Abstract
Objectives
Conducting contact tracing (CT) programs in low- and middle-income countries is challenging, and there is no evidence of their effectiveness in Latin America. We evaluated the effectiveness of CT on reducing fatality from COVID-19 in Colombia.
Study design
The study design is a retrospective cohort study with nation-wide data of suspected and confirmed cases of severe acute respiratory syndrome (SARS-CoV-2) infection and their registered contacts.
Methods
We analyzed confirmed and suspected COVID-19 cases and their chains of contact using a nation-wide registry from March 28, 2020 to January 13, 2021. To estimate the effect of CT on fatality, we adjusted a multilevel negative binomial model using the number of deaths and the number of people within a chain of contacts as the outcome variable and offset variable, respectively. Sensitivity analysis was conducted using different cutoff values of contacts traced and a logistic model for the effect of CT on death at an individual level.
Results
We analyzed 1.4 million cases, 542,936 chains of contact, and 46,087 deaths. Only, 5.8% of total cases and contacts were included in a chain of a case and five or more contacts. We found that tracing of at least five contacts per case reduces fatality by 48% (95% confidence interval: 45–51), and, at the current levels of tracing in Colombia, it prevents 1.8% of deaths. Results obtained from the sensitivity analysis were consistent with the reduction of fatality at an individual level and higher protective effect with the higher number of contacts traced.
Conclusions
In Colombia, tracing of at least five contacts per case reduces fatality from COVID-19. The coverage and intensity of tracing needs to be increased as a strategy to mitigate fatality in Colombia.
Keywords: SARS-CoV-2, COVID-19, Epidemiology, Fatality, Contact tracing, Colombia
Introduction
As of December 31, 2020, the severe acute respiratory syndrome (SARS-CoV-2) virus caused 88.8 million infections and the disease that it produces (COVID-19) led to a total of 1.8 million deaths.1 The National Institute of Health in Colombia registered 1,508,419 confirmed cases and 43,213 deaths as of that same date.2
Given that this is a new disease, there are not yet specific pharmacological treatments, and the vaccinations that have recently been introduced are not yet available to the general public in low- and middle-income countries. Therefore, since the pandemic began, governments have implemented non-pharmacological interventions (NPIs), including specific individual interventions, such as promoting hand washing3 and the use of masks;4 community interventions, such as imposing restrictions on activities in closed spaces and canceling mass gatherings; and, in general, a variety of restrictions on mobility to reduce the rate of close contacts, ranging from partial restrictions to sector isolation and complete lockdowns. Contact tracing (CT) of confirmed and suspected cases has also been proposed as a mitigation strategy.
CT with early isolation of suspected cases is a strategy that is functioning in the midst of the SARS-CoV-2 pandemic. When adequately implemented, it reduces the acceleration of transmission and decreases fatality.5 Nevertheless, its massive implementation requires great operational efforts, such that its cost-effectiveness has even been questioned.6
Identification and follow-up of COVID-19 cases, as well as close CT, are some of the public health surveillance actions that have been implemented in Colombia since the start of the epidemic. As a central component of the mitigation strategy, in August 2020, the Ministry of Health of Social Protection (MHSP) of Colombia implemented a particular CT program called the Tests, Tracing, and Select Sustainable Isolation Program (PRASS in Spanish). The aim of this program is to monitor confirmed and suspected cases along with their contacts, as well as provide early and adequate health care throughout the country. Colombia has an insurance-based health system in which insurance companies, called ‘Beneficiary Plan Administration Companies’ (EAPB in Spanish), provide individual health services to the population, such as those performed by the PRASS program. The State's role in the system is to regulate its operations to ensure that adequate health services are provided to enrollees. The PRASS program has been implemented in the framework of this insurance model, where responsibilities are shared among different actors and national regulations apply.
Currently, in Colombia, with the knowledge that vaccinations will not reduce transmission in the short term and general restrictions on mobility have negative socio-economic effects, the transmission of SARS-CoV-2 infection needs to be reduced by using strategies that effectively detect and trace cases and contacts. Nevertheless, the effectiveness of the contract tracing strategy and its impact on meeting that goal for the country has not yet been evaluated. As a first approximation, this study uses nation-wide data to evaluate the effectiveness and impact of CT on fatality from COVID-19.
Methods
A retrospective cohort study was performed with nation-wide data of suspected and confirmed cases of SARS-CoV-2 infection and their registered contacts in Colombia, having the deaths from COVID-19 as the outcome of interest.
