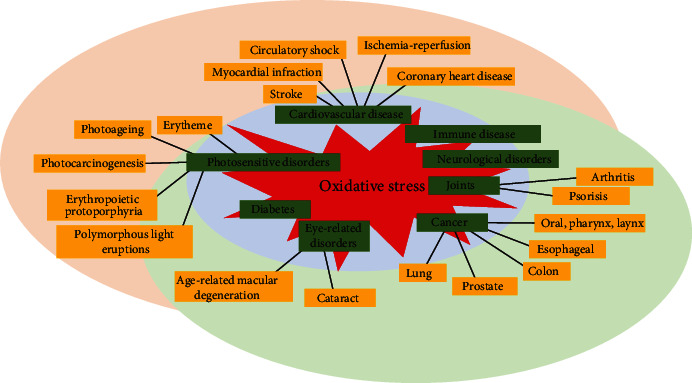
Examples of ROS and oxidative stress-induced diseases.
In addition, ROS has a big role in balancing the intracellular and extracellular Ca2+ levels [54, 59]. Cellular Ca2+ content is also known as one of the most versatile signals for the activation of protein kinase C (PKC) signal transduction cascades which are involved in the control of cellular processes and functions, such as contraction, secretion, metabolism, gene expression, cell survival, and cell death as well as the maintenance of plasma membrane fluidity by proton motive force [60] (Figure 2). ROS with free radical groups such as superoxide anion (O2·−), hydroxyl radical (·OH), and peroxynitrite (ONOO−) and the cellular Ca2+ level are well known to maintain the redox homeostasis and signaling events during normal physiological processes. Therefore, it is considered that the interaction between ROS and Ca2+ can be bidirectional [59–62]. Excessive and uncontrolled ROS signal or oxidative stress directly damages the plasma membrane fluidity and redox homeostasis [61, 62]. As a result, Ca2+ influx into the cytoplasm from the extracellular environment and disrupts the ion exchanging balance between the intra- and extra-cellular plasma membranes as well as mitochondria, which then leads to cell death or the apoptotic pathway by increasing the cytochrome c protein, which then in turn activates caspase 3 and caspase 9. The accelerated Ca2+ level disrupted the Ca2+ signal in the cellular cytoplasm, which dephosphorylates the protein and modulates the PKC signal transduction cascades, and is associated with many diseases and the aging process [60–62].
Primarily, antioxidants have the greatest defensive role in protecting the cell against oxidative damage [63]. Generally, antioxidants have a vital role in keeping optimal cellular functions and systemic health and well-being. However, under oxidative stress conditions, endogenous antioxidants in humans, although highly efficient, are not sufficient to protect the cell from the harmful effects of ROS [64]. Therefore, dietary antioxidants are required to maintain the optimal cellular defensive functions. The most efficient enzymatic antioxidants contain glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), present in cellular cytoplasm and mitochondria. Nonenzymatic exogenous antioxidants are mainly derived in nature from photosynthetic organisms, fruits, vegetables, and plants and belong to different families such as vitamins E and C, thiol antioxidants (glutathione, thioredoxin, and lipoic acid), melatonin, carotenoids, and natural flavonoids [64–67] (Figure 3).
Figure 3.
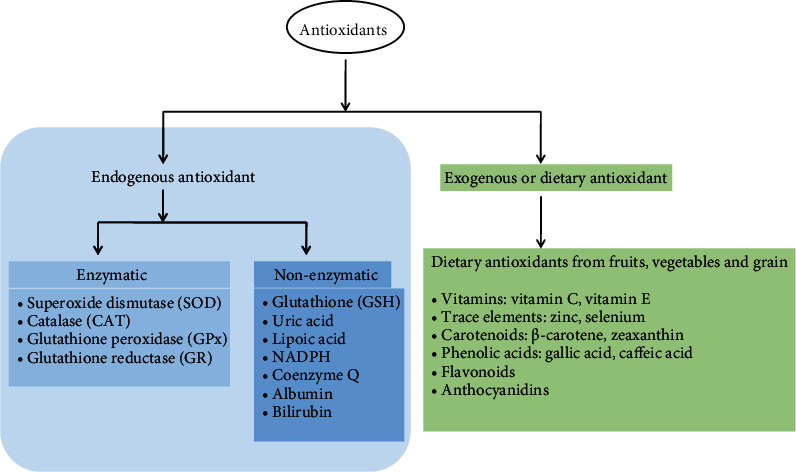
Classification of enzymatic and nonenzymatic antioxidants.
Antioxidants are considered as stable molecules that can donate an electron to a free radical and neutralize them, thereby scavenging the free radical and stopping the free radical from causing future damage [68]. Originally, antioxidants protect the cell from oxidative stress by applying one of the two mechanisms such as (a) the chain-breaking mechanism by which the primary antioxidant donates an electron to the free radical. (b) the second mechanism is the removal of ROS/reactive nitrogen species (RNS) initiators (secondary antioxidants) by quenching a chain-initiating catalyst. Antioxidants may provide their defensive action on biological systems at different levels including electron donation, metal ion chelation, radical scavenging, and repair or by gene expression regulation as well as prevent lipid peroxidation [69]. Moreover, antioxidants protect the cells by applying their strong multiphase efficacy system into the cell and cell membrane. It can strongly diffuse in both the aqueous and oil phases because of its polar and nonpolar paradox systems [70], known as a lipophilic, hydrophobic, and amphiphilic antioxidant (Figure 4). The efficacy of lipophilic antioxidants or polar antioxidants is to be more efficient than nonpolar or hydrophilic antioxidants. The lipophilic antioxidant can orient itself to the oil-water interface, where lipid peroxidation is induced whereas the hydrophilic antioxidant diffuses in the water phase, and therefore, it is less efficient [71]. Additionally, their activity also depends on the concentration of antioxidants, free radical types, and both the chemical structure and the reaction condition [69].
Figure 4.

Efficacy of active antioxidants at different phases.
Nowadays, a large number of natural antioxidant compounds such as carotenoids, ascorbic acid, flavonoids, and phenolic compounds are derived from seaweed sources [22, 72–75]. The antioxidative efficiency of seaweeds is higher than that of plants and fruits [76–78]. It is already well reported that the brown seaweeds are the richest sources of these antioxidant compounds with a unique structure, which has a greater level of hydrophilic and lipophilic nature [14, 17, 79] (Table 2).
Table 2.
Antioxidant efficacy of brown seaweeds compounds.
The redox equilibrium plays a pivotal role in the cell's physiological and pathological functions by balancing the ROS and antioxidant as well as ROS stability. ROS stability needs to activate or deactivate a variety of receptors, proteins, ions, and other signaling molecules [50, 53]. The excessive accumulation or depletion of ROS leads to instability of the redox balance that may influence many cellular signaling pathways and confers the cellular dysfunction as well as subsequently developing various pathologies [50]. To equilibrate the redox balance, antioxidants not only produce the antioxidant but also have a great ability to modulate the ROS sensitive cell signaling with the activation of the antioxidant responsive element ARE/Nrf2 pathway [54, 55]. However, numerous studies have already elucidated that the phytochemicals or natural compounds of antioxidants successively modulate the ROS-sensitive signaling pathway and restock the antioxidant into the cell through maintaining the redox balance [86]. The activation of such signaling pathways increased the expression of gene-encoding cytoprotective proteins, including phase II enzymes, antioxidant enzymes, growth factors, and proteins involved in the regulation of cellular energy metabolism. The activation of the Nrf2 pathway intersects with other intracellular signaling pathways such as the MAPK, PI3k/Akt, and NF-κB pathways [54, 55, 87] (Figure 5)
Figure 5.
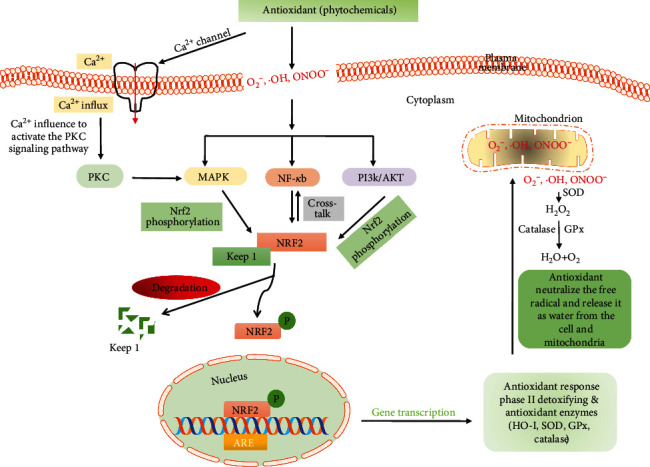
Antioxidant mechanism in the regulation of the Nrf2 signaling pathway with the crosstalk of the different signaling pathways.
On the other hand, antioxidants actively reduce the cellular ROS which may help to balance the cellular redox balance as well as the intra- and extracellular cytoplasmic Ca2+levels [87]. Antioxidants reduce the intracellular ROS level by balancing the ionic exchange between the plasma membrane and the cytoplasmic membrane as well as modulate the Ca2+ channel called IP inositol 1,4,5-trisphosphate receptor (IP3R) [54, 55, 59, 87]. As a result, the Ca2+ ion binds to the PKC enzyme and activates the PKC signaling cascades, as well as activates various signaling pathways and also triggers the release and translocation of Nrf2 from the cytosol to the nucleus, which is responsible for the expression of antioxidant genes, thus maintaining the cellular redox homeostasis [55, 59] (Figure 5).
Brown seaweeds are the largest type of macroalgae that belong to the phylum of Phaeophyta, which means “dusky plants.” Naturally, it is brown or yellow-brown and found in temperate or arctic waters. Brown algae typically have a root-like structure called a “holdfast” to anchor the algae to a surface. There are about 1500-2000 species of brown algae worldwide [3]. The huge biodiversity of algae and the richness of physiological traits with their unique adaptive properties are considered as a potential target in the research area [88]. Most of the algae are oxygenic autotrophs; they can quickly and continuously adapt themselves to extreme environments by synthesizing their bioactive compounds with protective functions, limiting or repairing potential damage from a harmful environment. These bioactive compounds act as primary and secondary sources of metabolites that provide the antioxidant and other bioactive functions as well as maintain their cellular homeostasis [3, 88]. It has been already proven in numerous research and clinical studies that the compound of brown algae species has antioxidative activity [23–25, 78, 81].
A large number of enzymatic and nonenzymatic antioxidants have been involved to detoxify the ROS and prevent the formation of highly reactive radicals such as hydroxyl radical (·OH) [88]. Enzymatic antioxidants mainly produced in a cell are usually high molecular weight substances such as SOD, glutathione reductase (GR), CAT, and ascorbate peroxidase. Primarily, enzymatic antioxidants inactivate the ROS intermediates and detoxify the ROS by supplying the NADPH for proper functioning (Figure 6). On the other hand, the dietary substances known as exogenous antioxidants (such as carotenoids, flavonoids, phenolic compounds, and ascorbic acid) scavenge the free radicals to break down the chain reaction responsible for lipid peroxidation [66–69]. The compounds of brown algae such as carotenoids, polyphenols, sulfated polysaccharides, and fucosterols have belonged as exogenous antioxidants, which may apply two defensive actions to cope with ROS before they can damage the cellular deference mechanism [89, 90] such as
The primary action is to break down the chain reaction, which results in free radicals becoming less reactive [66, 69]
The second mechanism is scavenging activity against superoxide and hydroxyl radicals by chelating/deactivating metals. The metal-binding proteins (such as albumin, ferritin, and myoglobin) inactivate the transition metal ions (such as copper, iron, manganese, zinc, and selenium act to upregulate the antioxidant enzyme activities) that catalyze the production of free radicals, quenching/scavenging the singlet and triplet oxygen (highly toxic) and removing ROS [66, 91, 92].
