Abstract
Objectives
Durability of the humoral immune response to SARS-CoV-2 has yet to be defined. We longitudinally evaluated during a 12-month period the antibody responses to SARS-CoV-2, and analysed predictors of antibody titres decline and seroreversion.
Methods
Prospective study conducted in a cohort of patients hospitalized for microbiologically-confirmed COVID-19. Blood and nasopharyngeal samples were sequentially obtained during hospital stay and at 1, 2, 6 and 12 months after patients’ discharge for measuring anti-spike (S) and anti-nucleocapsid (N) IgG antibody levels and SARS-CoV-2 RNA, respectively.
Results
80 non-vaccinated patients were analysed. At month 12 after discharge, 73 (91.2%) patients exhibited detectable S-IgG and 35 (43.8%) N-IgG antibody titres. A gradual wane was observed in S-IgG and N-IgG antibody titres. Linear regression showed that S-IgG decline was positively associated with peak antibody titres (coefficient [95% CI] 0.059 [0.05–0.067], p < 0.001), inversely with WHO severity score (coefficient [95% CI] −0.042 [-0.079/-0.004], p = 0.033), and there was a trivial positive association with age (coefficient [95% CI] 0.002 [0–0.005], p = 0.10); N-IgG decline was positively associated with peak antibody titres (coefficient [95% CI] 0.091 [0.078–0.105], p < 0.001). Logistic regression showed that seroreversion for S-IgG was inversely associated with peak S-IgG (OR 0.19; 95% CI, 0.04-0.45; p = 0.004); seroreversion for N-IgG was inversely associated with peak N-IgG (OR 0.71; 95% 0.53–0.90; p = 0.009) and positively with cycle threshold of RT-PCR (OR 1.14; 95% CI, 1.00–1.33; p = 0.062).
Conclusion
Anti-spike IgG antibodies remain detectable one year after hospitalization for COVID-19. Higher peak antibody titres and disease severity were associated with increased durability of detectable antibodies.
Keywords: COVID-19, SARS-CoV-2, Antibody responses, Anti-spike antibodies, S-IgG, Anti-nucleocapsid antibodies, One year, Humoral immune response, Antibody titers, Post-infection immunity
1. Introduction
Characterization of postinfection immunity is essential when planning strategies to face the COVID-19 pandemic. The majority of individuals infected with SARS-CoV-2 develop antibodies against the nucleocapsid (N) and the spike (S) proteins [1]. The S-protein is a primary target for neutralizing antibodies, which can block viral entry and infection of host cells [2]. While existing information is still limited, experimental and clinical data support that postinfection humoral immunity may protect against SARS-CoV-2 reinfection [[3], [4], [5], [6]]. However, durability of the humoral immune response has yet to be defined. To date, the longest observation period assessing the longevity of the antibody response has been of 6–8 months [[7], [8], [9]].
We longitudinally evaluated the antibody responses to SARS-CoV-2 during a 12-month period in a cohort of patients hospitalized with COVID-19, and analysed predictors of antibody titres decline and seroreversion.
2. Methods
A prospective study was conducted in a cohort of patients hospitalized for microbiologically-confirmed COVID-19 with a positive SARS-CoV-2 RNA test in a nasopharyngeal sample in the first wave, who were longitudinally followed-up during 12 months; details of the cohort with preliminary, short-term results are provided elsewhere [6,10]. The study was approved by the Ethical Committee of the Hospital General Universitario de Elche (Spain). Blood samples were sequentially obtained during hospital stay, and at 1, 2, 6 and 12 months after patients' discharge for measuring antibody levels. Nasopharyngeal samples to analyse SARS-CoV-2 RNA were also sequentially obtained until 6 months post-discharge. SARS-CoV-2–specific antibodies were measured in EDTA plasma samples. S-IgG and N-IgG were detected using commercial semiquantitative enzyme immunosassay kits (Anti-SARS-CoV-2 IgG ELISA, Euroimmun, Lubeck, Germany) in an automated instrument (Dynex DS2 ELISA system) following the manufacturer's instructions. Antibody levels were evaluated by calculating the ratio of the optical density (OD) of the patient sample over the OD of the calibrator (sample OD/calibrator OD =S/CO [absorbance/cut-off]). Ratio <1.1 was defined as negative and ≥1.1 as positive. SARS-CoV-2 RNA was detected by RT-PCR targeting the E, and N genes (AllplexTM 2019-nCoV Assay, Seegene, Seoul, Korea). Linear regression was performed to analyse factors associated with S-IgG and N-IgG antibody percent titres decline following peak levels, and logistic regression to analyse factors associated with seroreversion.