Population
Colombia is located in the north corner of South America. According to the National Administrative Department of Statistics (DANE, for its initials in Spanish), the total population is projected by 2020 in 50,372,424 inhabitants. The country is divided into 33 departments and districts which groups 1122 municipalities. Half of the population is women (51.2%), 77.1% of people live in urban areas, and 68.2% of Colombians are aged between 15 and 64 years. The first case of infection for SARS-CoV-2 was confirmed on March 6th in Bogotá.
Sources of information
This study used anonymized data from the MHSP information system that was designed specifically to address the SARS-CoV-2 pandemic, known as SEGCOVID19. This serves as the source of the PRASS program's integrated national registry on CT. This system combines information of suspected and confirmed cases reported to the National Public Health Surveillance System (SIVIGILA in Spanish) with their contacts identified through direct tracing by the EAPB. The system defines the chains of transmission as the index case and its respective contacts.
In addition, SEGCOVID19 crosses information with the national sampling system, which reports the results from all COVID-19 tests performed in the country, as well as with the database of enrollees in the health system and birth and death records. This cross information supplements the information on cases with the identification of results from diagnostic tests, health system enrollments for assigning who is responsible for follow-up (insurer and geographic entities), and the final vital status of each person.
While the MHSP administers this system of information, the EAPB and local authorities are required to report and contact the COVID-19 cases and their contacts and enter them into the system. The EAPB or local authorities must register in SEGCOVID19, in real time, the information related to suspected and confirmed cases, their demographic variables, clinical baseline data, and records of contacts for each case. These contacts are then visible to the EAPB responsible for the insurance for the purpose of identification, follow-up, clinical evaluation, and taking samples. Furthermore, because the system also makes it possible to follow-up on cases and contacts, it provides the best input of information on CT in the country.
The information for this analysis was taken from all observations at the national level that were registered from March 28, 2020 to January 13, 2021 by all the EAPBs and local authorities.
Variables and levels
Death from COVID-19 was the outcome of interest for this analysis. Exposure variables included individual-level data such as sex, age (in decades), presence of chronic comorbidities (cardiovascular disease, diabetes, cancer, immunodeficiencies), type of health insurance, socio-economic status, and place of residence. On a second level, the individuals were grouped into chains defined as a group consisting of a suspected or confirmed index case and its respective contacts who have been registered and contacted. The variables for the chain included the number of incident cases, the number of deaths in each chain, and the size of the chain. The main independent variable for the analysis was the size of the chain as an indirect indicator of the intensity of tracing. Based on a sensitivity analysis, this variable was dichotomized as a chain of five or more contacts per case, which was considered to be effective tracing. This conceptually makes sense because it recognizes at least two contacts other than those in the home, given that Colombia has an average of three people per household. An indicator variable was also included when the index case in a chain was a suspected case.
Statistical analysis
A multilevel negative binomial model was used with the number of deaths of individuals in each chain as the outcome variable and the number of people identified within the chain as the exposure variable. The model was adjusted by the aforementioned variables corresponding to the two levels. The model included random intercepts at the chain, insurance, and municipality level, as well as fixed effects. The intraclass correlation coefficient was calculated for each level as was relative risk (RR), with 95% confidence intervals (95% CIs). All assumptions of the model were verified, and the goodness-of-fit was evaluated. We used software Stata® version 16 to analyze the data.
To measure the impact on deaths, the preventable fraction (PF) was calculated based on the following formula, recommended by Benichou7 when the effect of confounding variables is present:
where Pd is the prevalence of exposure among deceased cases RR is the adjusted relative risk
Sensitivity analysis
We conducted a multilevel logistic model to calculate the individual risk of death associated with belonging to a chain of at least five contacts, adjusted by sex, age group, type of health insurance, socio-economic status, and comorbidities (diabetes, cardiovascular disease, chronic respiratory disease, cancer, and immunodeficiencies). We also conducted a sensitivity analysis using different cutoff values of the number of contacts traced to assess their effect on mortality from COVID-19.
Results
Between March 28, 2020 and January 13, 2021, SEGCOVID19 registered 1,404,294 suspected and confirmed cases, 542,936 of which had at least one registered contact (39.3%), and a total of 46,087 deaths due to COVID-19. A total of 1,029,020 contacts were registered, which is equivalent to a median of one contact per case (P25: 1 - P75: 3). The median age was 37 years for cases and 31 years for contacts. The percentage of men was 48.9% for cases and 46% for contacts. Health professionals accounted for 6.5% of cases and 2.4% of contacts. There was 62% prevalence of testing among contacts of cases during the study period. Table 1 summarizes the characteristics of the cases and contacts included in the study.
