Abstract
Animals, especially mammals, have played a critical role in the COVID-19 pandemic. The COVID-19 virus originated in animals, and the virus can jump back and forth between humans and animals. Moreover, animals have been central to the development of the various vaccines against the virus now employed around the world, continuing a long history. The interrelationships between animals and humans in both disease transmission and its prevention call for an interdisciplinary approach to medicine.
Keywords: COVID-19, Vaccines, Animals in science, Coronaviruses, One Health
Introduction
The COVID-19 pandemic continues to dominate our lives. However, the public remains largely unaware of the significant roles that animals play in the pandemic. In fact, there are three critical nodes of intersection between humans and animals in this disease, underlining the varied and often unnoticed inflection points between and among species. These intersections and inflections include a wide variety of mammalian species, both laboratory animals and wild ones. One node materialized in the origins of the virus: COVID-19 is a zoonosis, a disease that originated in animals and spilled over into humans. A second node emerged in the recent discovery that the virus can also jump from humans to animals. A third node, and the subject of this short essay, is the critical role of animals in the development of vaccines for COVID-19. These interrelationships between animals and humans in both disease transmission and its prevention call for an interdisciplinary approach to medicine that addresses both animals and humans, and call attention to the long history of vaccine research.
COVID-19 is part of a family of viruses known as coronaviruses from their spiky, crown-like outer layer, and seven coronaviruses have made the leap from animals to humans in the past two decades. The original pathway of communication of COVID-19 to humans is not yet fully understood, nor is the animal or animals that serve as a reservoir for it. Bats were early suspects, since they appear to have been the reservoir for other viruses, and recent studies appear to confirm that bats do constitute a reservoir for SARS-COV-2, the official term for the COVID-19 virus (Liu et al., 2021). A recent report by the World Health Organization suggested that animals sold for food at the market in Wuhan, China may have contracted the disease from bats and that this intermediate animal, which is still unknown, transmitted the disease to humans (Maxmen, 2021). Pangolins, rare scaly anteaters, were an early suspect for an intermediary. (In China, their scales are used in traditional medicine and their meat is eaten, even though they are protected by international law.) But this theory has since been discarded (Cyranoski, 2020).
Animals are a font of human diseases and have been throughout human history. The World Organization for Animal Health estimates that animal origins account for 60 percent of existing diseases and an even higher percentage of emerging diseases. Around five new diseases emerge each year, and at least three of these originate in animals. Within the past half-century scientists have recognized that influenza viruses circulate in both mammals and birds, and that the famous pandemic of 1918–19 began as a swine flu. There are many ways animal diseases can be communicated to humans, and over time, some of these then evolve into human diseases. Measles, for example, originated in a cattle disease known as rinderpest and appears to have diverged from it in the eleventh or twelfth century (Najera, 2019).
Because both humans and non-human mammals can be reservoirs of COVID-19, they therefore sit in reflexive relationship to one another. The November 2020 outbreak of COVID-19 on Danish mink farms offers a case in point. The mink contracted the virus from humans, and then humans in turn caught it from the mink. While both groups suffered, the outcome for the mink was especially dire. The Danish authorities ordered the killing of the country’s entire captive population of 17 million mink. In the Netherlands, 2.7 million mink have been culled, and mink farms across Europe have been found to be reservoirs for the virus (Koopmans, 2021). The family of Mustelids, which includes mink, ferrets, weasels, and martens, appears to be especially susceptible to SARS-CoV-2; we’ll hear more about ferrets below. Already by April 2020, human transmission of the virus to zoo animals, including lions and tigers, had occurred as well as to domestic dogs and cats (Gollakner and Capua, 2020). More recently, apes in zoos have also been infected. Domestic cats seem particularly susceptible to COVID-19 as they are to other respiratory viruses; while dogs can become infected, they do not get sick. A recent study confirmed that humans infected with COVID-19 could pass the virus on to their pet dogs and especially to their cats. The evidence indicates that it is unlikely that these companion animals could infect each other; however, they could act as reservoirs for the virus (Kannekens-Jager et al., 2021). The CDC warns owners of pet ferrets not to kiss them. Other coronaviruses can infect a wide range of animals, including pigs, cattle, horses, and turkeys, as well as dogs, cats, and mink (Stout et al., 2020). Investigation is ongoing to determine the relationships between these viruses and SARS-CoV-2, and whether animals exposed to these other viruses may have some protection against COVID-19 (Mobasheri, 2020).
