Highlights
-
•
CT pneumonia analysis program is an objective way to determine the disease severity.
-
•
The population over the age of 60 and with certain comorbidities such as DM, CHF, and COPD are more prone to severe disease than other patients.
-
•
CRP, Neutrophil/Lymphocyte, troponin levels are positive predictors for clinical worsening.
Keywords: COVID-19 pneumonia, Automated pneumonia analysis, Pneumonic scoring
Abstract
Purpose
The aim of this study is to define the role of an “Automated Multi Detector Computed Tomography (MDCT) Pneumonia Analysis Program’’ as an early outcome predictor for COVID-19 pneumonia in hospitalized patients.
Materials and Methods
A total of 96 patients who had RT-PCR proven COVID-19 pneumonia diagnosed by non-contrast enhanced chest MDCT and hospitalized were enrolled in this retrospective study. An automated CT pneumonia analysis program was used for each patient to see the extent of disease. Patients were divided into two clinical subgroups upon their clinical status as good and bad clinical course. Total opacity scores (TOS), intensive care unit (ICU) entry, and mortality rates were measured for each clinical subgroups and also laboratory values were used to compare each subgroup.
Results
Left lower lobe was the mostly effected side with a percentage of 78.12 % and followed up by right lower lobe with 73.95 %. TOS, ICU entry, and mortality rates were higher in bad clinical course subgroup. TOS values were also higher in patients older than 60 years and in patients with comorbidities including, Hypertension (HT), Diabetes Mellitus (DM), Chronic Obstructive Pulmonary Disease (COPD), Chronic Heart Failure (CHF) and malignancy.
Conclusion
Automated MDCT analysis programs for pneumonia are fast and an objective way to define the disease extent in COVID-19 pneumonia and it is highly correlated with the disease severity and clinical outcome thus providing physicians with valuable knowledge from the time of diagnosis.
1. Introduction
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2) a novel coronavirus causing COVID-19 was identified for the first time in December 2019, in Wuhan, the capital city of Hubei province in China [1]. The virus spread around the whole world in a short time and World Health Organization (WHO) declared the pandemic status by 11 March 2020. The number of people infected throughout the world has reached 140 millions as of April 2021 and the number of total deaths is more than 3 millions as reported by WHO [2].
Viruses are the most common cause of respiratory infections and MDCT is the main diagnostic tool for diagnosing viral pulmonary infections [3]. MDCT patterns of viral pneumonia are related to the pathogenesis of viral infections. Most viral pneumonia imaging patterns share similarity on the basis of viridae, because they possess a similar pathogenesis [3]. SARS-CoV-2 is the seventh known virus of coronaviridae that can be transmitted from human-to-human causing serious disease by involving multiple organs and mainly lungs, thus presenting with pneumonia [4].
The clinical signs and symptoms of viral pneumonia are often nonspecific, and also depend on the host’s immune status [5]. Radiological imaging of the pneumonia is important to detect and assess disease extent and to help to perform follow-up assessment and response to treatment [5]. It can be difficult to differentiate viral pneumonia from other infectious processes, and the cause of infection (eg, viral vs bacterial or fungal) cannot be reliably ascertained from its imaging appearance [6].
Real-time reverse transcription-polymerase chain reaction (RT-PCR) is the most commonly used test for detecting viral mRNA in COVID-19 patients by using nasopharyngeal swab to collect the samples. While sensitivity and specifity of RT-PCR is still controversial and false negative results might occur in the first few days, MDCT remains the most reliable diagnostic tool for diagnosing and following-up viral pneumonias [6,7]. In a recent large meta-analysis of 6218 patients from 68 studies in and outside China, the pooled sensitivity of chest MDCT and RT-PCR for the diagnosis of COVID-19 pneumonia were calculated as 94 % (95 % CI: 91–96 %) and 89 % (95 % CI: 81–94 %), respectively [8,11].
Pneumonia is the most common manifestation of COVID-19 and it is associated with a high morbidity and mortality [8]. Easy accessibility, rapid scanning techniques, high spatial resolution, post-processing techniques, high sensitivity and specifity makes MDCT preferred imaging modality for pneumonia. Ground glass opacities (GGO), crazy paving pattern and consolidations are the most common chest CT findings in patients with COVID-19 pneumonia [8,9]. The distribution of these CT findings is usually bilateral and multilobar with a predominant involvement of subpleural/peripheral and posterior regions of the lungs [8,10].
Chest MDCT imaging also plays a role in prognostic assessment to quantify the severity and outcome in COVID-19 pneumonia [7,8]. Automated pneumonia analysis softwares are enables physicians to determine the extent of disease and helps to manage the patient by prognosis prediction, risk priorisation, and assess the response to therapy [7].
In this study an automated MDCT pneumonia analysis program has been investigated as an early outcome predictor for hospitalized patients with COVID-19 pneumonia.