Figure 6.
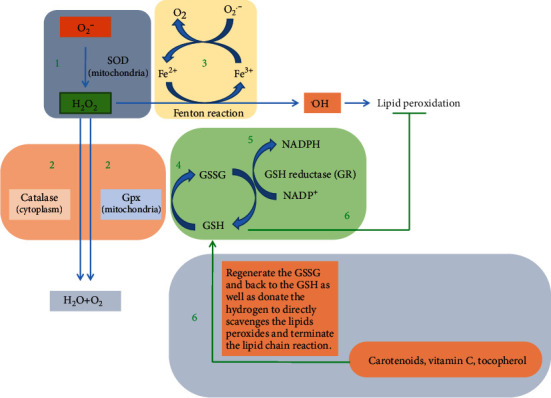
The enzymatic and nonenzymatic antioxidant protection mechanism. (1) Superoxide radical (O2·−) is formed by a single-electron reduction of oxygen. In a reaction catalyzed by superoxide dismutase (Cu/ZnSOD or MnSOD), superoxide radical binds an electron, which leads to the formation of hydrogen peroxide (H2O2). (2) In the further reduction of hydrogen peroxide (H2O2) to water and oxygen by catalase (CAT) and glutathione peroxidase (GPx) enzymes. (3) In the Fenton reaction, the H2O2 is then transformed into hydroxyl radical (HO·) by catalyzing the transition metal, which is further participating in the free radical chain reactions. (4) H2O2 is reduced by glutathione (GSH) and produced glutathione disulfide (GSSH). (5) The glutathione disulfide is then reduced by glutathione reductase (GR) using the hydrogen of NADPH which is oxidized to NADP+. (6) Besides, the nonenzymatic antioxidants such as carotenoids, vitamin C, and tocopherol support the regeneration of GSSG and back to GSH. Vitamin C, carotenoids, and tocopherol donate the hydrogen to free radicals that directly scavenges the free radical and terminates the lipid peroxidation chain reaction [66, 91, 92].
3. Antioxidant Effects of Brown Seaweed-Derived Compounds
3.1. Carotenoids
Carotenoids are a family of pigmented compounds that are naturally synthesized by plants, algae, fungi, bacteria, and archaea [29]. Over 1100 naturally occurring carotenoids are produced from those sources but not from animals [93]. Carotenoids are hydrophobic highly conjugated 40-carbon (with up to 15 conjugated double bonds) molecules. About more than 500 species of natural carotenoids are known and categorized into two groups: primary carotenoids which have pure hydrocarbons (those without any oxygen molecule), e.g., α-carotene, β-carotene, and lutein, and secondary carotenoids which are carbon (C), hydrogen (H), oxygen-containing xanthophylls (e.g., fucoxanthin, astaxanthin, canthaxanthin, and echinenone) are generally produced during the photosynthesis of seaweeds after exposure to specific environmental stimuli such as light and osmotic shock [39, 94–96]. The brown seaweeds are the rich source of carotenoids, which have been reported as powerful antioxidants with numerous beneficial health effects (Figure 7) [96, 97]
Figure 7.

Distribution, biological functions, and application of natural carotenoids derived from brown seaweeds.
Carotenoids (Crt) are very potent natural antioxidants. The antioxidant actions of carotenoids are based on their singlet oxygen quenching properties and the ability to trap free radicals, which mainly depend on the number of conjugated double bonds [98]. Furthermore, carotenoids can scavenge oxidizing free radicals via three primary reactions (Equations (1) to (3)), by its addition, electron transfer, addition, and hydrogen atom transfer.
The carotenoid antioxidant reactions are as follows: (i) electron transfer between the free radical (R·) and Crt, resulting in the formation of a Crt radical cation (Crt·+) (Equation (1)) or Crt radical anion (Crt·−) (Equation (2)); (ii) they can transfer the electrons forming a radical cation (RCrt·) (Equation (3)); and (iii) hydrogen atom transfer leading to a neutral Crt radical (Crt·) (Equation (4)) [38, 99].
| (1) |
| (2) |
| (3) |
| (4) |
Generally, the principal defense process in carotenoids is their lipophilic nature that allows them to penetrate through the cellular lipid bilayer membrane and cross to the blood-brain barrier, carrying out its biological function also in different regions of the human body, including the brain [39]. Because as a lipid-soluble molecule, carotenoids actively scavenge the lipid and aqueous phase radicals, mostly they are located in the apolar core of lipid membranes. Thus, carotenoids are associated with various types of membranes within the cell such as the outer cell membrane, but also the mitochondria and the nucleus. They also work in lysosomes. As a consequence, carotenoids play a major protecting role in the cell membranes and lipoproteins from damage by free radicals [100].
Several evidences suggested that carotenoids can act as redox agents in the case of oxidative stress due to their electrophile character and their conjugated double bonds that enhance the endogenous antioxidant Nrf2-Keap1 systems [101] which eventually help to maintain the redox homeostasis [80]. The redox homeostasis mainly depends on the nucleophile compounds, e.g., glutathione (GSH) and thiol (–SH) as well as their reductase enzymes GR, heme oxygenase-1 (HO-1), glutathione S-transferases (GSTs), and NAD(P)H quinine oxidoreductase 1 (NQO1). The GSH or –SH (thiol-) containing enzymes play a pivotal role in the free radical chain reaction that promotes to sustain the redox homeostasis (Figure 6) [102, 103]. Studies demonstrated that the carotenoids could increase the GSH level through the modulation of redox-sensitive –SH groups of Kelch-like ECH-associated protein 1 (Keap1). The Keap-Nrf2 signaling pathway plays an important role in the cellular defense against oxidative stress [104, 105]. Generally, under the normal physiological condition, Nrf2 is bound with their repressor protein Keap1 in the cytosol; which leads to its degradation by the ubiquitinylation process. Keap1 is a cysteine-rich protein, and its –SH residue conformation can be modified by different oxidants and electrophiles, thereby leading to the liberation of Nrf2 and transfer to the nucleus [80, 106]. At a higher oxidative stress condition, excessive ROS promotes the disassociation of Keap1 and Nrf2 either via the activation of PKC that assists to phosphorylate the Nrf2 or by oxidation of the cysteine residues of Keap1 that mainly govern the Keap1 activity [54–57, 107]. The carotenoids act as antioxidants through their electrophile C=C groups which conjugate with the aldehyde group (–CHO) of Keap1 modifying their thiol residues allowing the Nrf2 release into cytosol and translocation to the nucleus. Thus, Keap1 is inactivated, and newly synthesized Nrf2 binds to the ARE, which then leads to the expression of phase II enzymes and cytoprotective enzymes, e.g., GR, heme oxygenase-1 (HO-1), GSTs, NAD(P)H quinine oxidoreductase 1 (NQO1), SOD, and GPx [108] (Figure 8).
Figure 8.
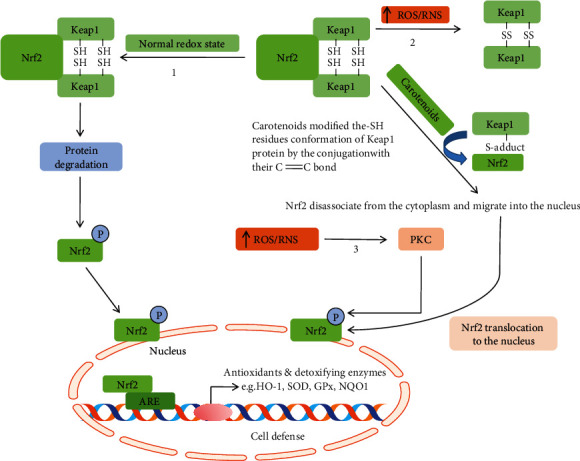
Electrophile activation of carotenoids of the Keap1-Nrf2 systems.
The scavenging function of carotenoids also has a signal-modulating role at the cellular signaling cascades such as the NF-κB, MAPK [109, 110], and PI3/Akt survival pathways as well the caspase pathway [111, 112]. Several studies suggest that carotenoids can act as modulators of redox-sensitive signaling cascades also able to progress the cell cycle through the direct modulation of cell cycle-related proteins as well as different signaling pathways which are usually involved in cell proliferation [108]. According to the studies, it can be hypothesized that β-carotene can modify the cellular redox status by changing the intracellular antioxidant status [112]. Studies showed that it can also delay the cell cycle G2/M phase by decreasing the expression of cyclin A in human colon adenocarcinoma cells [113] with increase of the antioxidant level which might help in decreasing the apoptotic protein as well as modifying the cellular growth [111]. It has been also reported that cell cycle progression and differentiation are dependent on the carotene dose, because it has a prooxidant role under high oxygen tension [113, 114]. Several indications suggested that carotenoids may also alter the expression of the apoptosis-related protein including the Bcl-2 and the caspase protein by a redox mechanism [111, 115]. Free radical species such as singlet oxygen [116] and nitric acid [117] have been reported to activate the caspase 8, an important protein degrading enzyme involved in the apoptotic cascade [108], whereas studies found carotenoids can initiate the caspase 3 activities in several cell lines, mainly by interacting with a single complex located on the cell membrane and inducing caspase 8. It was also reported that carotenoids protect the colon cancer cell from apoptosis by decreasing the expression of antiapoptotic protein Bcl-2 [112]. Such effects are mainly strictly related to apoptosis induction and ROS production by the carotenoids. This finding interestingly demonstrated that carotenoids have a role in an antioxidant pathway, whereby this protein can prevent programmed cell death by decreasing the ROS formation and lipid peroxidation products [115]. In vitro studies observed that β-carotene may increase the expression of the proapoptotic protein Bax in U-937 cells and HUVEC cells [112].
The NF-κB pathway is generally thought to be a primary oxidative-response pathway [112]. Studies have shown that carotenoids can inhibit the oxidative stress-induced NF-κB pathway with the addition of their electrophile groups and the cysteine residues of IκB kinase IKK and NF-κB subunits (p65) [118]. It has been reported that β-carotene prevents the phosphorylation of ERK, JNK, and P38 in oxidative stress [119]. Recently, it has been reported that β-carotene may affect cellular growth through the modulation of redox-sensitive transcription factor AP-1 [112]. Carotenoids may also modulate the antioxidant response regulatory gene (ARE) and can alter the gene expressions of phase II enzymes (i.e., HO-1, GSTs, NQO1, SOD, and GPx), resulting in cellular redox homeostasis [112] (Figure 9).
Figure 9.