But as interesting as I find the transits of disease between humans and non-human mammals, in the context of our current pandemic I am especially attentive to the critical roles of animals in the development of vaccines. Animals, especially mammals, have long played critical roles in the development of drugs and vaccines. Regulatory agencies in the US and elsewhere continue to require trials on animals before new therapies can be tested in humans. Moreover, scientists have increasingly recognized that animal diseases and their cures can tell us much about human diseases: one recent example is the anti-viral drug Remdesivir, first developed to treat cats for an infection caused by a feline coronavirus, and since found to be effective for humans with COVID-19 (Mobasheri, 2020). Much of the public focus on vaccine development has been on human clinical trials, which test the vaccine’s safety (first) and efficacy (second), usually in the form of double-blind trials in which neither the scientist not the human subject knows who is getting the vaccine and who is getting a placebo. These have been ongoing since summer 2020. But before getting to that stage, these potential vaccines had been tested on thousands of animals.
Pasteur and the model vaccine
The classic model of vaccine development goes back to Louis Pasteur in the 1880s. He made his rabies vaccine by passing the infectious agent, now known to be a virus, through the bodies of rabbits, dogs, and monkeys to weaken it. Pasteur then reversed this process to build immunity, beginning by injecting a very weak version of the virus and progressively increasing its virulence until the body—human or animal—could resist the disease. Because of the long incubation period of rabies, for which symptoms could emerge weeks after infection, this method prevented both the dreaded symptoms and almost certain death.
Pasteur, at the dawn of the germ theory of disease, did not fully understand how infection or immunity worked. He assumed, correctly, that rabies was caused by a microscopic entity, but unlike the bacteria with which he had previously worked, he could not see the rabies virus under the conventional optical microscopes then in use. In Pasteur’s time, “virus” simply meant any infectious agent, and its action was not thought to be any different than that of bacteria; it was just smaller. By the turn of the twentieth century, “filterable virus” identified a microbe that was invisible under optical microscopes and could seep through the pores of a porcelain laboratory filter.
Because infectious diseases differ in their microbial structure, their means of infection, and their effects on the body, scientists over the past century developed different methods to prevent them, cure them, or at least mitigate their effects. The one thing all of these methods have in common is that their development has required the use of animals, almost entirely mammals, ranging from mice to horses to apes. As I note in my forthcoming book Experimenting with Humans and Animals: From Aristotle to CRISPR, the scale of animal use multiplied over the twentieth century: while Pasteur used hundreds of rabbits, two decades later Paul Ehrlich used thousands of mice to develop his anti-syphilis drug known as Salvarsan. In the 1940s and 1950s, at least a million monkeys, and possibly many times that, gave their lives to develop the polio vaccine (Guerrini, 2022).
Influenza and viral understanding
If one vaccine could be said to be the iconic vaccine of the United States in the twentieth century, it was Jonas Salk’s polio vaccine, approved for use in 1955, closely followed by Albert Sabin’s vaccine a few years later. By the 1950s, scientists recognized that the cause of polio was a virus, although its action remained obscure. Salk’s injected vaccine employed a killed virus to provoke immunity; Sabin’s oral vaccine used a live but weakened virus. The model for the polio vaccine was influenza, a much more common virus. Attempts to create a vaccine for polio date from the 1930s but required an understanding of viruses that was only gradually attained, mainly by studying influenza. Historian of medicine John Eyler has pointed out, “Between 1935 and the early 1960s, the influenza virus was the most extensively studied virus infecting humans (polio was a close second)” (Eyler, 2006). Much of this work in the 1930s and 1940s continued to proceed on the assumption that viruses acted like bacteria. Work on influenza simultaneously sought a vaccine and figured out just what a virus was. Scientists also gained knowledge of viruses through intensive study of the plant disease known as tobacco mosaic, which was determined to be caused by a virus in 1930 (Creager, 2002).