Table 1.
Characteristics of COVID-19 suspected and confirmed cases and their contacts, Colombia, March 2020–January 2021.
| Variable | Cases n (%) |
Contacts n (%) |
|---|---|---|
| 1,404,294 | 1,029,010 | |
| Male sex | 687,823 (49.0) | 476,431 (46.3) |
| Age (median IQR) | 37 (27–53) | 31 (15–49) |
| Deaths | 42,120 (3.0) | 3967 (0.39) |
| Chronic diseases | ||
| Diabetes | 41,036 (2.9) | 10,703 (1.0) |
| Cardiovascular disease | 106,568 (7.6) | 29,097 (2.8) |
| Chronic respiratory disease | 15,911 (1.1) | 4852 (0.5) |
| Cancer | 9780 (0.7) | 2959 (0.3) |
| Immunodeficiency | 5 059 (0.4) | 1 020 (0.1) |
| Region | ||
| Andes region | 900,152 (64.1) | 246,700 (24.4) |
| Caribbean | 220,474 (15.7) | 56,912 (5.6) |
| Pacific | 151,663 (10.8) | 30,450 (3.01) |
| Amazonia | 21,064 (1.5) | 2911 (0.28) |
| Orinoquia | 35,107 (2.5) | 7412 (5.14) |
| Unknown | 71,618 (5.1) | 666,684 (65.9) |
The regions are made up of the following departments: Andes (Bogota, Cundinamarca, Tolima, Antioquia, Santander, Northern Santander, Caldas, Risaralda, Quindío, Boyaca, and Huila), Caribbean (Atlantic, Bolivar, Cesar, Cordoba, La Guajira, San Andres, and Providencia, Sucre), Pacific (Cauca, Choco, Nariño, and Valley of Cauca), Amazonia (Amazonas, Caqueta, Guainia, Guaviare, Putumayo, and Vaupes), Orinoquia (Arauca, Casanare, Meta, and Vichada). IQR, interquartile range.
The fatality during the study period was 3.3%, and the fatality rate was 83.7 per 100,000 residents in Colombia. A total of 5.8% of the people (initial cases and their contacts) were in chains with at least five contacts plus the initial case (chains of five or more contacts).
The results of the main model showed that when tracing five or more contacts per case, the likelihood of dying in that chain decreased on average by 48% (95% CI: 45–51), controlling for the effect of comorbidities, region of residence, type of health system regime, socio-economic status, and individual factors such as age and sex (Table 2 ). The results also showed that, in a chain, the risk of dying from COVID-19 decreased by 34% (95% CI: 32–36) when the chain's index case was a suspected case, adjusted by the effect of other variables.
Table 2.
Multilevel negative binomial model results for the effect of contact tracing on fatality from COVID-19 in Colombia, March 2020–January 2021.
| Variables | IRR | SE | P-value | 95% CI | |
|---|---|---|---|---|---|
| Chains with five or more contactsa | 0.52 | 0.014 | 0.000 | 0.49 | 0.55 |
| Index (suspected case) | 0.66 | 0.011 | 0.000 | 0.64 | 0.68 |
| Male sex | 1.20 | 0.009 | 0.000 | 1.17 | 1.21 |
| Age in years | |||||
| Less than 30 | 1.00 | ||||
| 30 to 49 | 1.29 | 0.015 | 0.000 | 1.25 | 1.41 |
| 50 to 69 | 3.17 | 0.037 | 0.000 | 3.10 | 3.24 |
| 70 to 79 | 6.63 | 0.101 | 0.000 | 6.43 | 6.83 |