3. Results
Of 95 patients admitted for COVID-19 with subsequent detectable antibody titres and available blood samples until month 12, 80 were analysed after excluding 15 patients vaccinated during follow-up. Median (Q1-Q3) age was 59.5 (52–69) years, 49 (61.2%) were male, and 49 (61.2%) had coexisting comorbid diseases. Median (Q1-Q3) initial SARS-CoV-2 cycle threshold of RT-PCR was 30.1 (26.8–34.6) for E gen, 32.6 (29.5–35.5) for N gen, and RNA shedding lasted a median (Q1-Q3) of 20 (6–47) days. On admission, patients showed a median WHO 7-point ordinal scale value of 4, and 13 (16.3%) patients required non-invasive or invasive mechanical ventilation (score >4 points) (Suppl. Table 1). Median (Q1-Q3) time from symptom onset to seropositivity was 15 (12–21) days for S-IgG and 13 (9–17) days for N-IgG. Peak S-IgG was 6.9 (5.9–11.8) S/CO and peak N-IgG 4.6 (4–6.8) S/CO. No correlation was found between initial SARS-CoV-2 cycle threshold and peak antibody levels; for S-IgG, r (Pearson) was −0.005 (95% confidence interval [CI], −0.246–0.235) for E gen and r = −0.031 (95% CI, −0.269–0.210) for N gen; for N-IgG, r = −0.135 (95% CI, −0.364–0.108) for E gen and r = −0.126 (95% CI, −0.355–0.117) for N gen. We neither observed a correlation between WHO 7-point ordinal scale values and peak S-IgG levels (r = 0.123; 95% CI, −0.100–0.335; 0.278) or N-IgG levels (r = 0.197; 95% CI, 0.024–0.401; p = 0.080). Peak antibody levels according to 7-point ordinal scale values are shown in supplementary Table 1. Patients with comorbidities tended to have a weaker S-IgG antibody response (peak S-IgG 6.4 [5.8–9.8] S/CO and 7.1 [6.4–13.8] S/CO in patients with or without comorbidities, respectively [p = 0.052]), with no differences in N-IgG response (peak N-IgG 4.5 [3.7–6.5] and 4.7 [4.1–7.6] in patients with or without comorbidities, respectively [p = 0.45]) (Suppl. Table 1).
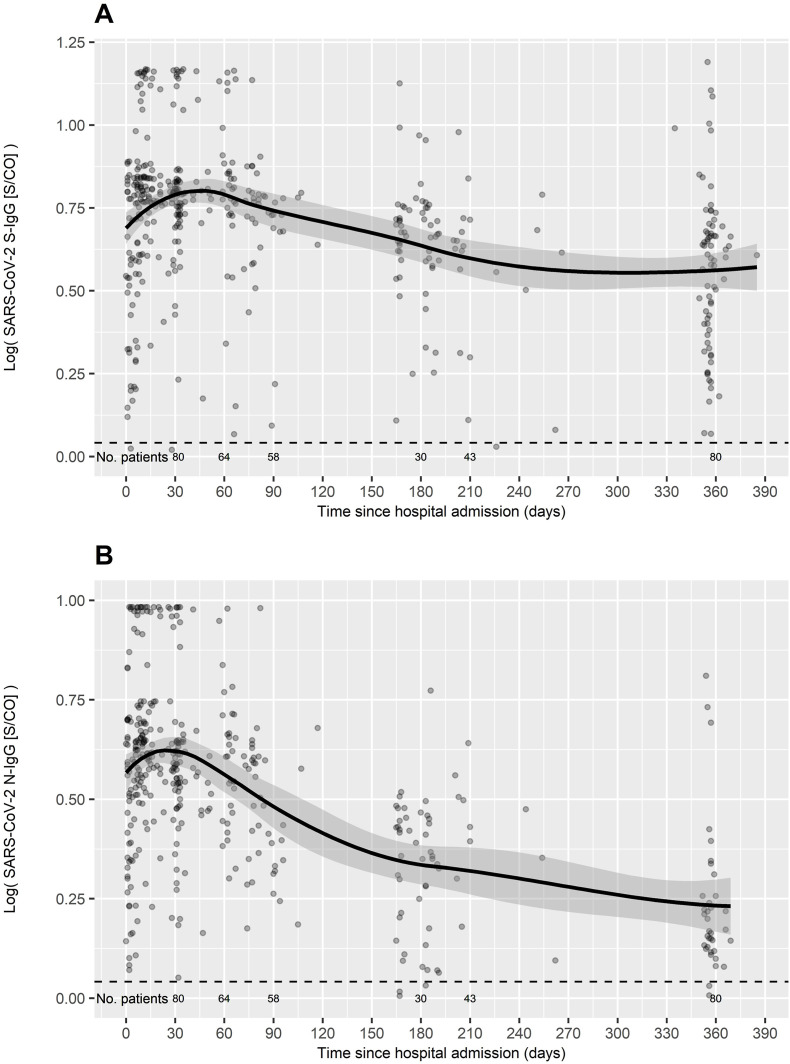
At month 12 after COVID-19 diagnosis, 73 (91.2%) patients exhibited detectable S-IgG and 35 (43.8%) N-IgG antibody titres (Table 1 ). Fig. 1 shows the S-IgG and N-IgG antibody kinetics, where a gradual wane was observed in antibody titres during the 12-month follow-up. Linear regression including age, sex, WHO severity score and therapy with steroids and/or tocilizumab showed that S-IgG decline was positively associated with peak antibody titres (coefficient [95% CI] 0.059 [0.05–0.067], p < 0.001), inversely with WHO severity score (coefficient [95% CI] −0.042 [-0.079/-0.004], p = 0.033), and there was a trivial positive association with age (coefficient [95% CI] 0.002 [0–0.005], p = 0.10); N-IgG decline was positively associated with peak antibody titres (coefficient [95% CI] 0.091 [0.078–0.105], p < 0.001).