Influenza was much more widespread and vastly more lethal than polio. When the “Spanish flu” devastated the world in the global pandemic of 1918–19, many researchers assumed its cause was bacterial and developed ineffective bacterial vaccines. Only in 1934 did researchers in Britain and the US determine that the cause of flu was a virus (Smith et al., 1933, Francis, 1934). These scientists, who included Thomas Francis of the Rockefeller Institute, studied the virus by injecting human nasal mucus into European ferrets (Francis, 1941, Eyler, 2006) (Fig. 1 ).
Fig. 1.

European ferret (Mustela putorius furo), photograph by Alfredo Gutiérrez. Wikimedia, GNU Free Documentation License, Creative Commons Attribution-Share Alike 4.0 International.
Ferrets have long been domesticated in Europe, where they are still used to “ferret” small mammals out of their lairs to be killed by hunters, mainly for control of agricultural pests such as rabbits and mice. Wild black-footed ferrets native to North America, on the other hand, are classified as endangered species. Ferrets are among the few mammals that can be infected with human flu and even in turn infect humans. Unlike many animals, ferrets can sneeze and therefore can spread flu by droplets. In the 1930s, a sneezing ferret infected a scientist with human flu (Fig. 2 ). Although they could get human flu, there was no ferret flu transmissible to humans.
Fig. 2.
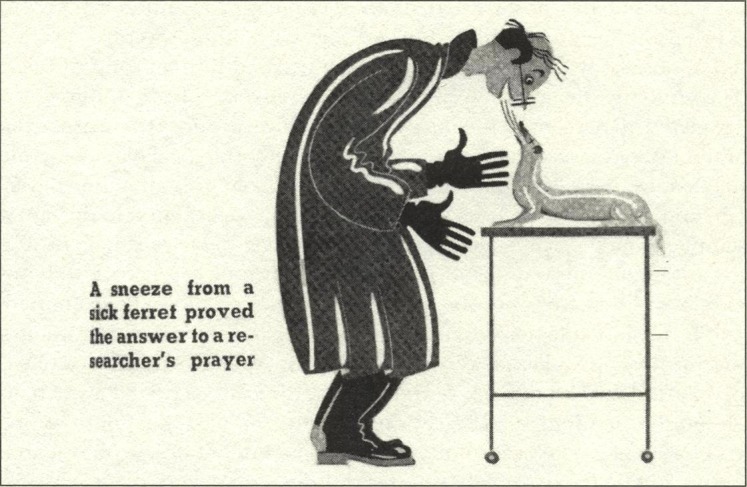
Sneezing ferret infecting a scientist with flu. Cartoon by George De Zayas in J.D. Ratcliff, “Cold Comfort,” Collier’s, 26 February 1938, p. 13. With permission, JTE Multimedia.
Francis and others cultivated the virus by injecting mice with ferret mucus. The injections of infectious mucus led both ferrets and mice to develop antibodies to the virus, apparently clearing the way for a vaccine. Several vaccines employing killed viruses were tested in humans during the late 1930s and early 1940s. Thousands of mice were sacrificed in this process because the only way to determine antibody levels was by injecting mice with the serum of vaccinated individuals. This confirmed the critical role of mice in disease research; mice had already proved central to research in genetics (Rader, 2004). However, mouse tissue caused allergic reactions in many people, leading to a search for an appropriate medium to cultivate a vaccine. These problems were resolved in the early 1940s: embryonated chicken eggs became the choice for cultivation (which is largely still the case), and new antibody tests eliminated the use of mice to reveal antibody concentration levels (Eyler, 2006).