2. Materials and methods
2.1. Patients and groups
This study is a single-center retrospective study approved by the local Clinical Research Ethics Committee (KA20/441) and an informed consent was taken from the patients. We retrospectively analyzed the data of the hospitalized patients who were diagnosed with COVID-19 by a positive RT-PCR test result and shown to have pneumonia by a non-contrast enhanced chest CT from June to November 2020 in our hospital. All patients were followed-up closely in an isolated ward with oxygen saturation measurement and routine blood tests and the treatment protocol was given according to the national guidelines as defined by Turkish Ministry of Health. Patients who had no pneumonic infiltration, massive pulmonary effusion and who had a contrast enhanced chest CT were excluded from this study. It was decided that pneumonic scoring would not be appropriate in patients with primary and metastatic lung malignancies, tuberculosis with dense fibrotic scars and large atelectasis since total aerated lung parenchyma would decrease and pneumonic infiltration opacities would be indistinguishable from the opacities of the underlying disease and would lead to inaccurate results. Therefore, these patients were excluded from this study. A total of 96 patients (60 men, 36 women, range of age: 28–100 years old) were divided into two subgroups according to their clinical course. RT-PCR test sample was taken from nasopharyngeal swab of outpatients at the time of application presenting with COVID-19 related symptoms (e.g. fever, cough) and patients who were already hospitalized for some other health conditions but developed pneumonia later.
According to the WHO clinical progression scale [12] patients were included into group 1 (named as good clinical course) if hospitalized with moderate disease meaning that patients either did not recieve oxygen therapy (score 4) or received oxygen therapy by nasal cannula or mask (score 5). Whereas group 2 (bad clinical course) included hospitalized patients with severe disease who entered intensive care unit (ICU) and recieved oxygen by high-flow or non-invasive ventilation (score 6) ; or intubation and invasive mechanical ventilation with pO2/FiO2 ≥ 150 or SpO2/FiO2 ≥ 200 (score 7); or recieved mechanical ventilation pO2/FiO2 < 150 or vasopressors (score 8); or recieved mechanical ventilation pO2/FiO2 < 150 and vasopressors, dialysis or extracorporeal membrane oxygenation (ECMO) (score 9) or who died at the end of the study (score 10). According to the above criteria there were defined 58 cases with good clinical course and 38 patients with a bad clinical course.
2.2. Chest MDCT protocol and assessment
All patients were assigned to a 64-slice multidetector CT scanner (Somatom® go.All; Siemens Healthineers, Forchheim, Germany). This scanner is reserved for COVID-19 suspected patients only. The CT room was disinfected after the examination of each patient was completed. All patients were examined in the supine position. MDCT images were then acquired during a single inspiratory breath-hold. The scanning range was from the apex of the lung to the costophrenic angle. Scan parameters were as follows: X-ray tube parameters 110 kVp, 76 mAs; rotation time 0.5 s; pitch 0,7; z cover 32 × 0,7 mm and a slice thickness of 3 mm with 1 mm reconstructions.
Chest MDCT imaging findings such as pleural thickening, subpleural lines, pleural effusion, pericardial effusion, mediastinal lymphadenopathy; parenchymal infiltration pattern as peripheral, peripheral-central, ground glass opacities, crazy paving pattern, consolidation and reverse halo sign were noted for all patients.
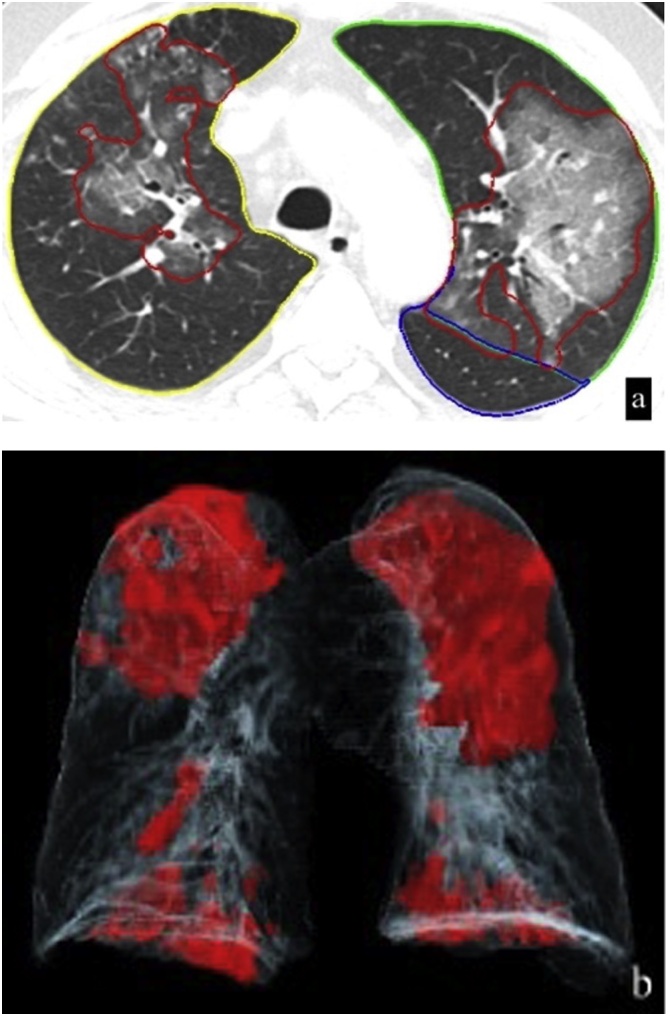