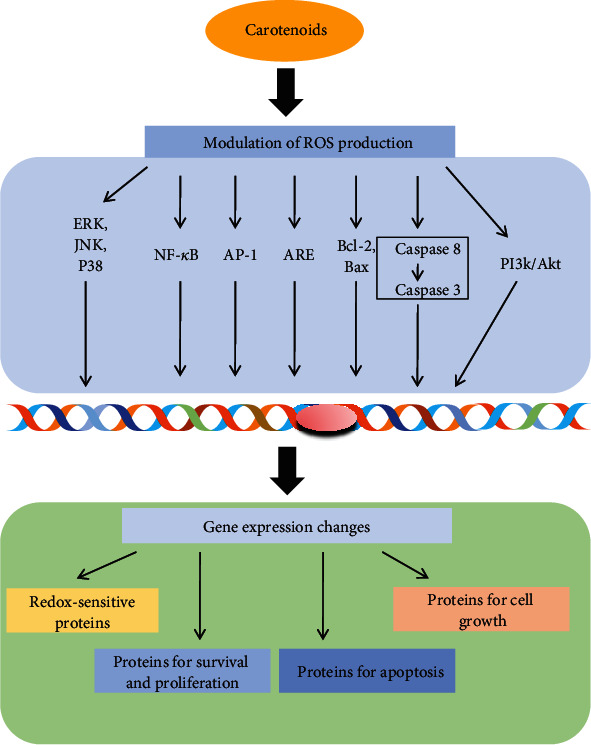
Scheme representing the multitude effects of carotenoids on the ROS-dependent signaling pathway and their related target protein gene expression. Carotenoid molecules may modulate the intracellular ROS production and can activate the redox-sensitive transcription factors or directly affect DNA damage which in turn may modify the gene expression.
Some of the studies indicate that carotenoids can also modulate oxidative stress-induced calcium signaling [120]. Carotenoids can decrease the calcium/calmodulin-dependent protein kinase IV (CAMKIV) enzyme by binding with the active site of CAMKIV; that form allows the cancer cell apoptosis [121]. CAMKIV is an enzyme belonging to the Ser/Thr kinase family. Generally, it plays a role in cell proliferation, migration, angiogenesis, and inhibition of apoptosis as well as calcium-dependent cell signaling. Elevated intracellular calcium ion concentration makes a complex form between Ca2+/calmodulin, which induces the phosphorylation of the transcription factor and causes various types of cancer [122, 123].
One of the well-known carotenoids is fucoxanthin, considered as a potential antioxidant because of its unique chemical structure including an allelic bond (C=C), epoxide group, and hydroxyl group [124]. It is well-known, and unique structural carotenoid pigments are present in brown seaweed that have not been found in other carotenoids [35, 125]. Fucoxanthin accounts for around 10% of the total natural production of seaweed carotenoids [126], which shows strong antioxidative properties by the inhibition of intracellular ROS formation, DNA damage, and apoptosis as well as exhibiting strong enhanced cell viability against H2O2-induced oxidative stress [127]. Several studies have demonstrated their experiments that fucoxanthin is safe and nontoxic to cells even at repeated doses [128–131]. In vivo studies also have shown that a fucoxanthin diet elevated the antioxidant activities including CAT, SOD with the mRNA expression of Nrf2 and its target genes such as NQO1 [132]. It has an active role in the modulation of signaling pathways including MAPK [133], NF-κB [134], and apoptosis caspase pathways [135] as well as induces cell cycle arrest [136]. Recently, it was shown that fucoxanthin suppresses H2O2-induced inflammation and oxidative damage in microglial cells via the attenuation of the phosphorylation of the MAPK signaling pathway, as well as by the free radical scavenging capacity of fucoxanthin and its ability to regulate the endogenous antioxidant system [137] It can also exert the cytoprotective effects against H2O2-induced oxidative stress through the activation of the PI3K-dependent Nrf2-signaling pathway along with the expression of mRNA and protein relative cytoprotective gene LO2 cells [138]. Sangeetha and collaborators in vivo analyzed the properties of fucoxanthin compared with β-carotene [139]. These two carotenoids decreased lipid peroxidation as well as enhanced the CAT and GST activities, showing the protective effect against Na+K+-ATPase activity. However, fucoxanthin exhibited higher antioxidant and protection properties than β-carotene [39, 139]. Many studies suggested that fucoxanthin increases the intracellular cytosolic Ca2+ that triggers cell apoptosis through modulation of the cell cycle arrest [135, 136] (Figure 10).
Figure 10.

Carotenoids and fucoxanthin effects on the cellular calcium level along with the multitude effects of fucoxanthin on cellular signaling pathways. Fucoxanthin protects the cell from oxidative stress by modulating several cell signaling pathways.
3.2. Polyphenols and Phlorotannins
Algal polyphenols are a large and diverse class of secondary metabolites that consist of around 8000 naturally occurring compounds that possess vital biological functions including antioxidant and free radical scavenging properties [27, 140]. These biological properties mainly shared by this molecule are their phenol groups [27]. The antioxidant radical scavenging activity of these compounds generally precedes through the hydrogen atom transfer or electron transfer mechanisms [141–143]. The polyphenol compounds have one or more hydroxyl groups (-OH), which directly bond to a phenolic or aromatic hydrocarbon group, and the hydrogen atom transfer mechanism indicates the ability of the phenolic derivatives (ArOH) to transfer an -H atom from the phenolic -OH group [144]. Generally, the stability of the resulting phenolic radicals (ArOH·) governs the radical scavenging ability of these compounds [27].
| (5) |
In the electron transfer mechanisms, the ability to transfer an electron governs the radical scavenging activity of phenolic derivatives. The stability of these radical cations depends on their ionization potential. The lower potential value indicates the better stability of the electron [27].
| (6) |
Given the simplified reaction mechanism (Figure 11), phenols can react with free radical species (R·) giving phenolic radicals that undergo resonance stabilization within the molecule by delocalizing the unpaired electron within the aromatic ring leading to a stable intermediate (indicated by the resonance hybrid). This resonance stabilization stabilizes the radical scavenging ability of phenol compounds [27]
Figure 11.
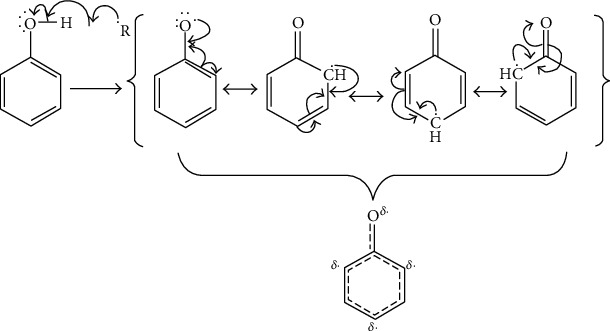
The proposed reaction between phenol and a free radical, which undergoes resonance stabilization; indicates the radical scavenging ability of compounds (i.e., curved half-headed arrows represent the transfer of a single electron).
The antioxidant effect of polyphenols has also the ability to enhance the enzymatic activity of different enzymes, including catalase, glutathione peroxidase, and superoxide dismutase, by their potent free radical scavenging properties and their ability to interact with other molecular targets, as they are capable of activating the Nrf2/ARE pathway [145] (Figure 12). Among algal polyphenolic compounds, phlorotannins have also been shown to have a high antioxidant property because of their highly hydrophilic components and the presence of –OH groups that can form hydrogen bonds with water. They have a wide range of molecular sizes between 126 and 650 kDa and can occur in various concentrations (0.5-20%) in brown algae [27, 146]. Brown microalgae accumulate a variety of phloroglucinol-based polyphenols as phlorotannins of low, intermediate, and high molecular weight containing both phenyl and phenoxy units, whichever can also be linked with each other or other algae such as red algae. Based on this linkage, phlorotannins can be classified into 4 major subclasses: (a) fuchalos and phlorethols (phlorotannins with an ether linkage), (b) fucols (with a phenyl linkage, (c) fucopholoroethols (with an ether and phenyl linkage), and (d) eckols (with an ether and phenyl linkage) [146]. Pholorotannins isolated from Ecklonia cava and their compounds (phlorofucofuroeckol A, dieckol, phloroglucinol, eckol, 7-pholoroeckol, 2-phloroeckol, and fucodipholoroethol G) are one of the most abundant sources of polyphenolic that have been mostly reported of biological activities including anti-inflammatory, antidiabetic, anticancer, antimicrobial, and antiviral, whereas most of them are focused on antioxidant activity [20, 146, 147]. Many researchers have shown that the eckol, phlorofucofuroeckol A, dieckol, and 8,8′-bieckol have shown potent inhibition of phospholipid peroxidation at 1 μM in a liposome system [147, 148], and these phlorotannins have significant radical scavenging activities against superoxide and DPPH radicals effectively compared with ascorbic acid and α-tocopherol [09]. There are several pieces of research that have shown that Ecklonia cava-derived phlorotannins and their bioactive compounds can modulate the ROS-mediated intracellular signaling pathway as well as gene expression and inhibit the enzymes [20, 145, 149, 150], which eventually protect the cellular damage from oxidative stress and apoptosis [20]. Studies also showed the phlorotannins may also protect the cancer cell by the inhibition of ROS-generating MMP-2 and MMP-9 via the NF-κB pathway [151]. It has been reviewed that phlorotannins can block the Ca2+ channel as well as reduce the Ca2 + influx which are stimulated by ROS (Figure 13) [20]. Due to several profound capabilities of E. cava, derived compounds are interesting to use as functional ingredients in pharmaceuticals and cosmeceuticals [147, 152, 153]
Figure 12.
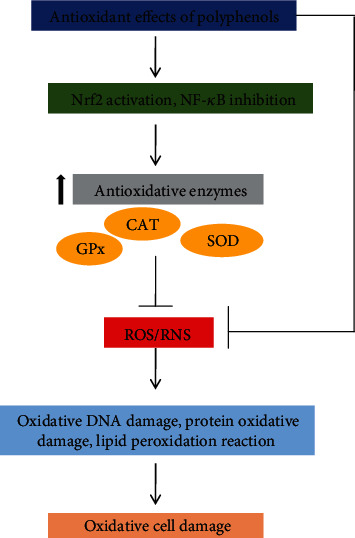
Potential mechanisms of polyphenols to the protection of the cell against oxidative stress.
Figure 13.
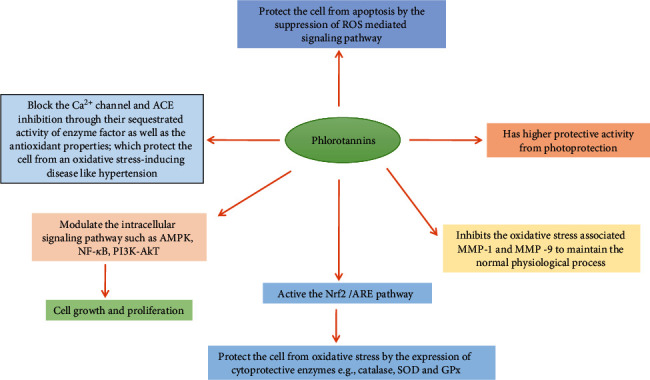
The biological role of phlorotannins on the function of the cell signaling pathway.