In 1941, the US Army created a Commission on Influenza headed by Thomas Francis, a 1940s version of the recent Operation Warp Speed, to develop a vaccine. Clinical trials of an effective flu vaccine occurred in 1943, and troops received the vaccine in 1945. The development of the first flu vaccine in the 1940s required a long process of cultivation and attenuation of the virus in animals, initially requiring “77 passages in mice; 717 passages in cell culture; 30 passages in chick embryos; five passages in ferrets; and an additional 50 passages in chick embryos” (Hannoun, 2013). In the 1930s and 1940s, following the model of Pasteur, animals served as receptacles, vials, and test tubes, as places to cultivate viruses but not themselves sources of disease, since most believed that influenza was unique to humans. Even if other animals could be infected with it in the lab, they could not contract it in the usual way. In the mid-1950s, however, the discovery of influenza antibodies in horses and ducks led to an entirely new understanding of flu viruses. By the mid-1960s, domesticated and wild birds provided evidence of 46 strains of flu. Apart from horses, flu was also discovered in pigs. Most of these strains were not infective in humans, but over time, human and animal viruses have recombined into new strains (Beveridge, 1993). This process of genetic reassortment of viruses happens constantly. When the 1945 vaccine was redeployed during an epidemic in 1947, it was almost entirely ineffective. Only in the early 1960s, following the flu pandemic of 1957, did the science of influenza move away from a bacterial model of infection and immunity to recognize the truly knotty nature of viruses.
The nature of coronaviruses
Scientists now understand that a virus is not a cell, but a piece of genetic information—DNA or RNA—encased in a protein package, sometimes with an additional fatty (lipid) layer surrounding it. Because they are not cells, they need to enter a host cell in order to take over its reproductive mechanism and reproduce. Both influenza viruses and the various coronaviruses that includes SARS and COVID-19 are RNA viruses. Viruses are highly adaptable, varying to suit their host and in response to antibodies the host develops to fight them. These processes of recombination and reassortment are why we need a newly formulated flu shot each year. Given the multiple variants of COVID-19 that have appeared, which make the virus more infective, or more deadly, or both, we may see many versions of COVID-19 vaccines, and like flu, we may need them on an annual basis.
There are many coronaviruses, so named, as noted above, from the corona of spikes on the surface of the virus particle. The first, discovered in the 1960s, were determined to cause minor cold-like symptoms. They were very common. But in 2003, a novel coronavirus known as SARS-CoV (an acronym for Severe Acute Respiratory Syndrome Coronavirus) emerged in east Asia. With an alarming death rate of 10 percent, it spread to more than two dozen countries in Asia, Europe, North and South America, infecting over 8000 people. The death rate for seasonal flu in 2019 was 1.8 per 100,000—or, as a percentage, 0.0018 (Centers for Disease Control and Prevention, 2017, Centers for Disease Control and Prevention, 2021). SARS therefore was quite deadly, but it was contained quickly. Its origins remain obscure, but bats are suspected, as well as civet cats (which are not felines, but members of a different taxonomic family).
SARS-CoV focused attention on human coronaviruses. In 2012, another one known as MERS (Middle East respiratory syndrome) made the leap from camels to humans in Saudi Arabia. There have been only about 2500 cases, but a whopping 35 percent of its victims have died. Toward the end of 2019, another coronavirus emerged, as we all know. What had already become known as COVID-19 was isolated in January 2020, and a few weeks later gained the official name SARS-CoV2 (Williams, 2020, Centers for Disease Control and Prevention, 2017).
Seeking a vaccine
In January and February 2020, researchers were more concerned with finding an animal reservoir and determining the structure and action of the virus than with developing a vaccine. A survey of articles in Science magazine on the nature of the virus for those months shows work with, among other animals, transgenic mice and ferrets, but also camelids such as llamas and alpacas, recalling the origins of MERS in camels. As the search for a vaccine ramped up in March 2020, the search for the best animal models for SARS-CoV-2 came to the fore (Cohen, 2020). Until an animal could be found that experienced the disease in the same way as humans did, and also transmitted it as humans did, vaccine development could be stalled.
An indication of the severity of the COVID crisis was that researchers who would ordinarily be competing began to collaborate across national and disciplinary lines, attacking the virus from several angles and with a number of different animals. Scientists turned back to research from the early 2000s on SARS; at that time Syrian hamsters were found to be easily infected but showed few symptoms. SARS-CoV-2 was another matter, not only infecting hamsters but making them sick. “The model’s prospects appear brighter,” commented a journalist: good news for humans but perhaps less good for the hamsters (Cohen, 2020).