Image analyses for pneumonic severity score was made by an automated lung opacity analysis program “CT Pneumonia Analysis” which is provided by (Siemens Healthineers, Forchheim, Germany), The CT Pneumonia Analysis prototype was performed on a non-contrast Chest MDCT axial data with 1 mm reconstructed slice thicknesses. Multiplanar reconstruction (MPR) images were obtained which contain lung segmentations and opacity areas with percentage of pneumonic infiltration and an opacity score. The algorithm automatically detects and quantifies abnormal tomographic patterns commonly present in lung infections, namely ground glass opacities (GGO) and consolidations. Based on 3D segmentations of lesions, lungs, and lobes, the algorithm quantifies the extent of overall abnormalities and the presence of high opacity abnormalities, both globally and lobe-wise. The severity of COVID-19 pneumonia was measured by measuring percentage of ground glass opacity (PO), percentage of high opacity (PHO, consolidation), total opacity score (TOS) which refers lobe-wise involvement of pneumonia for each 5 lobes and ranges between 0–4 for each lobe and totally ranges between 0–20 for all lung parenchyma (Fig. 1) [7]. Data process and calculation results take 1–2 min. After automatically measurement, the program allows the user to correct manually lung segment borders and false positive or false negative pneumonic opacities (Fig. 1).
Fig. 1.
A 52 years old woman presented with cough weakness and fatigue. Layout image of CT Pneumonia Analysis contains, axial, sagittal, coronal reformat images of lung parenchyma with color-coded lines (yellow - RUL, pink - ML, dark green - RLL, light green - LUL, blue - LLL) mark the borders of lung lobes and red lines which presents in both lungs, mark opacity areas of lung. Volume rendering image (right lower) demonstrates the spatial distribution of the opacities as red areas which involves both lungs. Summary tables of quantitative results show lung segmentations and opacity areas with percentage of pneumonic infiltration and an opacity score (TOS). On the left upper corner of the image lung segmentations and opacity areas editing tools are also available. (For interpretation of the references to colour in the Figure, the reader is referred to the web version of this article).
- Score 0: lobe is not affected
- Score 1: 0–25 % of the lobe affected
- Score 2: 25–50 % of the lobe affected
- Score 3: 50–75 % of the lobe affected
- Score 4: 75–100 % of the lobe affected
All CT examinations were independently reviewed by two chest radiologists with experience in Syngo via workstation (Siemens Healthineers, Forchheim, Germany). After automatically measurement of lung opacities, radiologists reviewed all results and edited images if needed. Although only in a few patients required minor contour adjustments to exclude hilar vascularities in images obtained after automated measurement, they were not large enough to affect scoring results.
2.3. Statistical analysis
Statistical analysis was performed in IBM SPSS version 25.0 program. Hypotheses were tested at α = 0.05 significance level. The compliance of the data to normal distribution was examined by Shapiro-Wilk test and the homogeneity of variances by Levene test. Descriptive statistics of all variables were calculated. In cases where the normal distribution assumption was not provided, Mann Whitney U test and Spearman's rank correlation analysis were performed. In examining the relationship between categorical variables, 2 × 2 Pearson Chi Square and Fisher's Exact test results were given. Receiver operating characteristic (ROC) Curve analysis results were examined in determining the cut-off point for the total opacity score.
3. Results
3.1. Demographic characteristics of the patients
A total of 96 patients were included in this study. 58 patients (38 % female, 62 % male) were in the good clinical course group with an average age of 63.75 years (range: 31–93). 38 patients (36.8% female, 63.2% male) were included in the bad clinical course group with an average age of 70.9 (range: 28–100) years. The average period of hospitalization was calculated as 134 days with a range from 1 to 41 days. When compared according to the clinical status the group with good prognosis had a hospitalization period of 11.53 ± 7.3 days and the group of patients with bad prognosis had a longer period equal to 16.29 ± 10.19 days. 40 (41.6%) of 96 patients were transferred to ICU during the hospitalization, but 2 of them were admitted in ICU for close follow-up only and did not meet the criteria to be included in the bad prognosis group according to the WHO definition. The overall mortality was 35.4 % (n = 34) and 77.5% of the patients admitted to the ICU (n = 31).