Table 1.
Characteristics of patients according to seroreversion of anti-spike and anti-nucleocapsid IgG antibodies.
| Anti-spike IgG antibodies | Anti-nucleocapsid IgG antibodies | |||||||
|---|---|---|---|---|---|---|---|---|
| 12 month non-seroreversion N = 73 | 12-month seroreversion N = 7 | P | Adjusted OR (95% CI) | 12 month non-seroreversion N = 35 | 12-month seroreversion N = 45 | P | Adjusted OR (95% CI) | |
| Sex, men | 44 (60.3) | 5 (71.4) | 0.700 | 19 (54.3) | 30 (66.7) | 0.355 | ||
| Age | 60 (53–69) | 52 (38–66.5) | 0.247 | 60 (58–71.5) | 57 (46–67) | 0.047 | 0.96 (0.91–1.02) | |
| Comorbidity | 43 (58.9) | 6 (85.7) | 0.239 | 22 (62.9) | 27 (60.0) | 0.821 | ||
| Highest WHO severity score (range) | 4 (4–6) | 4 (3–6) | 0.261 | 4 (4–6) | 4 (3–6) | 0.584 | ||
| ICU admission | 13 (17.8) | 0 | 0.592 | 6 (17.1) | 7 (15.6) | 1.0 | ||
| Hospital stay, days | 12 (9–17) | 6 (4–9.5) | 0.055 | 1.08 (0.86–1.44) | 13 (9.5–16) | 10.5 (6–13) | 0.015 | 0.96 (0.85–1.09) |
| Peak SARS-CoV-2 RNA, copies/sample | 5957 (1265–37,229) | 13,015 (2580–27,723) | 0.861 | 12,857 (5445–41,256) | 2989 (830–27,723) | 0.024 | ||
| Initial Ct, N-gen | 32.6 (29.5–35.4) | 32.6 (29.8–35.7) | 0.965 | 30.5 (28.8–34.1) | 33.8 (30.8–36.4) | 0.019 | 1.14 (1.00–1.33) | |
| Initial Ct, E-gen | 30.1 (26.9–33.4) | 30.4 (27.2–38.0) | 0.982 | 28.8 (26–30.7) | 32 (28.2–40) | 0.016 | ||
| Days to viral clearance | 20 (6–48) | 35 (4–46) | 0.862 | 20 (11–56) | 20.5 (4–37.8) | 0.303 | ||
| Days to seroconversion S-IgG | 15 (12–20) | 19 (12–67) | 0.335 | 16 (12–22) | 15 (12–19) | 0.652 | ||
| Days to seroconversion N-IgG | 12 (9–17) | 17 (5.5–36) | 0.623 | 11.5 (9–14.8) | 13 (10–18) | 0.238 | ||
| Peak S-IgG, S/CO | 6.9 (6.2–12.4) | 1.7 (1.6–3.3) | <0.001 | 0.19 (0.04–0.45) | 7.1 (5.9–13.5) | 6.6 (5.9–7.6) | 0.150 | |
| Peak N-IgG, S/CO | 4.7 (4.1–6.7) | 2.3 (1.6–3.8) | 0.008 | 0.72 (0.30–1.31) | 5.4 (4.4–9.4) | 4.3 (3.5–4.9) | <0.001 | 0.71 (0.53–0.90) |
| COVID-19 immune therapy | ||||||||
| Steroids | 15 (20.5) | 1 (14.3) | 1.0 | 8 (22.9) | 8 (17.8) | 0.587 | ||
| Tocilizumab | 49 (67.1) | 3 (42.9) | 0.232 | 23 (65.7) | 29 (64.4) | 1.0 | ||
Continuous variables are expressed as median (interquartile range) and categorical as number (%), unless indicated.