Each laboratory animal has its advantages and disadvantages in this kind of research. Mice are adaptable, their small size makes them easy to manipulate and transport, and their husbandry requirements have long been standardized. Because mice and rats form the vast majority of experimental animals globally—and have for well over a century—their anatomy and physiology are intimately known, with reams of data available. While hamsters (and guinea pigs) have similar advantages of size, they do not have this backdrop of data, and the availability of compatible chemical reagents required for diagnostic testing in these species is far less than for mice and rats. While primates are the most similar to humans in anatomy and physiology, with much data available, obtaining reagents has been problematic for these animals too. Moreover, the use of primates, especially apes, is controversial.
One big difference between 2020 and 2003 is the increased knowledge and availability of genetically engineered animals, especially mice and rats. Even before genetic engineering made custom-tailored animals possible, selective breeding of mice and rats supplied highly specialized animals to laboratories beginning in the early 1900s. The first transgenic mouse, implanted with DNA from another organism, made its appearance in 1981, over twenty years before the sequencing of the mouse genome in 2002. Rats followed in 1990 and 2004. Because mice and rats are so well understood both behaviorally and genetically, they would be highly desirable animal models for COVID-19 research, except that they do not naturally become infected with the virus because their virus receptors do not match it. By April 2020, genetically modified “knock-in” mice with human ACE-2 (the enzyme to which the virus binds) were appearing in multiple scientific papers. The technique known as CRISPR-Cas9, which employs a molecular “scissors” to snip out portions of a gene and replace them has become increasingly popular (Guerrini, 2022).
Ferrets are another choice for COVID research, given their long experience with influenza and other human respiratory viruses. A Chinese study published in May 2020 determined that the virus replicated in the upper respiratory tracts of ferrets but did not generally cause serious illness. Domestic cats, especially kittens, seemed more susceptible to the virus. However, obtaining the required nasal swabs from older cats proved to be difficult, as one might imagine, and “to avoid possible injury,” the researchers only collected feces from them. In the same study, dogs were found to have “low susceptibility” to SARS-CoV-2, while pigs, chickens, and ducks had none at all (Shi et al., 2020). Ferrets therefore came to be used extensively in vaccine research.
While there are currently over 200 vaccine candidates, they fall into four main categories (Rhodes, 2021). The first two, a dead virus and an attenuated live virus, follow the models of influenza and polio. However, the most successful COVID-19 vaccines thus far have followed two newer technologies: messenger RNA (or mRNA) and viral vectors. While mRNA vaccines are new, they are based on research over the past twenty years.1 With an mRNA vaccine, messenger RNA is injected into a muscle, where it instructs the muscle cells to make a piece of spike protein that resembles the spikes on the SARS-CoV-2 but is harmless. The cells then break down the messenger RNA. The harmless spike protein in turn provokes the immune system to make antibodies just as an actual viral protein would. The four main vaccines in use in North America and Western Europe are made by Moderna, Pfizer (employing technology developed by the German firm BioNTech), Johnson and Johnson, and AstraZeneca. The Moderna and Pfizer-BioNTech vaccines are both mRNA vaccines. The second vaccine technology, viral vectors, has been known since the 1990s. This vaccine uses a different virus, modified to be non-infectious (often an adenovirus, the virus behind the common cold) as a vector to deliver a gene that instructs cells to make antigens that in turn trigger production of antibodies. The Johnson and Johnson and AstraZeneca vaccines employ viral vectors.
While scientists have employed an array of small mammals, including rodents, mustelids such as ferrets, and cats to develop the vaccine, monkeys play a critical role in the final stages of vaccine development. Before clinical trials on humans, vaccines are commonly tested on monkeys, whose antibody responses resemble those of humans. But this routine use of monkeys has become increasingly difficult for several reasons. Public pressure and a new classification of captive as well as wild chimpanzees as endangered species led the US National Institutes of Health to end invasive chimpanzee research in 2015; many other countries had already done so (Kaiser, 2015). Chimpanzees are apes, not monkeys, but removing them from labs led to a spike in the number of monkeys used. In only two years, the number of monkeys used in US biomedical research reached a new high of nearly 75,000 animals, a 20 percent jump. Although monkeys remain only 0.5 percent of all research animals in the USA, the COVID pandemic has only increased demand for them (Fig. 3 ).