The patients included in this study had a variety of comorbidities (Table 1). The frequency of comorbidities was higher in the group admitted in ICU and there was only one patient who was 41 years old male without any previously known comorbidities who died at the 4th day of his hospitalization at ICU. This patient’s troponin levels were very high (the highest among 96 patients) and he died because of fulminant myocarditis.
Table 1.
Comorbidities of patients.
| Type of disease | No ICU | ICU |
|---|---|---|
| HT | 48 (50 %) | 24 (60 %) |
| DM | 26 (27.1 %) | 16 (40 %) |
| CAD | 25 (26 %) | 14 (35 %) |
| Malignancy | 12 (12.5 %) | 1 (2.5 %) |
| COPD | 12 (12.5 %) | 8 (20 %) |
| CHF | 9 (9.4 %) | 6 (15 %) |
| Patients with solid organ transplant | 7 (7.3 %) | 3 (7.5 %) |
| CKD | 9 (9.4 %) | 4 (10 %) |
| CLD | 5 (5.2 %) | 1 (2.5 %) |
| Other | 30 (31.3 %) | – |
| None | 9 (9.4 %) | 1 (2.5 %) |
HT: Hypertension, DM: Diabetes Mellitus, CAD: Coronary artery disease, CHF: Congestive heart failure, CKD: Chronic kidney disease, CLD: Chronic liver disease.
21 patients were re-hospitalized and 3 of them died during this period, whereas the other 18 cases were discharged.
3.2. Laboratory findings
Laboratory findings of 96 patients who were grouped according to the clinical course (Table 2). CRP and troponin levels were higher in the bad prognosis group whereas neutrophil to lymphocyte ratio was higher in the good prognosis group and the difference was found to be statistically significant (p < 0.05).
Table 2.
Laboratory findings variables according to clinical course status.
| Clinical Course | N | Mean | Median | Std. Deviation | Std. Error Mean | Mann Whitney U test | |
|---|---|---|---|---|---|---|---|
| CRP (mg/L) | Good | 57 | 76.8256 | 40.06 | 80.81793 | 10.70460 | U = 803.00, Z=−2.127 p < 0.05 |
| Bad | 38 | 106.2500 | 82.35 | 85.84618 | 13.92609 | ||
| D-Dimer (mcg/mL) | Good | 53 | 2.6710 | 0.96 | 5.83188 | 0.80107 | U = 850.00, Z=−1.070 p = 0.285 |
| Bad | 37 | 1.8949 | 1.13 | 1.91872 | 0.31543 | ||
| Leucocyte (109/L) | Good | 57 | 7.6447 | 6.67 | 4.00733 | 0.53078 | U = 958.00, Z=−0.747 p = 0.455 |
| Bad | 37 | 8.8335 | 7.06 | 5.99797 | 0.98606 | ||
| Lymphocyte (109/L) | Good | 57 | 1.0918 | 1.02 | 0.70895 | 0.09912 | U = 873.00, Z=−1.405 p = 0.160 |
| Bad | 37 | 4.7041 | 1.02 | 22.02690 | 3.62120 | ||
| Neutrophil/Lypmhocyte | Good | 57 | 24.3647 | 3.87 | 137.95595 | 1,8.27271 | U = 745.00, Z=−2.395 p < 0.05 |
| Bad | 37 | 13.6949 | 5.05 | 25.31935 | 4.16248 | ||
| Ferritin (ng/mL) | Good | 46 | 510.59 | 303.00 | 512.610 | 75.580 | U = 615.50, Z=−0.794 p = 0.427 |
| Bad | 30 | 656.53 | 390.00 | 620.795 | 113.341 | ||
| Creatine Kinase (units/L) | Good | 49 | 211.43 | 93.00 | 386.226 | 55.175 | U = 711.00, Z=−1.521 p = 0.128 |
| Bad | 36 | 95.47 | 68.00 | 109.893 | 18.316 | ||
| Procalcitonin (ng/mL) | Good | 43 | 3.3353 | 0.14 | 15.73704 | 2.39988 | U = 533.00, Z=−1.273 p = 0.203 |
| Bad | 30 | 3.6313 | 0.26 | 17.70238 | 3.23200 | ||
| Troponin (ng/mL) | Good | 53 | 95.89 | 8.00 | 493.650 | 67.808 | U = 722.00, Z=−2.123 p < 0.05 |
| Bad | 37 | 303.00 | 17.00 | 1428.080 | 234.775 |
3.3. Computed tomography findings
Left lower lobe was the most affected part of the lung with a percentage of 781% and the second was right lower lobe with a percentage 73,95 %. The lower lobe scores were higher than the middle and the upper lobe scores in all patients. The main radiologic feature was GGO (948%) which were distributed peripherally and centrally with lower lobe predilection (Table 3). The other radiological findings were consolidations, crazy paving pattern, sub-pleural lines, pleural thickening, pleural effusion, pericardial effusion and mediastinal lymphadenopathies (ie, lymph nodes with a short-axis diameter >1 cm) (Table 3, Figs. 1, 2). Mediastinal lymphadenopathy was seen in 25 patients and 17 (68 %) of them were classified in the bad clinical course group. Neither reverse halo nor fibrosis were seen in any patient.