| 80 or more | 8.04 | 0.125 | 0.000 | 7.80 | 8.29 |
| Comorbidities | |||||
| Diabetes | 1.34 | 0.024 | 0.000 | 1.31 | 1.41 |
| Cardiovascular disease | 1.23 | 0.017 | 0.000 | 1.19 | 1.25 |
| Chronic respiratory disease | 1.13 | 0.027 | 0.000 | 1.09 | 1.20 |
| Cancer | 1.36 | 0.041 | 0.000 | 1.26 | 1.42 |
| Immunodeficiency | 1.35 | 0.076 | 0.000 | 1.22 | 1.53 |
| Health system regime | |||||
| Contributory | 1.00 | ||||
| Subsidiary | 2.33 | 0.035 | 0.000 | 2.29 | 2.43 |
| Special | 1.91 | 0.060 | 0.000 | 1.79 | 2.03 |
| Non-registered | 1.91 | 0.027 | 0.000 | 1.87 | 1.99 |
| Socio-economic status | |||||
| 1 (very low) | 0.59 | 0.039 | 0.000 | 0.51 | 0.67 |
| 2 (low) | 1.37 | 0.058 | 0.000 | 1.24 | 1.47 |
| 3 (middle to low) | 1.34 | 0.056 | 0.000 | 1.23 | 1.44 |
| 4 (middle) | 1.16 | 0.048 | 0.000 | 1.07 | 1.26 |
| 5 (middle to high) | 1.07 | 0.049 | 0.140 | 0.97 | 1.17 |
| 6 (high) | 1.00 | ||||
| Non-registered strata | 0.93 | 0.038 | 0.000 | 0.86 | 0.91 |
| Region | |||||
| Andean | 1.00 | ||||
| Caribbean | 1.06 | 0.015 | 0.000 | 1.03 | 1.10 |
| Pacific | 1.15 | 0.020 | 0.000 | 1.11 | 1.18 |
| Amazonia | 1.23 | 0.050 | 0.000 | 1.13 | 1.33 |
| Orinoquia | 1.08 | 0.040 | 0.000 | 1.01 | 1.16 |
| Non-registered | 0.88 | 0.011 | 0.000 | 0.87 | 0.92 |
IRR, incidence rate ratio; SE, standard error; CI, confidence interval.
n = 2 433 304 and chains = 1 404 294.
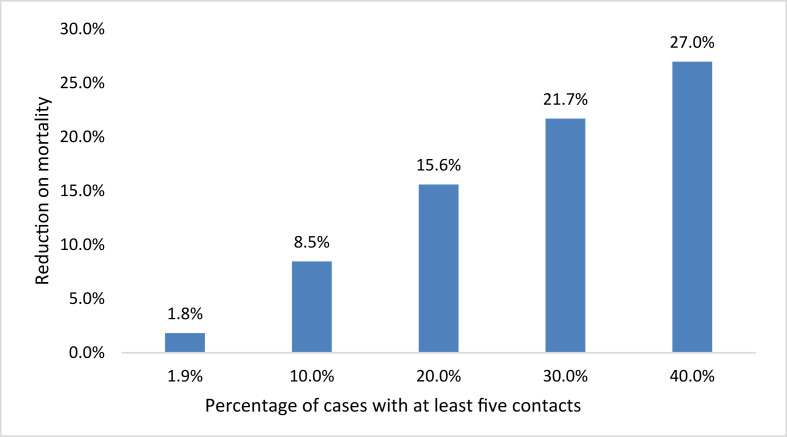
The adjusted PF was calculated as 1.8%, that is, 1.8% of total deaths (830) were estimated to have been prevented by tracing five or more contacts, adjusting by the effect of other covariables and at the observed tracing levels (5.7% of the total study population and 1.9% of deceased cases with tracing of five or more contacts). The calculation of the adjusted PF indicates that if the number of contacts in the tracing program increases progressively, then it is possible to prevent an average 27% of deaths when the coverage of tracing five or more contacts reach 40% of total cases (see Fig. 1).
Fig. 1.
Reduction in expected fatality if effective contact tracing increases (the number of people who are in chains of at least five contacts).
Sensitivity analysis
Table 3 presents the results of the logistic model that was used to evaluate the effect of CT on fatality. The results are consistent with those obtained with the negative binomial model, indicating that the risk of dying decreases on average by 55% (CI: 95% 51–58) when a person is part of a chain with at least five contacts, after controlling for the effect of sex, age, health system regime, comorbidities, region of residence, and socio-economic status. In this case, the odds ratio is similar to the RR because the accumulated incidence of death is under 5%.
Table 3.