Fig. 3.

Juvenile long-tailed macaque (Macaca fascicularis), photograph by Charles J. Sharp, Sharp Photography. Wikimedia, Creative Commons Attribution-Share Alike 4.0 International.
The most commonly used monkeys are rhesus macaques, cynomolgus macaques (also known as long-tailed or crab-eating macaques), and African green monkeys. Jonas Salk’s celebrated polio vaccine in the 1950s used so many rhesus macaques that their population in their native India crashed and India began to restrict their export in 1955. While some are bred in the US, some 60 percent of research monkeys, mainly cynomolgus macaques, have for the past several years been imported from China. China has been the major supplier of nonhuman primates used in experiments around the world for decades, with 32 breeding facilities across the country housing as many as 10,000 monkeys in each (Hsu, 2011). Cynomolgus macaques are not native to China but are imported from Cambodia for breeding. As science writer Sarah Zhang noted, “In China, breeding monkeys is cheaper and the animal-rights movement is also quieter” (Zhang, 2020). This supply had already been disrupted by US trade disputes with China, but it abruptly ended in February 2020, both because of COVID-related travel restrictions and because China simply stopped exporting them. Some of this gap has been filled by exports from Cambodia and Indonesia (Morton, 2011, Galland, 2021).
As the supply of monkeys has declined, they have become ever more precious to researchers, leading to more collaboration across laboratories and continued search for possible alternatives, many of which involve genetically engineering other species. The monkey supply crisis places in stark relief the continued reliance of humans on animals to develop vaccines as well as for other medical research. A 2018 poll from the Pew Research Center found Americans to be nearly evenly divided over the use of animals in research, with 52 percent opposing it (Strauss, 2018). However, such polls may not reflect differing opinions regarding different kinds of research, or differing opinions based on education level. The 2018 Pew poll found higher levels of support for animal use among those with higher levels of scientific knowledge.
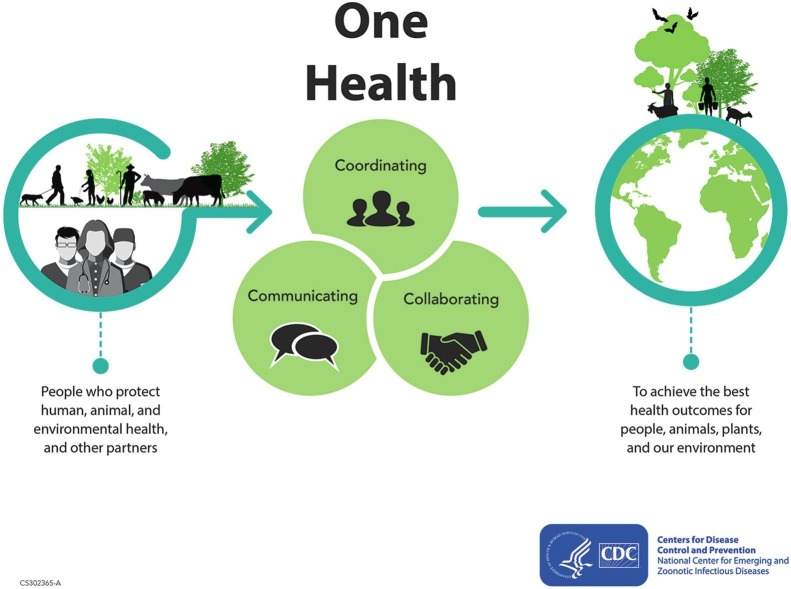
The role of animals in vaccine development has thus demonstrated yet again the continued, often unacknowledged, dependence of biomedical research on animals. Vaccine research has depended on animals from its outset in the nineteenth century. However, COVID-19 has also revealed critical intersections and inflections between humans and non-human animals in their experiences of disease and its transmission: animals infect humans, and humans infect animals, often their companion animals. COVID-19 has highlighted the ways in which human and animal medicine overlap, and how the boundaries between the two have begun to break down. In medicine, the movement known as “One Health” has emerged in the past two decades. One Health “is underpinned by the belief that some of the most important health threats faced today are not species specific, and consequently can only be tackled by interdisciplinary working across the domains of human medicine, veterinary medicine, and the life sciences” (1). It has now become a global movement devoted to cross-disciplinary collaboration to ensure human, animal, and environmental health (Fig. 4 ). Never before has it been quite so clear that we are all in this together: that human health, animal health, and indeed the health of the planet cannot be considered in isolation. Knowing the history of these intersections helps us to understand them.