Table 3.
Chest CT findings of patients at the time of admission.
| Frequency | Percentage(%) | |
|---|---|---|
| Ground Glass Opacity | 91 | 94.8 |
| Peripheral-Central distrubution | 75 | 78.1 |
| Pleural Thickennig | 74 | 77.1 |
| Consolidation | 62 | 64.6 |
| Crazy Paving | 37 | 38.5 |
| Pleural Effusion | 35 | 36.5 |
| Subpleural Lines | 34 | 35.4 |
| Mediastinal Lymphadenopathy | 25 | 26 |
| Peripheral disrtubution | 21 | 21.9 |
| Pericardial Effusion | 12 | 12.5 |
| Fibrosis | 0 | 0 |
| Reverse Halo | 0 | 0 |
Fig. 2.
A 62 years old man who has HT, DM and a history of renal transplantation presented with COVID-19 pneumonia. Axial chest CT image (a) of CT Pneumonia Analysis shows bilateral peripherally and centrally distributed ground glass opacities in both upper lobes marked within red lines. Color-coded lines (yellow – right upper lobe, green – left upper lobe, blue – left lower lobe) mark the borders of lung lobes. Volume rendering image (b) of same patient demonstrates the spatial distribution of the opacities as red areas which involves both lungs, predominantly upper lobes. (For interpretation of the references to colour in the Figure, the reader is referred to the web version of this article).
3.4. Pneumonia analysis and clinical outcome
TOS was found significantly higher in the bad clinical course, ICU entry and the mortality groups (Table 4). TOS values of patients who’s older than 60 years were significantly higher than the younger group (U = 487.50, Z=−3.989 p < 0.05) (Table 4).
Table 4.
Examination of total opacity score values according to clinical course, ICU entry, mortality, age and DM.
| N | Mean | Median | Std. Deviation | Std Error Mean | ||||
|---|---|---|---|---|---|---|---|---|
| TOS | Clinical course | Good | 58 | 3.84 | 3.00 | 3.619 | 0.479 | U = 606.00, Z=−3.639; p < 0.05 |
| Bad | 38 | 7.03 | 6.00 | 4.687 | 0.760 | |||
| ICU entry | Yes | 40 | 7.53 | 6.50 | 4.920 | 0.778 | U = 521.00, Z=−4.383; p < 0.05 | |
| No | 55 | 3.36 | 3.00 | 2.831 | 0.382 | |||
| Mortality | Yes | 34 | 7.56 | 6.50 | 4.507 | 0.773 | U = 499.50, Z=−4.266; p < 0.05 | |
| No | 62 | 3.81 | 3.00 | 3.625 | 0.460 | |||
| Age | ≤60 | 30 | 2.93 | 2.00 | 3.676 | 0.671 | U = 487.50, Z=−3.989 p < 0.05 | |
| ≥61 | 66 | 6.14 | 5.50 | 4.257 | 0.524 | |||
| HT | Yes | 48 | 5.65 | 5.00 | 4.185 | 0.604 | U = 947.00, Z=−1.508 p = 0.131 | |
| No | 48 | 4.63 | 3.00 | 4.456 | 0.643 | |||
| DM | Yes | 25 | 6.80 | 6.00 | 4.555 | 0.911 | U = 604.50, Z=−2.373 p < 0.05 | |
| No | 71 | 4.55 | 4.00 | 4.122 | 0.489 | |||
| CHF | Yes | 9 | 7.67 | 7.00 | 4.555 | 1.518 | U = 235.50, Z=−1.969 p < 0.05 | |
| No | 87 | 4.87 | 4.00 | 4.248 | 0.463 | |||
| COPD | Yes | 12 | 7.83 | 6.00 | 4.108 | 1.186 | U = 279.50, Z=−2.498 p < 0.05 | |
| N | 84 | 4.75 | 4.00 | 4.245 | 0.463 | |||
| Malignancy | Yes | 12 | 2.42 | 2.00 | 2.429 | 0.701 | U = 276.00, Z=−2.536 p < 0.05 | |
| No | 84 | 5.52 | 5.00 | 4.411 | 0.481 |
DM, CHF, COPD and the presence of a malignancy were the conditions in which TOS were significantly higher than the other patients (Table 4). There were no statistically difference between patients with or without HT for TOS (Table 4). The highest TOS among 96 patients was calculated as 18 and the patient was a 71 years old male with many comorbidities such as Rheumatoid Arthritis, DM and HT. He died at 5th day of his hospitalization in ICU. The second highest value of TOS was 17 and the patient was 55 years old male with HT and DM. He died at 24th day of his hospitalization in ICU. The third highest value of TOS was 16, the patient was an 81 years old male who was a renal transplant receiver with psoriatic arthritis. After 17 days hospitalization in ICU he improved and could be discharged from the hospital.