OR, odds ratio; CI, confidence interval; ICU, intensive care unit; Ct, cycle threshold of SARS-CoV-2 RT-PCR; S-IgG, anti-spike-IgG; N-IgG, anti-nucleocapsid-IgG; S/CO, absorbance/cutoff.
Fig. 1.
SARS-CoV-2 antibody kinetics. A, anti-spike IgG antibody titres; B, anti-nucleocapsid IgG antibody titres. S/CO, absorbance/cutoff; positive S/CO ≥ 1.1.
Median (Q1-Q3) time to seroreversion was 158 (109.5–201) days for S-IgG and 249 (180–351) days for N-IgG. Table 1 shows the factors associated with seroreversion for S-IgG and N-IgG. Seroreversion for S-IgG was inversely associated with peak S-IgG (p < 0.001) and peak N-IgG (p = 0.008), and there was a trend to an association with longer hospital stay (p = 0.055). Logistic regression showed that lower peak S-IgG predicted S-IgG seroreversion with an OR = 0.19; 95% CI = 0.04–0.45; and p = 0.004 (Table 1). Factors associated with N-IgG seroreversion in univariate analysis were younger age (p = 0.047), higher initial cycle threshold of RT-PCR for N gen (p = 0.019) and E gen (p = 0.016), lower peak N-IgG (p < 0.001) and longer hospital stay (p = 0.015). Logistic regression including the statistically significant variables showed an inverse association of seroreversion with peak N-IgG (OR 0.71; 95% CI, 0.53–0.90; p = 0.009), and a marginal positive association with cycle threshold of RT-PCR (OR 1.14; 95% CI, 1.00–1.33; p = 0.062).
4. Discussion
This longitudinal study shows that anti-spike IgG antibodies remain detectable one year after acute infection in the majority of patients hospitalized with COVID-19. Conversely, more than half of patients lost the anti-nucleocapsid IgG antibodies during the follow-up period. Long-term persistence of antibodies was associated with higher peak antibody titres and marginally with lower initial cycle threshold values of SARS-CoV-2 RT-PCR. Higher severity of disease predicted slower anti-spike antibodies decline.
Durability of the antibody response has significant implications in the COVID-19 pandemic. We observed that anti-spike and anti-nucleocapsid IgG antibodies exhibited distinct kinetics, evidenced by longer durability of the former. Anti-spike antibodies are capable of neutralizing viral entry and provide protective immunity. In a study conducted in healthcare workers with a 6-month follow-up, the presence of anti-spike IgG antibodies was associated with a substantial reduction in the risk of reinfection [11]. Our study demonstrates an enduring anti-spike antibody response, of at least 12 months after hospital discharge in the majority of patients with moderate/severe COVID-19, which could theoretically imply that reduction in the risk of reinfection might also be extended to that period of time. This long-lasting humoral immunity should have implications when establishing priorities for vaccination. Likewise, further investigation is needed to assess whether it might also be extrapolated to durability of vaccine and need for boosting doses.