Fig. 4.
One Health infographic, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID).
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I am grateful to the editor, to two anonymous reviewers, and to Michael A. Osborne and Barbara C. Canavan for their comments. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
The National Human Genome Research Institute defines messenger RNA as “a single-stranded RNA molecule that is complementary to one of the DNA strands of a gene. The mRNA is an RNA version of the gene that leaves the cell nucleus and moves to the cytoplasm where proteins are made” (NHGRI, 2021).
References
- Beveridge W.I.B. Unravelling the ecology of Influenza A virus. History and Philosophy of the Life Sciences. 1993;15:23–32. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Severe Acute Respiratory Syndrome (SARS), https://www.cdc.gov/sars/index.html (accessed 10 March 2021).
- Centers for Disease Control and Prevention National Center for Health Statistics, Influenza. 2021. https://www.cdc.gov/nchs/fastats/flu.htm accessed 10 March 2021.
- Cohen J. From mice to monkeys, animals studied for coronavirus answers. Science. 2020;368(6488):221–222. doi: 10.1126/science.368.6488.221. [DOI] [PubMed] [Google Scholar]
- Creager A.N. University of Chicago Press; Chicago: 2002. The life of a virus: Tobacco mosaic virus as an experimental model, 1930–1965. [Google Scholar]
- Cyranoski, D. (2020, 7 February). Did pangolins spread the China coronavirus to people? Nature, 10.1038/d41586-020-00364-2. [DOI] [PubMed]
- Eyler J.M. DeKruif’s boast: Vaccine trials and the construction of a virus. Bulletin of the History of Medicine. 2006;80(3):409–438. doi: 10.1353/bhm.2006.0092. [DOI] [PubMed] [Google Scholar]
- Francis T., Jr. Transmission of influenza by filterable virus. Science. 1934;80(2081):457–459. doi: 10.1126/science.80.2081.457-a. [DOI] [PubMed] [Google Scholar]
- Francis T., Jr. Epidemic influenza. Bulletin of the New York Academy of Medicine. 1941;17:268–279. [PMC free article] [PubMed] [Google Scholar]
- Galland, G. (2021, 9 March). Importation of non-human primates [Webinar]. Rapid Response by Laboratory Animal Research Institutions During the COVID-19 Pandemic: Lessons Learned. National Academy of Science, Institute for Laboratory Animal Research, https://www.nationalacademies.org/event/03-09-2021/rapid-response-by-laboratory-animal-research-institutions-during-the-covid-19-pandemic-lessons-learned#sectionWebFriendly.
- Gollakner R., Capua I. Is COVID-19 the first pandemic that evolves into a panzootic? Veterinaria Italiana. 2020;56(1):11–12. doi: 10.12834/VetIt.2246.12523.1. [DOI] [PubMed] [Google Scholar]
- Guerrini A. 2nd edition. Johns Hopkins University Press; Baltimore: 2022. Experimenting with humans and animals: From Aristotle to CRISPR. (in production) [Google Scholar]
- Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Review of Vaccines. 2013;12(9):1085–1094. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- Hsu C.K. In: Animal Research in a Global Environment. National Research Council, editor. National Academies Press; Washington, D.C.: 2011. China as a resource for NHP; pp. 224–227. [Google Scholar]
- Kaiser J. An end to US chimp research. Science. 2015;350(6264):1013. doi: 10.1126/science.350.6264.1013. [DOI] [PubMed] [Google Scholar]
- Kannekens-Jager, M., De Groot, Y., Kooistra, H. S., Egberink, H. F., Zhao, S., Wagenaar, J. A., Duim, B., Broens, E. M. (2021, 9-12 July). High prevalence of SARS-CoV-2 in dogs and cats living in COVID-19 positive households. Paper presented at the European Congress of Clinical Microbiology & Infectious Diseases (online).