3.3. Sulfated Polysaccharides
Marine SPs are polymeric carbohydrates with large hydrophilic molecules. They have a fundamental role in living marine organisms such as energy storage and protection or as a structural molecule, which are mainly produced by photosynthesis [85]. The algal polysaccharides possess potent bioactivities because of their unique physicochemical properties, such as high content of fucose, galactose, uronic acid, and sulfate [154]. Microalgae can accumulate carbohydrates to more than 50% of their dry weight, due to the high photoconversion efficiency of the photosynthetic process [155]. These compounds are present in high concentrations and vast diversity in microalgae [156]. A vast number of researchers have reported that the SPs and its compounds could alleviate oxidative stress-mediated diseases, such as liver injury, diabetes, obesity, neurodegenerative disease colitis, and cancer [155–161]. This effect could be explained by three distinct mechanisms, including scavenging the ROS and regulating the antioxidant system or oxidative stress-mediated signaling pathways and also implying the complicated interactions of marine-derived antioxidant polysaccharides in reducing the oxidative stress [46, 47] (Figure 14). The antioxidant activity of SPs depends on their structural property, such as the degree of sulfating, their molecular weight, types of the major sugars, and glycosidic branching [46, 162]. The low molecular weight SPs have been shown to be potent antioxidants rather than the high molecular weight SPs [163]. It has been also reported that low molecular weight (~30 kDa) SPs can promote cell proliferation [164] as well as cell protection from the oxidative stress-induced apoptosis, which are directly and indirectly related to their antioxidant properties [46, 165]. Currently, SP has become a hot research area in the field of regenerative medicine and tissue engineering application due to its unique structure and specific features as antioxidants, antitumors, immunomodulatory, inflammation, anticoagulant, antiviral, antiprotozoal, and antibacterial [46, 47, 162, 165].
Figure 14.

Overview of sulfated polysaccharide (fucoidan) derived from brown seaweeds in alleviating oxidative stress-mediated disease.
Among the three types of SPs extracted from seaweeds, the brown seaweeds are the richest source of antioxidants [46, 47]. The major bioactive SP extracted from brown seaweeds is fucan which is usually defined as fucoidan; it is water-soluble and composed of significant quantities of L-fructose and sulfated ester groups accompanied with a little percentage of other monosaccharides, e.g., arabinose, xylose, glucose, galactose, and mannose. These constitutes of polysaccharides are known to be one of the major components of the brown algae cell wall that protects the cell in stress or low tide environments [46, 165].
Numerous in vivo and in vitro studies have revealed that fucoidan derived from brown algae can alleviate body damage from oxidative stress through the regulation of the antioxidant system in the body [45, 165, 166]. The excellent antioxidant activity of fucoidan is mainly dependent on their desulfation or oversulfation that allows the development of derivatives of fucoidans [46, 165, 166]. The DPPH free radical scavenging assay is widely used to evaluate the in vitro antioxidant activity [167]. Polysaccharides provide the hydrogen or electrons to DPPH free radicals to form stable molecules (DPPH-H) [168]. The electron-withdrawing group of polysaccharides and the specific structures activate the hydrogen atoms on sugar residues [169]. Many studies have already found that sulfated polysaccharides from brown algae have strong DPPH free radical scavenging activity and reduction ability. Furthermore, SPs also have strong ferrous ion-chelating and reducing power activities [170, 171]. Many studies have revealed that fucoidan inhibits oxidative stress by downregulating the malondialdehyde (MDA) level and upregulating the SOD level [172]. Moreover, studies have documented that fucoidan extract can reduce the levels of lipid peroxidation markers MDA and TBARS in alcoholic rats and increase the levels of the nonenzymatic antioxidant GSH and the enzymatic antioxidant SOD, CAT, and GPx in the liver, ultimately reducing the oxidative damage caused by alcohol. In addition, fucoidan also reduces the mRNA expression of TNF-α, IL-1β, and MMP-2 to inhibit the production of ROS in the liver, thus alleviating the development of nonalcoholic fatty liver disease [173]. Similarly, fucoidans inhibit the proliferation and induced apoptosis through the downregulation of the PI3K/AKT pathway as well as the reduction of Bcl-2 and Bcl-xl and the rise of the expression of Bax, caspase 3, and caspase 9 activation [174]. It can also inhibit the epidermal growth factor (EGF) signaling in several cancer cell types, by preventing binding their receptor site that promotes to block the activation of activator protein-1 (AP-1) which also diminishes the transactivation activity of ERK1/ERK2 and JNK signaling protein [175]. Another study also showed that fucoidan can inhibit apoptosis and improve the cognitive ability in Alzheimer's disease model mice by upregulating the expression of the apoptosis-inhibiting proteins by activating the SOD activity and increasing the GSH levels [176]. Recently, a study found that fucoidan can improve atherosclerotic cardiovascular disease by reducing the production of ROS by inhibiting NADPH oxidase (Figure 15) [177]. Besides, fucoidan can also inhibit ROS production through the regulation of the antioxidant defense system, thereby alleviating oxidative stress-related diseases. A study revealed that low molecular weight fucoidan regulates the activity of SOD and CAT by activating the SIRT1/AMPK/PGC1α signaling pathway, inhibiting the superoxide production and the lipid peroxidation, reducing TNF-α and transcription factor NF-κB, and then inhibiting oxidative stress in the liver (Figure 15) [164]. Furthermore, fucoidan significantly increased the expression of MnSOD and decreased the ROS level through the PI3/AKT pathway and finally enhanced the survival and angiogenesis of mesenchymal stem cells in the ischemia model [178]. Interestingly, it was reported that it could upregulate the expression of HO-1 and SOD-1 genes by activating Nrf2 and then attenuate the oxidative stress in HaCaT cells [163]. Research also showed that the Turbinaria ornata protects the human carcinoma cell through the modulation of PPAR gamma, NF-κB, and oxidative stress [179]. Another study also proved that the Turbinaria ornata protects the hepatic injury from azoxymethane-induced oxidative stress by exerting multiple pathways including the abolishment of inflammation and oxidative damage and the activation of PPAR gamma with increase of the antioxidant enzymes like SOD and GPx activities [180]. Thus, we could be review by all of these studies that fucoidan derived from brown algae not only has significant antioxidant activity but also modulates the oxidative stress-mediated diseases by regulating the antioxidant defense systems and the oxidative stress-related signaling pathway in cellular and experimental animal models [22].
Figure 15.
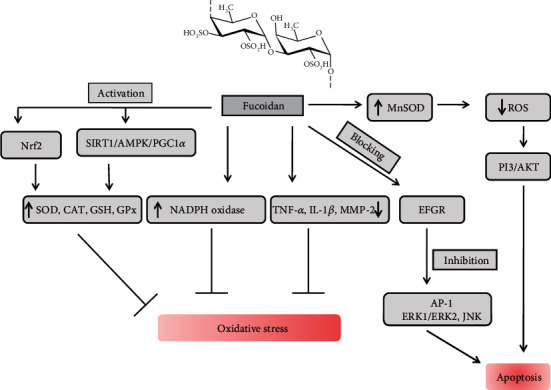
Fucoidan prevents oxidative stress and cell apoptosis by regulating the antioxidant system and signal-modulating pathway.
3.4. Sterols
There are abundant sterols in brown seaweeds. These compounds occur in the free form, esterified with fatty acids, or are involved in glycosylated conjugates [49, 181]. Sterols are amphipathic and triterpene compounds that can reach about 5.1% of the total biomass in the microalgae [181, 182]. Most of them contain 28 or 29 carbons and 1 or 2 carbon-carbon double bonds, typically one in the sterol nucleus and sometimes a second in the alkyl side chain [181]. Moreover, fucosterol displays a high diversity of the unique compounds of phytosterols, such as brassicasterol, sitosterol, and stigmasterol [182, 183]. Some species contain a mixture of ten or even more phytosterols [183]. Sterol composition varies depending on the algae strain and can be modulated by light intensity, temperature, or growth phase [83]. Sterols have received much attention in the last few years because of their cholesterol-lowering properties [181]. Fucosterol is structural and functionally similar to cholesterol; however, it contains an alkyl; fucosterol, a phytosterol found in brown seaweeds, is well recognized for its various beneficial biological activities [49].
Studies showed that fucosterol elevates the activities of free radical scavenging enzymes such as SOD, CAT, and GPx against hydrogen peroxide. It can also restore the SOD and GPx cellular defensive enzymes which help to prevent cell membrane oxidation [48]. It can also suppress the nitric oxide (NO) and ROS generation through the suppression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) expression because increasing iNOS and COX-2 is responsible for inflammatory disease [49]. Moreover, fucosterol exhibits inhibitory activity against acetyl- and butyryl-cholinesterases (AChE and BChE, respectively) [184, 185] and β-secretase enzyme which is responsible for Alzheimer's disease, although this enzyme production level is mainly associated with increasing the oxidative stress [186].
In the study of pharmacology and in silico analysis, it was revealed that the fucosterol action could intervene in the disease progression through modulating the biological processes as well as the signaling pathway, such as cytokine-mediated signaling pathway, apoptotic process, transcription regulation, inflammatory response, aging, response to lipopolysaccharide, NF-κB activity, and also cellular response to ROS, which are closely associated with the disease pathophysiology. Studies showed that fucosterol could pass through the cell membrane to reach directly to various intracellular targets. The pharmacological network data reported that fucosterol showed a close association with the target proteins of many crucial pathways at the molecular and cellular levels [187]. Another report demonstrated that fucosterol attenuates the oxidative stress through the upregulation of antioxidant enzymes such as GPX1, SOD, CAT, and HO-1 via Nrf2/ARE and PI3/Akt signaling activation [49, 188].
Fucosterol treatment can inhibit the oxidative stress-induced MAPK and NF-κB signaling pathway along with reducing the levels of inflammatory mediators PGE2 and COX-2, as well as proinflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8 [188]. Studies represented an excellent role of fucosterol in cancer cell proliferation, cell cycle, and apoptosis [189]. It was found that fucosterol causes the arrest of the cancer cells at the stage of the G2/M phase that prevents the cancer cells to enter the mitosis stage which can prevent cell growth and proliferation [190]. The Raf/MEK/ERK signaling pathway contributes to cancer cell proliferation. It also showed that fucosterol induced cell cycle arrest, via the inhibition of Raf/MEK/ERK signaling pathways that ultimately prevents cell proliferation [191]. Fucosterol can induce cancer cell apoptosis by increasing the expression of cleaved caspase 3, caspase 9, and Bax and decreasing the expression of Bcl-2, MMP-2, and MMP-9 [189, 192] (Figure 16). Research showed that the species of fucosterol named Padina pavonica exerted an excellent antidiabetic and antioxidant effect via the activation of PPARγ mechanisms [193]. Fucosterol treatment can protect the cell from DNA damage after oxidative stress [188]
Figure 16.
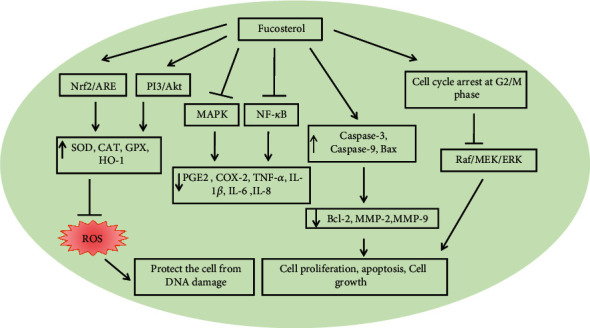
Overview of the biological role of fucosterol derived from brown seaweeds in alleviating the oxidative stress-mediated signaling pathway.