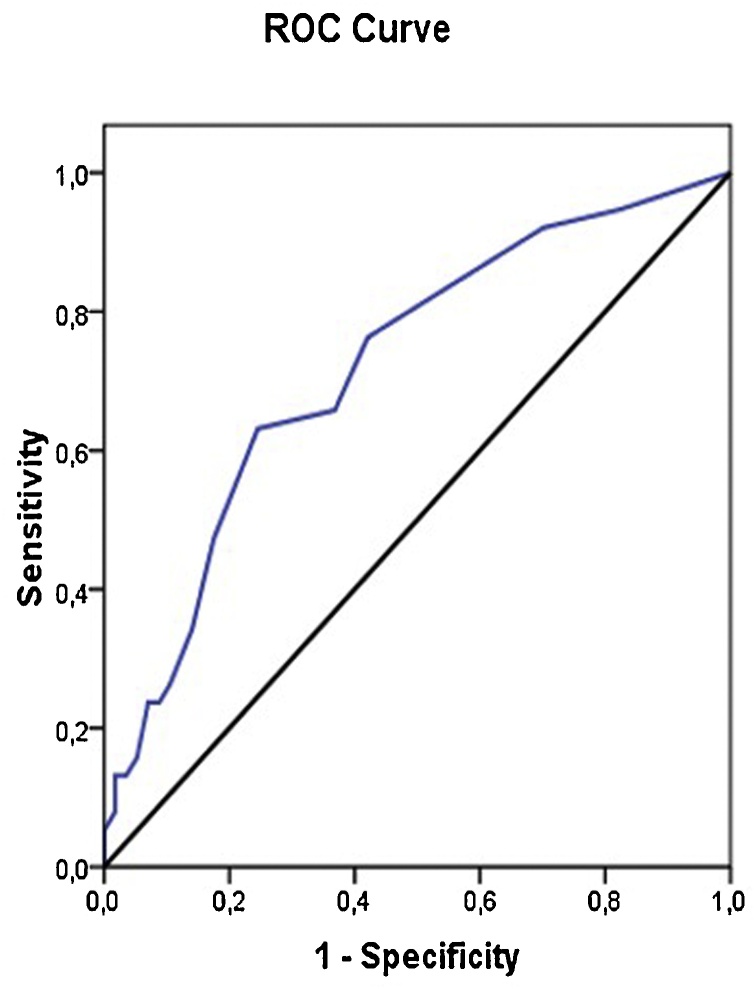
Cut-off value for TOS between good clinical course and bad clinical course subgroups was found as 5 according to ROC curve analysis with a sensitivity 63.6% and specifity 75.44 % (Fig. 3). Whereas ROC curve analysis results for cut-off values of TOS regarding the mortality was also found to be 5 with a sensitivity 67.65 % and specifity 74.19 % (Fig. 4).
Fig. 3.
TOS ROC curve for clinical course. AUC = 0.72, Specificity 75.44 %, sensitivity 63.16 %, Cut-off value = 5.
Fig. 4.
TOS ROC curve for mortality. AUC = 0.763, specificity 74.19 %, sensitivity 67.65 %, Cut-off value = 5.
As a result of Spearman’s rank correlation, there was a statistically significant moderate positive correlation between CRP and TOS value (rs = 0.53; p < 0.05).
4. Discussion
Our study was designed to investigate the role of an automated pneumonia analysis program as “CT Pneumonia Analysis’’ in prediction of clinical outcome and the possible role might it have in the management of the hospitalized patients with COVID-19 pneumonia. All of 96 patients were hospitalized at admission or they were diagnosed for COVID-19 pneumonia while they were inpatients for some other reasons.
In accordance with other published studies the main radiological feature was GGO which can be explained by the early stage of the disease and distributed peripherally and centrally with lower lobe predilection [[14], [15], [16], [17], [18], [19], [20], [21], [22]]. Pleural thickening, consolidation, crazy paving, pleural effusion, sub-pleural lines, mediastinal lymphadenopathy, pericardial effusion were the other chest CT findings.
In this study we found that automated pneumonia analysis program results are compatible with other prognostic factors such as age and co-morbidities. TOS were higher in bad clinical course group, patients older then 60 years, patients with DM, CHF, COPD and malignancy and it was compatible with ICU entry and mortality status. CRP, Neutrophil/Lymphocyte, troponin levels were found as positive predictors of clinical worsening whereas higher D-dimer levels were correlated with mortality status [22,28,29].
Like other previous studies, our hypothesis was that automated diagnosis and quantification programs for pneumonia are valuable in predicting clinical outcome, helps physicians in triaging patients and to start appropriate treatment as soon as possible [13,22,23].