Logistic model results for the effect of contact tracing on fatality from COVID-19 in Colombia, March 2020–January 2021.
| Variable | OR | SE | P-value | 95% CI | |
|---|---|---|---|---|---|
| Chains with five or more contacts | 0.45 | 0.016 | 0.000 | 0.42 | 0.49 |
| Index (suspected case) | 0.73 | 0.013 | 0.000 | 0.71 | 0.76 |
| Male sex | 2.10 | 0.022 | 0.000 | 2.06 | 2.14 |
| Age in years | |||||
| Less than 30 | 4.30 | 0.168 | 0.00 | 3.98 | 4.64 |
| 30 to 49 | 30.13 | 1.102 | 0.000 | 28.04 | 32.37 |
| 50 to 69 | 114.22 | 4.258 | 0.000 | 106.18 | 122.88 |
| 70 to 79 | 240.46 | 9.008 | 0.000 | 223.44 | 258.78 |
| 80 or more | |||||
| Comorbidities | |||||
| Diabetes | 1.62 | 0.028 | 0.000 | 1.57 | 1.68 |
| Cardiovascular disease | 1.21 | 0.017 | 0.000 | 1.18 | 1.24 |
| Chronic respiratory disease | 1.58 | 0.037 | 0.000 | 1.50 | 1.65 |
| Cancer | 1.76 | 0.055 | 0.000 | 1.65 | 1.87 |
| Immunodeficiency | 2.01 | 0.126 | 0.000 | 1.78 | 2.28 |
| Health system regime | |||||
| Contributory | 1.00 | ||||
| Subsidiary | 1.60 | 0.024 | 0.000 | 1.56 | 1.65 |
| Special | 1.30 | 0.038 | 0.000 | 1.23 | 1.37 |
| Non-registered | 1.75 | 0.023 | 0.000 | 1.70 | 1.79 |
| Region | |||||
| Andean | |||||
| Caribbean | 1.18 | 0.017 | 0.000 | 1.14 | 1.21 |
| Pacific | 1.09 | 0.018 | 0.000 | 1.06 | 1.13 |
| Amazonia | 1.29 | 0.049 | 0.000 | 1.20 | 1.39 |
| Orinoquia | 1.16 | 0.041 | 0.000 | 1.08 | 1.24 |
| Unknown | 0.18 | 0.005 | 0.000 | 0.17 | 0.18 |
| Socio-economic status | |||||
| 1 (very low) | 0.20 | 0.028 | 0.000 | 0.15 | 0.26 |
| 2 (low) | 1.99 | 0.099 | 0.000 | 1.80 | 2.19 |
| 3 (middle to low) | 1.89 | 0.092 | 0.000 | 1.71 | 2.07 |
| 4 (middle) | 1.37 | 0.067 | 0.005 | 1.24 | 1.50 |
| 5 (middle to high) | 1.17 | 0.064 | 0.000 | 1.05 | 1.30 |
| 6 (high) | 1.00 | ||||
| Undefined | 0.88 | 0.044 | 0.012 | 0.80 | 0.97 |
Pseudo R2 = 0.3245. OR, odds ratio; SD, standard error; CI, confidence interval.
The results of the analysis using the negative binomial model and different cutoff values for the number of contacts as the exposure variable are presented in supplementary material (Table S1). All effect measures were below the null value (RR < 1) and statistically significant indicating an overall protective effect of CT on COVID-19 mortality. Although there is not a specific dose--response pattern, the CT with the higher number of contacts (more than eight or nine contacts) showed the highest protective effect.
Discussion
This study aimed to assess the effectiveness of the CT strategy on COVID-19 fatality in Colombia. The results show that when the intensity of CT reaches five or more contacts per case, the risk of death from COVID-19 decreases on average by 48% in that chain of transmission. Furthermore, this study estimated that roughly 1.8% of deaths were prevented between March 2020 and January 2021 due to exposure to a CT chain of five or more contacts per case. The risk of death for COVID-19 is decreased on average by 88% when the intensity of CT reaches more than nine contacts. This is the first study to assess the effectiveness and impact of CT by using a national integrated database of confirmed and suspected COVID-19 cases and their contacts in Colombia.