4. Conclusion
The research on brown seaweeds and their bioactive compounds' biological spectrum has been widely raised in recent years. Brown seaweeds and their derived compounds such as phlorotannins, SPs, fucosterol, and carotenoid pigments including fucoxanthin radical scavenging property are now clinically emphasized as a novel therapeutic compound. Antioxidants not only scavenge the free radical but also have numerous roles in the signal transduction pathway. From this point of view, we have attempted to gather plenty of information on how brown seaweeds and their antioxidant compounds modulate the oxidative stress signaling pathway as well as regulate the antioxidant system. However, the mechanisms of seaweeds are still obscure in the case of stress-causing disease. Thus, more studies should be focused on the investigation of activation and modulation as well as specific effects on the signaling pathway. The potentiality and structural features of the seaweed compound can have different effects on the cell. Additionally, long-term toxicity and drug delivery approaches should be evaluated.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
All authors contributed to the article and approved the submitted version.
References
- 1.Pereira L. Biological and therapeutic properties of the seaweed polysaccharides. International Biology Review. 2018;2(2) doi: 10.18103/ibr.v2i2.1762. [DOI] [Google Scholar]
- 2.Hoek C., Mann D., Jahns H. M., Jahns M. Algae: an Introduction to Phycology. Cambridge University Press; 1995. [Google Scholar]
- 3.Kandale A., Meena A. K., Rao M. M., et al. Marine algae: an introduction, food value and medicinal uses. Journal of Pharmacy Research. 2011;4(1):219–221. [Google Scholar]
- 4.Kelman D., Posner E. K., McDermid K. J., Tabandera N. K., Wright P. R., Wright A. D. Antioxidant activity of Hawaiian marine algae. Marine Drugs. 2012;10(12):403–416. doi: 10.3390/md10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeong S. C., Jeong Y. T., Lee S. M., Kim J. H. Immune-modulating activities of polysaccharides extracted from brown algaeHizikia fusiforme. Bioscience, Biotechnology, and Biochemistry. 2015;79(8):1362–1365. doi: 10.1080/09168451.2015.1018121. [DOI] [PubMed] [Google Scholar]
- 6.Heo S., Park E., Lee K., Jeon Y. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresource Technology. 2005;96(14):1613–1623. doi: 10.1016/j.biortech.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Wells M. L., Potin P., Craigie J. S., et al. Algae as nutritional and functional food sources: revisiting our understanding. Journal of Applied Phycology. 2017;29(2):949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M. T., Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 9.Uddin M. S., Al Mamun A., Kabir M. T., et al. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. European Journal of Pharmacology. 2020;886, article 173412 doi: 10.1016/j.ejphar.2020.173412. [DOI] [PubMed] [Google Scholar]
- 10.Khansari N., Shakiba Y., Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents on Inflammation & Allergy Drug Discovery. 2009;3(1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 11.Sies H. Biochemistry of oxidative stress. Angewandte Chemie International Edition in English. 1986;25(12):1058–1071. doi: 10.1002/anie.198610581. [DOI] [Google Scholar]
- 12.Liu Z., Ren Z., Zhang J., et al. Role of ROS and nutritional antioxidants in human diseases. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padayatty S. J., Katz A., Wang Y., et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American College of Nutrition. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty K., Maneesh A., Makkar F. Antioxidant activity of brown seaweeds. Journal of Aquatic Food Product Technology. 2017;26(4):406–419. doi: 10.1080/10498850.2016.1201711. [DOI] [Google Scholar]
- 15.Kahl R., Kappus H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 1993;196(4):329–338. doi: 10.1007/BF01197931. [DOI] [PubMed] [Google Scholar]
- 16.Jose G. M., Kurup G. M. In vitro antioxidant properties of edible marine algae Sargassum swartzii, Ulva fasciata and Chaetomorpha antennina of Kerala coast. Pharmaceutical Bioprocessing. 2016;4(6):100–108. [Google Scholar]
- 17.Sansone C., Brunet C. Promises and challenges of microalgal antioxidant production. Antioxidants. 2019;8(7):p. 199. doi: 10.3390/antiox8070199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan C. X., Ho C. L., Phang S. M. Trends in seaweed research. Trends in Plant Science. 2006;11(4):165–166. doi: 10.1016/j.tplants.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Alghazwi M., Kan Y. Q., Zhang W., Gai W. P., Garson M. J., Smid S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. Journal of Applied Phycology. 2016;28(6):3599–3616. doi: 10.1007/s10811-016-0908-2. [DOI] [Google Scholar]
- 20.Gómez-Guzmán M., Rodríguez-Nogales A., Algieri F., Gálvez J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Marine Drugs. 2018;16(8):p. 250. doi: 10.3390/md16080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo S. Y., Hwang J. H., Yang S. H., et al. Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin. Marine Drugs. 2019;17(5):p. 311. doi: 10.3390/md17050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galasso C., Gentile A., Orefice I., et al. Microalgal derivatives as potential nutraceutical and food supplements for human health: a focus on cancer prevention and interception. Nutrients. 2019;11(6):p. 1226. doi: 10.3390/nu11061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.el-Sheekh M. M., el-Shenody R. A. E. K., Bases E. A., el Shafay S. M. Comparative assessment of antioxidant activity and biochemical composition of four seaweeds, Rocky Bay of Abu Qir in Alexandria, Egypt. Food Science and Technology. 2020;41(Supplement 1):29–40. doi: 10.1590/fst.06120. [DOI] [Google Scholar]
- 24.Cox S., Abu-Ghannam N., Gupta S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. International Food Research Journal. 2010;17(1):205–220. [Google Scholar]
- 25.Peng J., Yuan J. P., Wu C. F., Wang J. H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Marine Drugs. 2011;9(10):1806–1828. doi: 10.3390/md9101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S. K., Pangestuti R. Advances in Food and Nutrition Research, Vol. 64. Academic Press; 2011. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae; pp. 111–128. [DOI] [PubMed] [Google Scholar]
- 27.Fernando I. S., Kim M., Son K. T., Jeong Y., Jeon Y. J. Antioxidant activity of marine algal polyphenolic compounds: a mechanistic approach. Journal of Medicinal Food. 2016;19(7):615–628. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- 28.Stiger-Pouvreau V., Bourgougnon N., Deslandes E. Seaweed in Health and Disease Prevention. Academic Press; 2016. Carbohydrates from seaweeds; pp. 223–274. [Google Scholar]
- 29.Li Y. X., Kim S. K. Utilization of seaweed derived ingredients as potential antioxidants and functional ingredients in the food industry: an overview. Food Science and Biotechnology. 2011;20(6):1461–1466. doi: 10.1007/s10068-011-0202-7. [DOI] [Google Scholar]
- 30.Qi H., Zhang Q., Zhao T., et al. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. International Journal of Biological Macromolecules. 2005;37(4):195–199. doi: 10.1016/j.ijbiomac.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q., Li N., Zhou G., Lu X., Xu Z., Li Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacological Research. 2003;48(2):151–155. doi: 10.1016/S1043-6618(03)00103-8. [DOI] [PubMed] [Google Scholar]
- 32.Wijesekara I., Pangestuti R., Kim S. K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydrate Polymers. 2011;84(1):14–21. doi: 10.1016/j.carbpol.2010.10.062. [DOI] [Google Scholar]
- 33.Tanna B., Mishra A. Nutraceutical potential of seaweed polysaccharides: structure, bioactivity, safety, and toxicity. Comprehensive Reviews in Food Science and Food Safety. 2019;18(3):817–831. doi: 10.1111/1541-4337.12441. [DOI] [PubMed] [Google Scholar]
- 34.Sanjeewa K. K., Fernando I. P. S., Samarakoon K. W., et al. Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae. 2016;31(3):277–287. doi: 10.4490/algae.2016.31.6.29. [DOI] [Google Scholar]
- 35.Yan X., Chuda Y., Suzuki M., Nagata T. Fucoxanthin as the major antioxidant inHijikia fusiformis, a common edible seaweed. Bioscience, Biotechnology, and Biochemistry. 1999;63(3):605–607. doi: 10.1271/bbb.63.605. [DOI] [PubMed] [Google Scholar]
- 36.Kong Z. L., Sudirman S., Hsu Y. C., Su C. Y., Kuo H. P. Fucoxanthin-rich brown algae extract improves male reproductive function on streptozotocin-nicotinamide-induced diabetic rat model. International Journal of Molecular Sciences. 2019;20(18):p. 4485. doi: 10.3390/ijms20184485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattaya S., Benjakul S., Prodpran T. Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. International Aquatic Research. 2015;7(1):1–6. doi: 10.1007/s40071-014-0085-3. [DOI] [Google Scholar]
- 38.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6(2):466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galasso C., Corinaldesi C., Sansone C. Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants. 2017;6(4):p. 96. doi: 10.3390/antiox6040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim A. R., Shin T. S., Lee M. S., et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. Journal of Agricultural and Food Chemistry. 2009;57(9):3483–3489. doi: 10.1021/jf900820x. [DOI] [PubMed] [Google Scholar]
- 41.Mwangi H. M., Van Der Westhuizen J., Marnewick J., Mabusela W. T., Kabanda M. M., Ebenso E. E. Isolation, identification andradical scavenging activity of phlorotannin derivatives from brown algae, Ecklonia maxima: an experimental and theoretical study. Free Radicals and Antioxidants. 2013;3:S1–S10. [Google Scholar]
- 42.Li Y., Qian Z. J., Ryu B. M., Lee S. H., Kim M. M., Kim S. K. Chemical components and its antioxidant properties in vitro: An edible marine brown alga, Ecklonia cava. Bioorganic & Medicinal Chemistry. 2009;17(5):1963–1973. doi: 10.1016/j.bmc.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 43.Shibata T., Ishimaru K., Kawaguchi S., Yoshikawa H., Hama Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. Journal of Applied Phycology. 2008;20(5):705–711. doi: 10.1007/s10811-007-9254-8. [DOI] [Google Scholar]
- 44.Chitra R., Ali M. S., Anuradha V., Shantha M. Antioxidant activity of polysaccharide from Sargassum sp. IOSR Journal of Pharmacy. 2018;8(8) [Google Scholar]
- 45.Wang L., Oh J. Y., Hwang J., Ko J. Y., Jeon Y. J., Ryu B. M. In vitro and in vivo antioxidant activities of polysaccharides isolated from celluclast-assisted extract of an edible brown seaweed, Sargassum fulvellum. Antioxidants. 2019;8(10):p. 493. doi: 10.3390/antiox8100493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel S. Therapeutic importance of sulfated polysaccharides from seaweeds: updating the recent findings. 3 Biotech. 2012;2(3):171–185. doi: 10.1007/s13205-012-0061-9. [DOI] [Google Scholar]