In a recent study, Ran Y. et al. investigated the importance of a semiquantitative chest CT severity scoring (CT-SS) program to differentiate clinical forms of COVID-19 pneumonia. In contrast with our study in their study the lung opacities were subjectively evaluated on chest CT images while it was fully automated in our study. In accordance with our study they found CT scoring usefull to expedite triage of patients in need of hospital admission [13].
Marko F. et al. made a similar approach on semiquantitative chest CT pneumonic scoring and it’s correlation with severity and short-term prognosis in COVID-19 patients [22]. In accordance with our study they found CT scoring could help to stratify patient’s risk and predict short-term outcome of COVID -19 patients.
A similar approach was made by Furkan U. et al. who assessed the severity of COVID-19 by quantitative and semiquantitative methods, but in a different way by calculating the involved lung volume with a formula including total lung from which the healthy lung part is subtracted and divided then by total lung volume to obtain a ratio [24]. In contrast with our study they used an external software to calculate pneumonic scores which could make some delay to get results in a pandemic burden. They also reported in a similar way that the left lower lobe of lung was the most involved part in accordance with our study [24].
Our study investigated a fully automated pneumonic scoring prototype program as called “CT pneumonia Analysis’’ (Siemens Healthineers, Forchheim, Germany) which doesn’t require any user intervention that affects results. The program provides fast and reliable results, as well as measuring the extent of the disease throughout the lobe, the right and left lung, as well as the total lung, and visually demonstrating the extent of the disease with MPR and VRT images which is useful in daily practice.
While different scoring programs are defined by different manufacturers, the main result from different studies is similar [13,22,23].
The limitations of this study were the small number of patients included and only the CT and laboratory values at admission were used.
It is important to know clinical outcomes of COVID-19 pneumonia among different patient subgroups and to define the most vulnerable patients. Management of the patients with COVID-19 pneumonia is highly dependent on the fast diagnosis and revealing of the severity of lung involvement. Chest radiographs can cause false negativeness for early abnormalities of the COVID-19 pneumonia and also might not be enough to reveal the disease severity [4,13,23,25]. On the other hand, RT-PCR test results take a while and false negativeness is another issue. Moreover, it is well known that chest CT findings of COVID-19 pneumonia has similarities with and cannot be distinguished from other viral-atypical pneumonias [23,26,27]. In the conditions of a pandemic, chest CT scan has a unique place as the main, fastest and most reliable diagnostic tool available to diagnose and to reveal the disease severity of COVID-19 pneumonia.
MDCT automated pneumonia analysis programs which are being used nowadays need to be developed and improved in order to differentiate consolidation from nodules, masses, fibrosis, atelectasis and the other etiological agents of the pneumonia.
5. Conclusion
MDCT automated analysis programs for pneumonia are fast and objective diagnostic tools in the pandemic disease burden and very useful and reliable in patient’s risk stratification and determination of the early outcome. Lung involvement is positively correlated with bad clinical course, ICU entry and mortality. Hospitalized patients with co-morbidities and older than 60 years are the most vulnerable patients for severe disease and should be monitored closely for clinical worsening. Future larger studies will enlighten the value of CT scoring systems in diagnosing and management of the COVID-19 pneumonia.
CRediT authorship contribution statement
Rahime Sezer: Conceptualization, Methodology, Validation, Resources, Analysis, Writing. Dorina Esendagli: Conceptualization, Methodology, Validation, Resources, Writing and Editing. Cigdem Erol: Resources, Review and Editing. Koray Hekimoglu: Supervision, Writing - Reviewing and Editing
Ethical statement
This study is a single-center retrospective study approved by the local Clinical Research Ethics Committee (KA20/441) and an informed consent was taken from the patients.
Funding or financial
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
We have no conflict of interest to declare.
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Coronavirus diease (COVID-19) Situation dashboard https://www.who.int/redirect-pages/page/novel-coronavirus-(covid-19)-situation-dashboard.