- Koopmans M. SARS-CoV-2 and the human-animal interface: Outbreaks on mink farms. Lancet Infectious Diseases. 2021;21(1):18–19. doi: 10.1016/S1473-3099(20)30912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., Tan, S., Niu, S., Wang, J., Wua, L., Sun, H., Zhang, Y., Pan, X., Qua, X., Du, P., Meng, Y., Jia, Y., Chen, Q., Deng, C., Yan, J., Wang, H-W., Wang, Q., Qi, J., and Gao, G.F. (2021). Cross-species recognition of SARS-CoV-2 to bat ACE2, PNAS 5 January, https://doi.org/10.1073/pnas.2020216118. [DOI] [PMC free article] [PubMed]
- Maxmen, A. (2021). WHO report into COVID pandemic origins zeroes in on animal markets, not labs. Nature News, 30 March, https://www.nature.com/articles/d41586-021-00865-8 (accessed 31 March 2021). [DOI] [PubMed]
- Mobasheri, A. (2020). COVID-19, companion animals, comparative medicine, and One Health, Frontiers in Veterinary Science, 7 (August), article 522. DOI: https://doi.org/10.3389/fvets.2020.00522. [DOI] [PMC free article] [PubMed]
- Morton W. In: Animal research in a global environment. National Research Council, editor. National Academies Press; Washington D.C.: 2011. The United States; pp. 219–223. [Google Scholar]
- Najera, R. F. (2019, February 20). A brief history of measles. The history of vaccines, College of Physicians of Philadelphia. https://www.historyofvaccines.org/content/blog/brief-history-measles (accessed 20 March 2021).
- National Human Genome Research Institute (NHGRI) Messenger RNA (mRNA) 2021. https://www.genome.gov/genetics-glossary/messenger-rna accessed 5 July 2021.
- Rader K.A. Princeton University Press; Princeton: 2004. Making mice. [Google Scholar]
- Rhodes J. University of Chicago Press; Chicago: 2021. How to make a vaccine. An essential guide for COVID-19 & beyond. [Google Scholar]
- Strauss M. Americans are divided over the use of animals in scientific research. Fact Tank. 2018;16 August https://www.pewresearch.org/fact-tank/2018/08/16/americans-are-divided-over-the-use-of-animals-in-scientific-research/ (accessed 10 April 2021) [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B.…Bu G. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368(6494):1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W., Andrewes C.H., Laidlaw P.P. A virus obtained from influenza patients. The Lancet. 1933;222(5732):66–68. doi: 10.1016/S0140-6736(00)78541-2. [DOI] [Google Scholar]
- Stout A.E., André N.M., Jaimes J.A., Millet J.K., Whittaker G.R. Coronaviruses in cats and other companion animals: Where does SARS-CoV-2/COVID-19 fit? Veterinary Microbiology. 2020;247:108777. doi: 10.1016/j.vetmic.2020.108777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, S. (2020). A brief history of human coronavirus, The Scientist, 2 June, https://www.the-scientist.com/news-opinion/a-brief-history-of-human-coronaviruses-67600 (accessed 10 March 2021).
- 1.Woods A., Bresalier M., Cassidy A., Dentinger R.M. In: Animals and the shaping of modern medicine: One health and its histories. Woods A., Bresalier M., Cassidy A., Mason Dentinger R.M., editors. Palgrave Macmillan; London: 2018. Introduction: Centring animals within medical history; pp. 1–26. [PubMed] [Google Scholar]
- Zhang, S. (2020, 31 August). America is running low on a crucial resource for COVID-19 vaccines, The Atlantic (online), https://www.theatlantic.com/science/archive/2020/08/america-facing-monkey-shortage/615799/ (accessed 15 March 2021).