- 47.Zhong Q., Wei B., Wang S., et al. The antioxidant activity of polysaccharides derived from marine organisms: an overview. Marine Drugs. 2019;17(12):p. 674. doi: 10.3390/md17120674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S., Lee Y. S., Jung S. H., Kang S. S., Shin K. H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Archives of Pharmacal Research. 2003;26(9):719–722. doi: 10.1007/BF02976680. [DOI] [PubMed] [Google Scholar]
- 49.Abdul Q. A., Choi R. J., Jung H. A., Choi J. S. Health benefit of fucosterol from marine algae: a review. Journal of the Science of Food and Agriculture. 2016;96(6):1856–1866. doi: 10.1002/jsfa.7489. [DOI] [PubMed] [Google Scholar]
- 50.Uddin M. S., Upaganlawar A. B. Oxidative Stress and Antioxidant Defense Biomedical Value in Health and Diseases. USA: Nova Science Publishers; 2019. [Google Scholar]
- 51.Zhang H., Gomez A. M., Wang X., Yan Y., Zheng M., Cheng H. ROS regulation of microdomain Ca2+ signalling at the dyads. Cardiovascular Research. 2013;98(2):248–258. doi: 10.1093/cvr/cvt050. [DOI] [PubMed] [Google Scholar]
- 52.Sena L. A., Chandel N. S. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang J., Wang X., Vikash V., et al. ROS and ROS-mediated cellular signaling. Oxidative Medicine and Cellular Longevity. 2016;2016:18. doi: 10.1155/2016/4350965.4350965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ray P. D., Huang B. W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular Signalling. 2012;24(5):981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin J., Ren W. K., Wu X. S., et al. Oxidative stress-mediated signaling pathways: a review. Journal of Food, Agriculture and Environment. 2013;11(2):132–139. [Google Scholar]
- 56.Kensler T. W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annual Review of Pharmacology and Toxicology. 2007;47(1):89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 57.Lee J.-M., Johnson J. A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. Journal of Biochemistry and Molecular Biology. 2004;37(2):139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 58.Jung K.-A., Kwak M. K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15(10):7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Y., Wei C. L., Zhang W. R., Cheng H. P., Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacologica Sinica. 2006;27(7):821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 60.Hempel N., Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feno S., Butera G., Vecellio Reane D., Rizzuto R., Raffaello A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxidative Medicine and Cellular Longevity. 2019;2019:18. doi: 10.1155/2019/9324018.9324018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honda H., Kondo T., Zhao Q. L., Feril L. B., Jr., Kitagawa H. Role of intracellular calcium ions and reactive oxygen species in apoptosis induced by ultrasound. Ultrasound in Medicine & Biology. 2004;30(5):683–692. doi: 10.1016/j.ultrasmedbio.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Davies K. J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50(4):279–289. doi: 10.1080/15216540051081010. [DOI] [PubMed] [Google Scholar]
- 64.Wilson D., Nash P., Buttar H., et al. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: an overview. Antioxidants. 2017;6(4):p. 81. doi: 10.3390/antiox6040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu D. P., Li Y., Meng X., et al. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. International Journal of Molecular Sciences. 2017;18(1):p. 96. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirończuk-Chodakowska I., Witkowska A. M., Zujko M. E. Endogenous non-enzymatic antioxidants in the human body. Advances in Medical Sciences. 2018;63(1):68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Bouayed J., Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxidative Medicine and Cellular Longevity. 2010;3(4):p. 237. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos-Sánchez N. F., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidants. IntechOpen; 2019. Antioxidant compounds and their antioxidant mechanism. [Google Scholar]
- 69.Krinsky N. I. Mechanism of action of biological antioxidants. Experimental Biology and Medicine. 1992;200(2):248–254. doi: 10.3181/00379727-200-43429. [DOI] [PubMed] [Google Scholar]
- 70.Frankel E. N., Huang S. W., Kanner J., German J. B. Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. Journal of Agricultural and Food Chemistry. 1994;42(5):1054–1059. doi: 10.1021/jf00041a001. [DOI] [Google Scholar]
- 71.Laguerre M., Bayrasy C., Panya A., et al. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Critical Reviews in Food Science and Nutrition. 2015;55(2):183–201. doi: 10.1080/10408398.2011.650335. [DOI] [PubMed] [Google Scholar]
- 72.Newman D. J., Cragg G. M. Marine natural products and related compounds in clinical and advanced preclinical trials. Journal of Natural Products. 2004;67(8):1216–1238. doi: 10.1021/np040031y. [DOI] [PubMed] [Google Scholar]
- 73.Aklakur M. Natural antioxidants from sea: a potential industrial perspective in aquafeed formulation. Reviews in Aquaculture. 2018;10(2):385–399. doi: 10.1111/raq.12167. [DOI] [Google Scholar]
- 74.al-Saif S. S. A. L., Abdel-Raouf N., el-Wazanani H. A., Aref I. A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi Journal of Biological Sciences. 2014;21(1):57–64. doi: 10.1016/j.sjbs.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volkman J. K. Sterols in microalgae. In The Physiology of Microalgae. In: Borowitzka M. A., Beardall J., Raven J. A., editors. Developments in Applied, Phycology. Dordrecht, the Netherlands: Springer International Publishing; 2016. pp. 485–505. [Google Scholar]
- 76.Chen L., Xin X., Zhang H., Yuan Q. Phytochemical properties and antioxidant capacities of commercial raspberry varieties. Journal of Functional Foods. 2013;5(1):508–515. doi: 10.1016/j.jff.2012.10.009. [DOI] [Google Scholar]
- 77.Assunção M. F. G., Amaral R., Martins C. B., et al. Screening microalgae as potential sources of antioxidants. Journal of Applied Phycology. 2017;29(2):865–877. doi: 10.1007/s10811-016-0980-7. [DOI] [Google Scholar]
- 78.Ali H. E. A., Shanab S. M. M., Shalaby E. A. A., Eldmerdash U., Abdullah M. A. Applied Mechanics and Materials. Vol. 625. Stafa-Zurich, Switzerland: Trans Tech Publications; 2014. Screening of microalgae for antioxidant activities, carotenoids and phenolic contents; pp. 156–159. [Google Scholar]
- 79.Ak I., Turker G. Antioxidant activity of five seaweed extracts. New Knowledge Journal of Science. 2018;7(2):149–155. [Google Scholar]
- 80.Barros M. P., Rodrigo M. J., Zacarias L. Dietary carotenoid roles in redox homeostasis and human health. Journal of Agricultural and Food Chemistry. 2018;66(23):5733–5740. doi: 10.1021/acs.jafc.8b00866. [DOI] [PubMed] [Google Scholar]
- 81.Hermund D. B. Antioxidant properties of seaweed-derived substances. Bioactive Seaweeds for Food Application - Natural Ingredients for Healthy Diets. 2018. pp. 201–221.
- 82.Cotas J., Leandro A., Monteiro P., et al. Seaweed phenolics: from extraction to applications. Marine Drugs. 2020;18(8):p. 384. doi: 10.3390/md18080384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fábregas J., Arán J., Morales E. D., Lamela T., Otero A. Modification of sterol concentration in marine microalgae. Phytochemistry. 1997;46(7):1189–1191. doi: 10.1016/S0031-9422(97)80009-X. [DOI] [Google Scholar]
- 84.Sanjeewa K. A., Jeon Y. J. Edible brown seaweeds: a review. Journal of Food Bioactives. 2018;2:37–50. doi: 10.31665/jfb.2018.2139. [DOI] [Google Scholar]
- 85.Raposo M. F., de Morais R., Bernardo de Morais A. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Marine Drugs. 2013;11(1):233–252. doi: 10.3390/md11010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gupta V. K., Singh R., Sharma B. Phytochemicals mediated signalling pathways and their implications in cancer chemotherapy: challenges and opportunities in phytochemicals based drug development: a review. Biochemical Compounds. 2017;5(1):1–5. doi: 10.7243/2052-9341-5-2. [DOI] [Google Scholar]
- 87.Marengo B., Nitti M., Furfaro A. L., et al. Redox homeostasis and cellular antioxidant systems: crucial players in cancer growth and therapy. Oxidative Medicine and Cellular Longevity. 2016;2016:16. doi: 10.1155/2016/6235641.6235641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olasehinde T. A., Olaniran A. O., Okoh A. I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Marine Drugs. 2019;17(11):p. 609. doi: 10.3390/md17110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbosa M., Valentão P., Andrade P. Bioactive compounds from macroalgae in the new millennium: implications for neurodegenerative diseases. Marine Drugs. 2014;12(9):4934–4972. doi: 10.3390/md12094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vinayak R. C., Sabu A. S., Chatterji A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evidence-based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1093/ecam/neq024.673083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goiris K., Muylaert K., De Cooman L. Handbook of Marine Microalgae. Academic Press; 2015. Microalgae as a novel source of antioxidants for nutritional applications; pp. 269–280. [Google Scholar]
- 92.Mehta S. K., Gowder S. J. Members of antioxidant machinery and their functions. Basic Principles and Clinical Significance of Oxidative Stress. 2015;11:59–85. [Google Scholar]
- 93.Yabuzaki J. Carotenoids database: structures, chemical fingerprints and distribution among organisms. Database. 2017;2017 doi: 10.1093/database/bax004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miyashita K., Hosokawa M. Health impact of marine carotenoids. Journal of Food Bioactives. 2018;1:31–40. doi: 10.31665/JFB.2018.1125. [DOI] [Google Scholar]
- 95.Poojary M. M., Barba F., Aliakbarian B., et al. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Marine Drugs. 2016;14(11):p. 214. doi: 10.3390/md14110214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Novoveská L., Ross M. E., Stanley M. S., Pradelles R., Wasiolek V., Sassi J. F. Microalgal carotenoids: a review of production, current markets, regulations, and future direction. Marine Drugs. 2019;17(11):p. 640. doi: 10.3390/md17110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takaichi S. Carotenoids in algae: distributions, biosyntheses and functions. Marine Drugs. 2011;9(6):1101–1118. doi: 10.3390/md9061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young A. J., Lowe G. L. Carotenoids—antioxidant properties. [DOI] [PMC free article] [PubMed]
- 99.Noviendri D., Hasrini R. F., Octavianti F. Carotenoids: sources, medicinal properties and their application in food and nutraceutical industry. Journal of Medicinal Plant Research. 2011;5(33):7119–7131. [Google Scholar]
- 100.Britton G. Structure and properties of carotenoids in relation to function. The FASEB Journal. 1995;9(15):1551–1558. doi: 10.1096/fasebj.9.15.8529834. [DOI] [PubMed] [Google Scholar]