The implementation of NPIs has successfully reduced virus transmission and deaths during a period in the COVID-19 pandemic that has lacked effective treatment and vaccines. NPIs involve a range of measures, including isolation of symptomatic individuals, CT, quarantine of exposed people, social distancing, face mask use, travel restrictions, the cancellation of mass gatherings, city and nationwide lockdowns, and the closure of schools, workplaces, and other spaces.9 There is cumulative evidence of the protective effect of social distancing, face masks, and eye protection to prevent virus transmission in healthcare and community settings.10 In Europe, NPIs, particularly lockdowns, had a large effect on reducing transmission and fatality, as well as decreasing the time-varying reproduction number to below 1, or at least delaying transmission while health services were increasing their response capacity.11
Mobility restrictions and lockdowns have also had secondary effects on the social and mental well-being of populations. During periods of lockdown, there is evidence of increased rates of violence against women,12 suicides,13 and food insecurity,14 as well as mental health disorders in children and adolescents.15 Lockdowns also negatively impact education, with increased rates of school dropout,16 and the economy, with deepening social inequalities,17 , 18 which leads to health inequalities.19 In addition, in low- and middle-income countries, the capacity of health systems and close intergenerational contacts limit the benefits of NPIs involving the use of restrictions for mitigation and suppression.20 For these reasons, there is a need to design and implement mitigation strategies that have fewer socio-economic impacts, particularly in low- and middle-income countries in Latin America.21
Studies in different regions of the world have reported evidence that supports the benefits of testing, CT, quarantine, and isolation to prevent COVID-19. Studies consistently report that quarantine is the most effective measure, which when integrated with other public health measures, such as testing and CT, increases the effectiveness of test, trace, and isolate programs.22 , 23 , 24 CT is also considered to be one of the most effective strategies for controlling the COVID-19 epidemic.25 Modeling studies have estimated that CT coupled with quarantine of people exposed to confirmed or suspected cases prevents 44%–96% of cases and 31%–76% of deaths, as compared with no intervention.26
The findings herein suggest that identifying and tracing five or more contacts related to a confirmed or suspected case reduces the risk of fatality in that chain of transmission on average by 48%. Nevertheless, the PF of this effect is still low (1.8%), probably due to the low prevalence of CT during the study period (5.7%). Different factors may explain this low prevalence, including the program's low coverage in urban areas, limitations in the human and technological resources needed to conduct CT, and a lack of follow-up on program responsibilities that are shared by all involved parties, particularly by EAPB, which provide primary health services to 95% of the population.27 One measure that is needed to better control transmission and reduce fatality is to implement strong health system regulations, given that the current healthcare provider system is market driven and fragmented. Another possible measure is looking for operational alternatives that improve coverage and increase the intensity of CT.
Automated CT is an alternative to manual procedures and could potentially reduce transmission. Nevertheless, a high population uptake is needed for automated CT to be effective, and this may be limited in low- and middle-income countries where internet and mobile phones are not widely available.28 In addition, privacy and equity concerns exist that need to be considered.29 The successful experiences with controlling the COVID-19 epidemic in Taiwan,30 South Korea,31 and Singapore32 involved automated CT and strong government regulations. In Taiwan, traditional CT was supplemented by automated CT, which helped to identify 88% of secondary cases and reduced effective reproductive number (Rt) to under 1.33 In Colombia, the application CoronApp mobile application was implemented in March 2020 to supplement traditional CT, but its use did not reach high coverage (10%), and its contribution to identifying secondary cases and reducing contacts has not been evaluated.34
CT in combination with other measures increases COVID-19 control. Using a mathematical modeling study, Kucharski et al.35 estimated that isolation and CT is more likely to control COVID-19 transmission than mass random testing. They estimated a 64% reduction in mean transmission for self-isolation and household quarantine with tracing of all contacts.35 However, to maximize the effectiveness of CT, another mathematical modeling study suggests that this component should be integrated with widespread coverage, early testing, and isolation. Given that testing programs miss 75% of cases, prevention by isolating contacts is estimated to be effective only under a high level of testing and CT.22 Therefore, CT with isolation of symptomatic patients alone may not be enough to control the epidemic,36 especially in communities of young people where the dynamics of infection reflect high infectivity during the incubation period.37 The results herein show a 62% prevalence of testing among contacts of cases during the study period. The PRASS program aims to test all symptomatic contacts and those belonging to high-risk groups (older than 60 years or comorbidities), leaving out asymptomatic people who might transmit the infection. Therefore, strategies to increase COVID-19 testing coverage will probably also contribute to interrupting chains of transmission and prevent more deaths.