Seroreversion was associated with lower peak postinfection antibody titres, which supports the central role of the magnitude of the antibody response in the durability of detectable levels [12]. Despite the higher decline linked with greater peak antibody levels in our study, the highest titres also predicted a higher probability of persistent detectable antibodies up to one year. Severity of disease was another factor involved in the rate of decline of anti-spike antibody levels in our cohort, with an inverse association, and we found a trend to faster anti-spike antibodies decrease with older age. Aging has been associated with reduced production of naïve B cells from the bone marrow, and reduced growth and survival of pro-B lymphocytes as well as common lymphoid progenitors [13]. Finally, higher cycle threshold of SARS-CoV-2 RT-PCR was marginally associated with seroreversion, to which a weaker coexistent immune response might have had a contributing role.
The study included hospitalized patients, and results might not be generalizable to other scenarios, which is a limitation. However, detectable antibody titres have been described in asymptomatic or mildly symptomatic outpatients up to 8 months after infection [7], suggesting that several factors are involved in antibody dynamics. We did not measure the neutralizing activity of antibodies; however, we have recently introduced a new test that quantifies neutralizing antibodies concentration, and have found a strong correlation (>95%) with S-IgG concentrations in another sample of patients. Strengths are the longitudinal design and sequential samples obtained in all patients during a long time period.
In conclusion, our results demonstrate a durable anti-spike IgG antibody response at least one year after diagnosis in the majority of patients hospitalized with COVID-19. Higher peak antibody titres and severity of disease were associated with a higher likelihood of long-term detectable antibodies. This information contributes to expand knowledge of SARS-CoV-2 immune response and has direct implications in the adoption of preventive strategies and public health policies.
Funding
This work was supported by the RD16/0025/0038 project as a part of the Plan Nacional Research Development Innovation (R + D + I) and cofinanced by Instituto de Salud Carlos III - Subdirección General de Evaluación y Fondo Europeo de Desarrollo Regional; Instituto de Salud Carlos III (Fondo de Investigaciones Sanitarias [grant number PI16/01740; PI18/01861; CM 19/00160, COV20-00005]).
Author statement
Mar Masiá: Conceptualization, Methodology, Writing – original draft preparation, Supervision Marta Fernández: Investigation, Writing – review & editing Guillermo Telenti: Data curation, Writing - Review Vanesa Agulló: Investigation, Writing - Review José A. García: Formal analysis, Writing – review & editing Sergio Padilla: Software, Formal analysis, Writing – review & editing Javier García-Abellán: Data curation, Writing – review & editing Antonio Galiana: Investigation, Writing – review & editing, Nieves Gonzalo-Jiménez: Investigation, Writing – review & editing Félix Gutiérrez: Conceptualization, Methodology, Writing – original draft preparation, reviewing and editing, Funding acquisition, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaut.2021.102703.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju B., Zhang Q., Ge J., Wang R., Sun J., Ge X., et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature. 2020;584:115–119. doi: 10.1038/s41586-020-2380-z. [DOI] [PubMed] [Google Scholar]
- 3.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng W., Bao L., Liu J., Xiao C., Liu J., Xue J., et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.02107-20. A. e02107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masiá M., Padilla S., Galiana A., Fernández-González M., Gutiérrez F. Incidence of delayed asymptomatic COVID-19 recurrences in a 6-month longitudinal study. J. Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choe P.G., Kim K.H., Kang C.K., Suh H.J., Kang E., Lee S.-.Y., et al. Antibody responses 8 Months after asymptomatic or mild SARS-CoV-2 infection. Emerg. Infect. Dis. 2021;27:928–931. doi: 10.3201/eid2703.204543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bal A., Trabaud M.A., Fassier J.B., Rabilloud M., Saker K., Langlois-Jacques C., et al. Six-month antibody response to SARS-CoV-2 in healthcare workers assessed by virus neutralization and commercial assays. Clin. Microbiol. Infect. 2021;27:933–935. doi: 10.1016/j.cmi.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masiá M., Telenti G., Fernández M., García J.A., Agulló V., Padilla S., et al. SARS-CoV-2 seroconversion and viral clearance in patients hospitalized with COVID-19: viral load predicts antibody response. Open Forum Infect. Dis. 2021;8 doi: 10.1093/ofid/ofab005. ofab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumley S.F., O'Donnell D., Stoesser N.E., Matthews P.C., Howarth A., Hatch S.B., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N. Engl. J. Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nature Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajaj V., Gadi N., Spihlman A.P., Wu S.C., Choi C.H., Moulton V.R. Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 2021;11:571416. doi: 10.3389/fphys.2020.571416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.