- 101.Linnewiel K., Ernst H., Caris-Veyrat C., et al. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radical Biology & Medicine. 2009;47(5):659–667. doi: 10.1016/j.freeradbiomed.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Ursini F., Maiorino M., Forman H. J. Redox homeostasis: the golden mean of healthy living. Redox Biology. 2016;8:205–215. doi: 10.1016/j.redox.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sies H., Berndt C., Jones D. P. Oxidative Stress. Annual Review of Biochemistry. 2017;86(1):715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 104.Akaboshi T., Yamanishi R. Certain carotenoids enhance the intracellular glutathione level in a murine cultured macrophage cell line by inducing glutamate-cysteine-ligase. Molecular Nutrition & Food Research. 2014;58(6):1291–1300. doi: 10.1002/mnfr.201300753. [DOI] [PubMed] [Google Scholar]
- 105.Barros M. P., Marin D. P., Bolin A. P., et al. Combined astaxanthin and fish oil supplementation improves glutathione-based redox balance in rat plasma and neutrophils. Chemico-Biological Interactions. 2012;197(1):58–67. doi: 10.1016/j.cbi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 106.Kaulmann A., Bohn T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutrition Research. 2014;34(11):907–929. doi: 10.1016/j.nutres.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 107.Niture S. K., Khatri R., Jaiswal A. K. Regulation of Nrf2--an update. Free Radical Biology & Medicine. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palozza P., Serini S., Ameruso M., Verdecchia S. Carotenoids. Basel: Birkhäuser; 2009. Modulation of intracellular signalling pathways by carotenoids; pp. 211–234. [Google Scholar]
- 109.Ben-Dor A., Steiner M., Gheber L., et al. Carotenoids activate the antioxidant response element transcription system. Molecular Cancer Therapeutics. 2005;4(1):177–186. [PubMed] [Google Scholar]
- 110.Palozza P., Serini S., Torsello A., et al. β-Carotene regulates NF-κB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. The Journal of Nutrition. 2003;133(2):381–388. doi: 10.1093/jn/133.2.381. [DOI] [PubMed] [Google Scholar]
- 111.Palozza P. Can β-carotene regulate cell growth by a redox mechanism? An answer from cultured cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1740(2):215–221. doi: 10.1016/j.bbadis.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 112.Palozza P., Serini S., Calviello G. Carotenoids as modulators of intracellular signaling pathways. Current Signal Transduction Therapy. 2006;1(3):325–335. doi: 10.2174/157436206778226950. [DOI] [Google Scholar]
- 113.Palozza P., Serini S., Maggiano N., et al. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis. 2002;23(1):11–18. doi: 10.1093/carcin/23.1.11. [DOI] [PubMed] [Google Scholar]
- 114.Palozza P., Serini S., Torsello A., et al. Regulation of cell cycle progression and apoptosis by ?-carotene in undifferentiated and differentiated HL-60 leukemia cells: possible involvement of a redox mechanism. International Journal of Cancer. 2002;97(5):593–600. doi: 10.1002/ijc.10094. [DOI] [PubMed] [Google Scholar]
- 115.Kane D. J., Sarafian T., Anton R., et al. Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science. 1993;262(5137):1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 116.Zhuang S., Lynch M. C., Kochevar I. E. Caspase-8 mediates caspase-3 activation and cytochrome c release during singlet oxygen-induced apoptosis of HL-60 cells. Experimental Cell Research. 1999;250(1):203–212. doi: 10.1006/excr.1999.4501. [DOI] [PubMed] [Google Scholar]
- 117.Chlichlia K., Peter M. E., Rocha M., et al. Caspase activation is required for nitric oxide–mediated, CD95 (APO-1/Fas)–dependent and independent apoptosis in human neoplastic lymphoid cells. Blood. 1998;91(11):4311–4320. doi: 10.1182/blood.V91.11.4311. [DOI] [PubMed] [Google Scholar]
- 118.Linnewiel-Hermoni K., Motro Y., Miller Y., Levy J., Sharoni Y. Carotenoid derivatives inhibit nuclear factor kappa B activity in bone and cancer cells by targeting key thiol groups. Free Radical Biology & Medicine. 2014;75:105–120. doi: 10.1016/j.freeradbiomed.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Jang S. H., Lim J. W., Kim H. Beta-carotene inhibits Helicobacter pylori-induced expression of inducible nitric oxide synthase and cyclooxygenase-2 in human gastric epithelial AGS cells. Journal of Physiology and Pharmacology. 2009;60(Supplement 7):131–137. [PubMed] [Google Scholar]
- 120.Cho K. S., Shin M., Kim S., Lee S. B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxidative Medicine and Cellular Longevity. 2018;2018:13. doi: 10.1155/2018/4120458.4120458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naz H., Islam A., Ahmad F., Hassan M. I. Calcium/calmodulin-dependent protein kinase IV: a multifunctional enzyme and potential therapeutic target. Progress in Biophysics and Molecular Biology. 2016;121(1):54–65. doi: 10.1016/j.pbiomolbio.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 122.Naz H., Khan P., Tarique M., et al. Binding studies and biological evaluation of β-carotene as a potential inhibitor of human calcium/calmodulin-dependent protein kinase IV. International Journal of Biological Macromolecules. 2017;96:161–170. doi: 10.1016/j.ijbiomac.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 123.Rzajew J., Radzik T., Rebas E. Calcium-involved action of phytochemicals: carotenoids and monoterpenes in the brain. International Journal of Molecular Sciences. 2020;21(4, article 1428) doi: 10.3390/ijms21041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hosokawa M., Okada T., Mikami N., Konishi I., Miyashita K. Bio-functions of marine carotenoids. Food Science and Biotechnology. 2009;18(1):p. 1. [Google Scholar]
- 125.Dembitsky V. M., Maoka T. Allenic and cumulenic lipids. Progress in Lipid Research. 2007;46(6):328–375. doi: 10.1016/j.plipres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 126.Matsuno T. Aquatic animal carotenoids. Fisheries Science. 2001;67(5):771–783. doi: 10.1046/j.1444-2906.2001.00323.x. [DOI] [Google Scholar]
- 127.Heo S. J., Ko S. C., Kang S. M., et al. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. European Food Research and Technology. 2008;228(1):145–151. doi: 10.1007/s00217-008-0918-7. [DOI] [Google Scholar]
- 128.Beppu F., Niwano Y., Tsukui T., Hosokawa M., Miyashita K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. The Journal of Toxicological Sciences. 2009;34(5):501–510. doi: 10.2131/jts.34.501. [DOI] [PubMed] [Google Scholar]
- 129.Iio K., Okada Y., Ishikura M. Bacterial reverse mutation test and micronucleus test of fucoxanthin oil from microalgae. Shokuhin Eiseigaku Zasshi. 2011;52(3):190–193. doi: 10.3358/shokueishi.52.190. [DOI] [PubMed] [Google Scholar]
- 130.Rijstenbil J. W. Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Marine Ecology Progress Series. 2003;254:37–48. doi: 10.3354/meps254037. [DOI] [Google Scholar]
- 131.Beppu F., Niwano Y., Sato E., et al. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH) The Journal of Toxicological Sciences. 2009;34(6):693–698. doi: 10.2131/jts.34.693. [DOI] [PubMed] [Google Scholar]
- 132.Ha A. W., Na S. J., Kim W. K. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutrition Research and Practice. 2013;7(6):475–480. doi: 10.4162/nrp.2013.7.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pangestuti R., Vo T. S., Ngo D. H., Kim S. K. Fucoxanthin ameliorates inflammation and oxidative Reponses in microglia. Journal of Agricultural and Food Chemistry. 61(16):3876–3883. doi: 10.1021/jf400015k. [DOI] [PubMed] [Google Scholar]
- 134.Briglia M., Calabró S., Signoretto E., et al. Fucoxanthin induced suicidal death of human erythrocytes. Cellular Physiology and Biochemistry. 2015;37(6):2464–2475. doi: 10.1159/000438599. [DOI] [PubMed] [Google Scholar]
- 135.Kumar S. R., Hosokawa M., Miyashita K. Fucoxanthin: a marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Marine Drugs. 2013;11(12):5130–5147. doi: 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Satomi Y., Nishino H. Implication of mitogen-activated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790(4):260–266. doi: 10.1016/j.bbagen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 137.Zhang H., Tang Y., Zhang Y., et al. Fucoxanthin: a promising medicinal and nutritional ingredient. Evidence-based Complementary and Alternative Medicine. 2015;2015:10. doi: 10.1155/2015/723515.723515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X., Cui Y. J., Qi J., et al. Fucoxanthin exerts Cytoprotective effects against hydrogen peroxide-induced oxidative damage in L02 cells. BioMed Research International. 2018;2018:11. doi: 10.1155/2018/1085073.1085073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sangeetha R. K., Bhaskar N., Baskaran V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Molecular and Cellular Biochemistry. 2009;331(1-2):59–67. doi: 10.1007/s11010-009-0145-y. [DOI] [PubMed] [Google Scholar]
- 140.Frere-Pelegrin Y., Robledo D. Bioactive phenolic compounds from algae. In: Hernândez-Ledesma B., Herrero M., editors. bioactive compounds from marine foods: plant and animal sources. Chichester, UK: John Wiley & Sons Ltd; 2013. [DOI] [Google Scholar]
- 141.Kozlowski D., Trouillas P., Calliste C., Marsal P., Lazzaroni R., Duroux J. L. Density functional theory study of the Conformational, electronic, and antioxidant properties of natural Chalcones. The Journal of Physical Chemistry. A. 2007;111(6):1138–1145. doi: 10.1021/jp066496+. [DOI] [PubMed] [Google Scholar]
- 142.Trouillas P., Marsal P., Svobodová A., et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin Flavonolignans: a joint experimental and theoretical study. The Journal of Physical Chemistry A. 2008;112(5):1054–1063. doi: 10.1021/jp075814h. [DOI] [PubMed] [Google Scholar]
- 143.Wright J. S., Johnson E. R., DiLabio G. A. Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. Journal of the American Chemical Society. 2001;123(6):1173–1183. doi: 10.1021/ja002455u. [DOI] [PubMed] [Google Scholar]
- 144.Mwangi H. M., van der Westhuizen J., Marnewick J., Mabusela W. T., Kabanda M. M., Ebenso E. E. Isolation, identification and radical scavenging activity of phlorotannin derivatives from brown algae, Ecklonia maxima: an experimental and theoretical study. Free Radicals and Antioxidants. 2013;3 doi: 10.1016/j.fra.2013.10.006. [DOI] [Google Scholar]
- 145.Sánchez M., Romero M., Gómez-Guzmán M., Tamargo J., Pérez-Vizcaino F., Duarte J. Cardiovascular effects of flavonoids. Current Medicinal Chemistry. 2019;26(39):6991–7034. doi: 10.2174/0929867326666181220094721. [DOI] [PubMed] [Google Scholar]
- 146.Li Y. X., Wijesekara I., Li Y., Kim S. K. Phlorotannins as bioactive agents from brown algae. Process Biochemistry. 2011;46(12):2219–2224. doi: 10.1016/j.procbio.2011.09.015. [DOI] [Google Scholar]
- 147.Kojima-Yuasa A. Polyphenols: Mechanisms of Action in Human Health and Disease. Academic Press; 2018. Biological and pharmacological effects of polyphenolic compounds from Ecklonia cava; pp. 41–52. [Google Scholar]
- 148.Shibata T., Ishimaru K., Kawaguchi S., Yoshikawa H., Hama Y. Nineteenth International Seaweed Symposium. Dordrecht: Springer; 2007. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae; pp. 255–261. [Google Scholar]
- 149.Wijesekara I., Yoon N. Y., Kim S. K. Phlorotannins from Ecklonia cava (Phaeophyceae): biological activities and potential health benefits. BioFactors. 2010;36(6):408–414. doi: 10.1002/biof.114. [DOI] [PubMed] [Google Scholar]