The present study provides strong evidence by using data from a national integrated information system on COVID-19 in Colombia (SEGCOVID19), which allowed for controlling for other potential sociodemographic confounders of COVID-19 fatality. In particular, CT was evaluated with more than one million records, which represent all the suspected and confirmed cases and their contacts that were reported to the country's public health surveillance system during the study period. Nonetheless, limitations should be considered when interpreting the results. First, because the data reported to the national health system (SIVIGILA) are for surveillance purposes, there is a significant degree of underreporting. Preliminary results from a seroprevalence study based on population sampling in 11 cities, conducted by the National Institute for Health, estimated that SIVIGILA captures an average 10%–20% of total prevalent cases.38 Another limitation is the absence of individual data related to other NPIs such as the use and adherence of hand washing and use of masks as these data are not available in the dataset used for the analysis. Regarding the community-based NPIs such as mobility restrictions, they were widely used in different cities according to the pandemic situation and are expected to be similar within municipalities. Despite these interventions are associated with lower probability of a contagion, they are not related to the CT process, and therefore, they are not acting as potential confounders for our analyses. Therefore, we consider that the absence of this information is not a source of potential bias in our study.
In conclusion, the findings herein show that CT reduces the risk of fatality in the chains of transmission. A reduction in the fatality risk was observed despite the low prevalence of CT. These findings support the need to strengthen government regulations of CT, overcome its operational limitations, increase its intensity, and reach a CT coverage and intensity that contributes to stopping chains of transmission and preventing deaths.
Author statements
Ethical approval
The data were obtained anonymized from public secondary records from SEGCOVID19, database is available in a public repository (https://doi.org/10.6084/m9.figshare.14265380), and it was analyzed by the MSPS for public health purposes. The present analysis followed international and national guidelines for the protection of humans in health research. It also adhered to the guidelines defined by the MHSP for the analysis of SEGCOVID19 data.8
Funding
This study did not have a specific funding grant. Study design, data collection, data analysis, data interpretation, and writing the report were conducted as part of the work of the Direction of Epidemiology and Demography of the Ministry of Health and Social Protection of Colombia
Competing interests
J.A.F.-N., C.P.-M., and M.R.-B. worked at the Ministry of Health and Social Protection. Authors do not have other conflicts of interest to declare.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2021.07.013.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Johns Hopkins University . 2020. Coronavirus map.https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Instituto Nacional de Salud . 2020. COVID-19 en Colombia.https://www.ins.gov.co/Noticias/paginas/coronavirus.aspx [Google Scholar]
- 3.Centers for Disease Control and Prevention . 2020. Hand hygiene recommendations.https://www.cdc.gov/coronavirus/2019-ncov/hcp/hand-hygiene.html [Google Scholar]
- 4.Centers for Disease Control and Prevention . 2020. Considerations for wearing masks.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html#:%7B∼%7D:text=Wear [Google Scholar]
- 5.Fetzer T., Graeber T. Does contact tracing Work ? Quasi-experimental evidence from an excel error in england. MedRxiv. 2020 doi: 10.1101/2020.12.10.20247080. [DOI] [Google Scholar]
- 6.Reddy K.P., Shebl F.M., Foote J.H.A., et al. Cost-effectiveness of public health strategies for COVID-19 epidemic control in South Africa: a microsimulation modelling study. Lancet Glob Heal. 2020;9:e120–e129. doi: 10.1016/S2214-109X(20)30452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 8.República de Colombia M de S y PS . Lineamientos para el análisis de los datos registrados en SEGCOVID19. 2020. p. 52. [Google Scholar]
- 9.Patiño-Lugo D.F., Vélez M., Salazar P.V., et al. Non-pharmaceutical interventions for containment, mitigation and suppression of covid-19 infection. Colomb Méd. 2020;51:1–25. doi: 10.25100/cm.v51i2.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu D.K., Akl E.A., Duda S., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaxman S., Mishra S., Gandy A., et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 12.Agüero J.M. COVID-19 and the rise of intimate partner violence. World Dev; 2020. p. 105217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sher L. The impact of the COVID-19 pandemic on suicide rates. QJM. 2020;113:707–712. doi: 10.1093/qjmed/hcaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Agriculture Organization . Food and Agriculture Organization; Santiago, Chile: 2020. Comunidad de Estados Latinoamericanos y Caribeños. Seguridad Alimentaria bajo la Pandemia de COVID-19. [DOI] [Google Scholar]