- 150.Valdés L., Cuervo A., Salazar N., Ruas-Madiedo P., Gueimonde M., González S. The relationship between phenolic compounds from diet and microbiota: impact on human health. Food & Function. 2015;6(8):2424–2439. doi: 10.1039/C5FO00322A. [DOI] [PubMed] [Google Scholar]
- 151.Zhang C., Li Y., Qian Z. J., Lee S. H., Li Y. X., Kim S. K. Dieckol from Ecklonia cava regulates invasion of human fibrosarcoma cells and modulates MMP-2 and MMP-9 expression via NF-κB pathway. Evidence-based Complementary and Alternative Medicine. 2011;2011:8. doi: 10.1155/2011/140462.140462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thomas N. V., Kim S. K. Beneficial effects of marine algal compounds in cosmeceuticals. Marine Drugs. 2013;11(12):146–164. doi: 10.3390/md11010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Holdt S. L., Kraan S. Bioactive compounds in seaweed: functional food applications and legislation. Journal of Applied Phycology. 2011;23(3):543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- 154.Bernaerts T. M., Gheysen L., Kyomugasho C., et al. Comparison of microalgal biomasses as functional food ingredients: focus on the composition of cell wall related polysaccharides. Algal Research. 2018;32:150–161. doi: 10.1016/j.algal.2018.03.017. [DOI] [Google Scholar]
- 155.Zhu S., Wang Y., Huang W., et al. Enhanced accumulation of carbohydrate and starch in Chlorella zofingiensis induced by nitrogen starvation. Applied Biochemistry and Biotechnology. 2014;174(7):2435–2445. doi: 10.1007/s12010-014-1183-9. [DOI] [PubMed] [Google Scholar]
- 156.Guzmán S., Gato A., Lamela M., Freire-Garabal M., Calleja J. M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytotherapy Research. 2003;17(6):665–670. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- 157.Huleihel M., Ishanu V., Tal J., Arad S. M. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. Journal of Applied Phycology. 2001;13(2):127–134. doi: 10.1023/A:1011178225912. [DOI] [Google Scholar]
- 158.Yim J. H., Son E., Pyo S., Lee H. K. Novel sulfated polysaccharide derived from red-tide microalga Gyrodinium impudicum strain KG03 with immunostimulating activity in vivo. Marine Biotechnology. 2005;7(4):331–338. doi: 10.1007/s10126-004-0404-6. [DOI] [PubMed] [Google Scholar]
- 159.Chen B., You W., Huang J., Yu Y., Chen W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World Journal of Microbiology and Biotechnology. 2010;26(5):833–840. doi: 10.1007/s11274-009-0240-y. [DOI] [Google Scholar]
- 160.Dvir I., Stark A. H., Chayoth R., Madar Z., Arad S. M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp. in rats. Nutrients. 2009;1(2):156–167. doi: 10.3390/nu1020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gardeva E., Toshkova R., Minkova K., Gigova L. Cancer Protective Action of Polysaccharide, Derived from Red MicroalgaPorphyridium Cruentum—A Biological Background. Biotechnology and Biotechnological Equipment. 2009;23(Supplement 1):783–787. doi: 10.1080/13102818.2009.10818540. [DOI] [Google Scholar]
- 162.Imjongjairak S., Ratanakhanokchai K., Laohakunjit N., Tachaapaikoon C., Pason P., Waeonukul R. Biochemical characteristics and antioxidant activity of crude and purified sulfated polysaccharides fromGracilaria fisheri. Bioscience, Biotechnology, and Biochemistry. 2016;80(3):524–532. doi: 10.1080/09168451.2015.1101334. [DOI] [PubMed] [Google Scholar]
- 163.Ryu M. J., Chung H. S. Fucoidan reduces oxidative stress by regulating the gene expression of HO-1 and SOD-1 through the Nrf2/ERK signaling pathway in HaCaT cells. Molecular Medicine Reports. 2016;14(4):3255–3260. doi: 10.3892/mmr.2016.5623. [DOI] [PubMed] [Google Scholar]
- 164.Zheng Y. Y., Liu T., Wang Z., Xu Y., Zhang Q., Luo D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. International Journal of Biological Macromolecules. 2018;112:929–936. doi: 10.1016/j.ijbiomac.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 165.Juárez-Portilla C., Olivares-Bañuelos T., Molina-Jiménez T., et al. Seaweeds-derived compounds modulating effects on signal transduction pathways: a systematic review. Phytomedicine. 2019;63:p. 153016. doi: 10.1016/j.phymed.2019.153016. [DOI] [PubMed] [Google Scholar]
- 166.Senthilkumar K., Manivasagan P., Venkatesan J., Kim S. K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. International Journal of Biological Macromolecules. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 167.Qi H., Zhao T., Zhang Q., Li Z., Zhao Z., Xing R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta) Journal of Applied Phycology. 2005;17(6):527–534. doi: 10.1007/s10811-005-9003-9. [DOI] [Google Scholar]
- 168.Wang J., Zhang Q., Zhang Z., Zhang J., Li P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. International Journal of Biological Macromolecules. 2009;44(2):170–174. doi: 10.1016/j.ijbiomac.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 169.Wang J., Liu L., Zhang Q., Zhang Z., Qi H., Li P. Synthesized oversulphated, acetylated and benzoylated derivatives of fucoidan extracted from Laminaria japonica and their potential antioxidant activity in vitro. Food Chemistry. 2009;114(4):1285–1290. doi: 10.1016/j.foodchem.2008.10.082. [DOI] [Google Scholar]
- 170.Mak W., Hamid N., Liu T., Lu J., White W. L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydrate Polymers. 2013;95(1):606–614. doi: 10.1016/j.carbpol.2013.02.047. [DOI] [PubMed] [Google Scholar]
- 171.Berteau O., Mulloy B. Sulfated fucans fresh perspectives: structures functions and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13(6):29R–240. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 172.Wang Q., Song Y., He Y., et al. Structural characterisation of algae Costaria costata fucoidan and its effects on CCl4-induced liver injury. Carbohydrate Polymers. 2014;107:247–254. doi: 10.1016/j.carbpol.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 173.Heeba G. H., Morsy M. A. Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environmental Toxicology and Pharmacology. 2015;40(3):907–914. doi: 10.1016/j.etap.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 174.Xue M., Ji X., Xue C., et al. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3β pathway in vivo and in vitro. Biomedicine & Pharmacotherapy. 2017;94:898–908. doi: 10.1016/j.biopha.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 175.Lee N. Y., Ermakova S. P., Choi H. K., et al. Fucoidan from Laminaria cichorioides inhibits AP-1 transactivation and cell transformation in the mouse epidermal JB6 cells. Molecular Carcinogenesis. 2008;47(8):629–637. doi: 10.1002/mc.20428. [DOI] [PubMed] [Google Scholar]
- 176.Wei H. Y., Gao Z., Zheng L., et al. Protective effects of fucoidan on Aβ25–35 and d-gal-induced neurotoxicity in PC12 cells and d-gal-induced cognitive dysfunction in mice. Marine Drugs. 2017;15(3):p. 77. doi: 10.3390/md15030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang X., Pei L. L., Liu H. B., et al. Fucoidan attenuates atherosclerosis in LDLR-/- mice through inhibition of inflammation and oxidative stress. International Journal of Clinical and Experimental Pathology. 2016;9:6896–6904. [Google Scholar]
- 178.Han Y. S., Lee J. H., Jung J. S., et al. Fucoidan protects mesenchymal stem cells against oxidative stress and enhances vascular regeneration in a murine hindlimb ischemia model. International Journal of Cardiology. 2015;198:187–195. doi: 10.1016/j.ijcard.2015.06.070. [DOI] [PubMed] [Google Scholar]
- 179.Mahmoud A. M., Abdella E. M., el-Derby A. M., Abdella E. M. Protective effects of Turbinaria ornata and Padina pavonia against azoxymethane-induced colon carcinogenesis through modulation of PPAR gamma, NF-κB and Oxidative Stress. Phytotherapy Research. 2015;29(5):737–748. doi: 10.1002/ptr.5310. [DOI] [PubMed] [Google Scholar]
- 180.Abdella E. M., Mahmoud A. M., el-Derby A. M. Brown seaweeds protect against azoxymethane-induced hepatic repercussions through up-regulation of peroxisome proliferator-activated receptor gamma and attenuation of oxidative stress. Pharmaceutical Biology. 2016;54(11):2496–2504. doi: 10.3109/13880209.2016.1160938. [DOI] [PubMed] [Google Scholar]
- 181.Moreau R. A., Whitaker B. D., Hicks K. B. Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Progress in Lipid Research. 2002;41(6):457–500. doi: 10.1016/S0163-7827(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 182.Ahmed F., Zhou W., Schenk P. M. Pavlova lutheri is a high-level producer of phytosterols. Algal Research. 2015;10:210–217. doi: 10.1016/j.algal.2015.05.013. [DOI] [Google Scholar]
- 183.Volkman J. K., Barrett S. M., Blackburn S. I. Eustigmatophyte microalgae are potential sources of C29 sterols, C22-C28n-alcohols and C28-C32n-alkyl diols in freshwater environments. Organic Geochemistry. 1999;30(5):307–318. doi: 10.1016/S0146-6380(99)00009-1. [DOI] [Google Scholar]
- 184.Jung W.-K., Ahn Y. W., Lee S. H., et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF- κB pathways. Food and Chemical Toxicology. 2009;47(2):410–417. doi: 10.1016/j.fct.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 185.Heneka M., Obanion M. Inflammatory processes in Alzheimer's disease. Journal of Neuroimmunology. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 186.Jung W.-K., Heo S. J., Jeon Y. J., et al. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. Journal of Agricultural and Food Chemistry. 2009;57(10):4439–4446. doi: 10.1021/jf9003913. [DOI] [PubMed] [Google Scholar]
- 187.Hannan M., Dash R., Sohag A. A. M., Moon I. S. Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Marine Drugs. 2019;17(11):p. 639. doi: 10.3390/md17110639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fernando I. S., Jayawardena T. U., Kim H. S., et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh) Environmental Research. 2019;172:150–158. doi: 10.1016/j.envres.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 189.Mao Z., Shen X., Dong P., et al. Fucosterol exerts antiproliferative effects on human lung cancer cells by inducing apoptosis, cell cycle arrest and targeting of Raf/MEK/ERK signalling pathway. Phytomedicine. 2019;61:p. 152809. doi: 10.1016/j.phymed.2018.12.032. [DOI] [PubMed] [Google Scholar]
- 190.Park J. I. Growth arrest signaling of the Raf/MEK/ERK pathway in cancer. Frontiers of Biology. 2014;9(2):95–103. doi: 10.1007/s11515-014-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Su C. C. Tanshinone IIA inhibits gastric carcinoma AGS cells by decreasing the protein expression of VEGFR and blocking Ras/Raf/MEK/ERK pathway. International Journal of Molecular Medicine. 2018;41 doi: 10.3892/ijmm.2018.3407. [DOI] [PubMed] [Google Scholar]
- 192.Mo W., Wang C., Li J., et al. Fucosterol protects against concanavalin A-induced acute liver injury: focus on P38 MAPK/NF-κB pathway activity. Gastroenterology Research and Practice. 2018;2018:13. doi: 10.1155/2018/2824139.2824139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Germoush M. O., Elgebaly H. A., Hassan S., Kamel E. M., Bin-Jumah M., Mahmoud A. M. Consumption of terpenoids-rich Padina pavonia extract attenuates hyperglycemia, insulin resistance and oxidative stress, and upregulates PPARγ in a rat model of type 2 diabetes. Antioxidants. 2020;9(1):p. 22. doi: 10.3390/antiox9010022. [DOI] [PMC free article] [PubMed] [Google Scholar]