- 15.Benjamin S., Lachal J., Radjack R., et al. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatr Res. 2020;291:113264. doi: 10.1016/j.psychres.2020.113264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia S. UNDP LAC C19 PDS; Bogotá: 2020. COVID-19 and primary and secondary education: the impact of the crisis and public policy implications for Latin America and the Caribbean. No. 20. [Google Scholar]
- 17.Adams-Prassl A., Boneva T., Golin M., Rauh C. Inequality in the impact of the coronavirus shock: evidence from real time surveys. J Publ Econ. 2020:189. doi: 10.1016/j.jpubeco.2020.104245. [DOI] [Google Scholar]
- 18.Puerto S., Kim K. Young workers will be hit hard by COVID-19's economic fallout. Int. Labor Organ. Blog. 2020 https://iloblog.org/2020/04/15/young-workers-will-be-hit-hard-by-covid-19s-economic-fallout/ [Google Scholar]
- 19.Bambra C., Riordan R., Ford J., Matthews F. The COVID-19 pandemic and health inequalities. J Epidemiol Community Health. 2020;74:964–968. doi: 10.1136/jech-2020-214401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker P.G.T., Whittaker C., Watson O.J., et al. The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science. 2020;369(80):413–422. doi: 10.1126/science.abc0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia P.J., Alarcón A., Bayer A., et al. COVID-19 response in Latin America. Am J Trop Med Hyg. 2020;103:1765–1772. doi: 10.4269/ajtmh.20-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girum T., Lentiro K., Geremew M., Migora B., Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop Med Health. 2020;48 doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferguson N.M., Laydon D., Nedjati-gilani G., Imai N., Ainslie K., Baguelin M. 2020. Report 9 - impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand.https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-9-impact-of-npis-on-covid-19/ London. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang B., Xia F., Tang S., et al. The effectiveness of quarantine and isolation determine the trend of the COVID-19 epidemic in the final phase of the current outbreak in China. Int J Infect Dis. 2020;95:288–293. doi: 10.1016/j.ijid.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keeling M.J., Hollingsworth T.D., Read J.M. Efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19) J Epidemiol Community Health. 2020;74:861–866. doi: 10.1136/jech-2020-214051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nussbaumer-Streit B., Dobrescu A.I.M., Chapman A., et al. Measures to control COVID-19 : a rapid review (Review) Cochrane Database Syst Rev. 2020:1–44. doi: 10.1002/14651858.CD013574.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giovanella L., Vega R., Tejerina-Silva H., et al. ¿Es la atención primaria de salud integral parte de la respuesta a la pandemia de Covid-19 en Latinoamérica? Trab Educ e Saúde. 2021:19. doi: 10.1590/1981-7746-sol00310. [DOI] [Google Scholar]
- 28.Braithwaite I., Callender T., Bullock M., Aldridge R. Automated and partly automated contact tracing: a systematic review to inform the control of COVID-19. Lancet Digit Heal. 2020;2:e607–e621. doi: 10.1016/S2589-7500(20)30184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapa S., Halamka J., Raskar R. Contact tracing to manage COVID-19 spread—balancing personal privacy and public health. Mayo Clin Proc. 2020;95:1320–1322. doi: 10.1016/j.mayocp.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbrook R. Contact tracing, testing, and control of COVID-19 - learning form Taiwan. JAMA Intern Med. 2020:180. doi: 10.1016/S0140-6736. [DOI] [PubMed] [Google Scholar]
- 31.Park Y., Choe Y., Park O., et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26:2465–2468. doi: 10.3201/eid2610.201315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee V., Chiew C., Khong W. Interrupting transmission of COVID-19: lessons from containment efforts in Singapore. J Trav Med. 2020;27 doi: 10.1093/jtm/taaa039. 0.1093/jtm/taaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jian S.W., Cheng H.Y., Huang X.T., Liu D.P. Contact tracing with digital assistance in Taiwan's COVID-19 outbreak response. Int J Infect Dis. 2020;101:348–352. doi: 10.1016/j.ijid.2020.09.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Instituto Nacional de Salud . 2020. Orientaciones para la Vigilancia en Salud Pública de la Covid19.https://www.ins.gov.co/Noticias/Coronavirus/Estrategia.VSP.COVID-19 23072020.pdf Bogotá. [Google Scholar]
- 35.Kucharski A.J., Klepac P., Conlan A.J.K., et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H. Contact tracing assessment of COVID-19 transmission dynamics in taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang L., Zhang X., Zhang X., et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect. 2020;80:e1–e13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Instituto Nacional de Salud . 2020. Seroprevalencia de SARS-CoV-2 durante la epidemia en Colombia.https://www.ins.gov.co/estudio-nacional-de-seroprevalencia/reporte.html [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.