- 3.Koo H.J., Lim S., Choe J., Choi S.H., Sung H., Do K.H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 4.Dawoud M.M., Dawoud T.M., Ali N.Y.A. Chest CT in COVID-19 pneumonia: a correlation of lung abnormalities with duration and severity of symptoms. Egypt J. Radiol. Nucl. Med. 2020;51:246. [Google Scholar]
- 5.Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260(July (1)):18–39. doi: 10.1148/radiol.11092149. [DOI] [PubMed] [Google Scholar]
- 6.Zitek T. The appropriate use of testing for COVID-19. West. J. Emerg. Med. 2020;21(April 13 (3)):470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaganti S., Balachandran A., Chabin G., Cohen S., Flohr T., Georgescu B., Grenier P., Grbic S., Liu S., Mellot F., Murray N., Nicolaou S., Parker W., Re T., Sanelli P., Sauter A.W., Xu Z., Yoo Y., Ziebandt V., Comaniciu D. Quantification of Tomographic Patterns associated with COVID-19 from Chest CT. [DOI] [PMC free article] [PubMed]
- 8.Larici A.R., Cicchetti G., Marano R., Merlino B., Elia L., Calandriello L., Del Ciello A., Farchione A., Savino G., Infante A., Larosa L., Colosimo C., Manfredi R., Natale L. Corrigendum to “Multimodality imaging of COVID-19 pneumonia: from diagnosis to follow-up. A comprehensive review” [Eur. J. Radiol. 131 (October) (2020) 109217] Eur. J. Radiol. 2021;134(January) doi: 10.1016/j.ejrad.2020.109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams H.J.A., Kwee T.C., Yakar D. Chest CT imaging signature of COVID- 19 infection: in pursuit of the scientic evidence. Chest. 2020;(June 25) doi: 10.1016/j.chest.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J., Zhong Z., Li H. CT imaging features of 4121 patients with COVID- 19: a meta-analysis. J. Med. Virol. 2020;92(7):891–902. doi: 10.1002/jmv.25910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H., Hong H., Yoon S.H. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta- analysis. Radiology. 2020;296(3):E145–E155. doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 2020;20(August (8)):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. Epub 2020 Jun 12. Erratum in: Lancet Infect Dis. 2020 Oct;20(10):e250. PMID: 32539990; PMCID: PMC7292605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang R., Li X., Liu H., Zhen Y., Zhang X., Xiong X.Z., Luo Y., Gao C., Zeng W. Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol.: Cardiothorac. Imaging. 2020;2(March (2)) doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng M.Y., Lee E.Y.P., Yang J. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol. Cardiothorac. Imaging. 2020;2(1) doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed I.A.I., Hasan H.A., Abdel-Tawab M. CT characteristics and laboratory findings of COVID-19 pneumonia in relation to patient outcome. Egypt J. Radiol. Nucl. Med. 2021;52:28. [Google Scholar]
- 16.Pan Y., Guan H., Zhou S., Wang Y., Li Q., Zhu T. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur. Radiol. 2020;30(6):3306–3309. doi: 10.1007/s00330-020-06731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song F., Shi N., Shan F., Zhang Z., Shen J., Lu H. Emerging 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020209021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung M., Bernheim A., Mei X., Zhang N., Huang M., Zeng X. CT imaging features of 2019 novel coronavirus (2019-NCoV) Radiology. 2020 doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020;295(1):18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabri Y.Y., MMT F., Nossair E.Z., El-Mandooh S.M., Hegazy A.A., Tadros S.F. CT findings of 795 COVID-19 positive cases: a multicenter study in Egypt. Egypt J. Radiol. Nucl. Med. 2020;51(1):237. [Google Scholar]
- 21.Sultan O.M., Al-Tameemi H., Alghazali D.M., Abed M., Ghniem M.N.A., Hawiji D.A. Pulmonary ct manifestations of COVID-19: changes within 2 weeks duration from presentation. Egypt J. Radiol. Nucl. Med. 2020;51(1):1–7. [Google Scholar]
- 22.Francone M., Iafrate F., Masci G.M., Coco S., Cilia F., Manganaro L., Panebianco V., Andreoli C., Colaiacomo M.C., Zingaropoli M.A., Ciardi M.R., Mastroianni C.M., Pugliese F., Alessandri F., Turriziani O., Ricci P., Catalano C. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020;30(December (12)):6808–6817. doi: 10.1007/s00330-020-07033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malpani Dhoot N., Goenka U., Ghosh S., Jajodia S., Chand R., Majumdar S., Ramasubban S. Assigning computed tomography involvement score in COVID-19 patients: prognosis prediction and impact on management. BJR Open. 2020;2(August 20 (1)) doi: 10.1259/bjro.20200024. PMID: 33178981; PMCID: PMC7583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ufuk F., Demirci M., Uğurlu E., Çetin N., Yiğit N., Sarı T. Evaluation of disease severity with quantitative chest CT in COVID-19 patients. Diagn. Interv. Radiol. 2021;27(March(2)):164. doi: 10.5152/dir.2020.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Q., Liu Q., Xu H., Lu H., Liu S., Li H. Imaging of coronavirus disease 2019: a Chinese expert consensus statement. Eur. J. Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections fromWuhan, China: key points for the radiologist. Radiology. 2020;(295):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo H.J., Lim S., Choe J., Choi S.-H., Sung H., Do K.-H. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:19–39. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 28.Xia X., Wen M., Zhan S., He J., Chen W. [An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19] Nan Fang Yi Ke Da Xue Xue Bao. 2020;40(March 30 (3)):333–336. doi: 10.12122/j.issn.1673-4254.2020.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular Implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(July 1 (7)):811–818. doi: 10.1001/jamacardio.2020.1017. Erratum in: JAMA Cardiol. 2020 Jul 1;5(7):848. PMID: 32219356; PMCID: PMC7101506. [DOI] [PMC free article] [PubMed] [Google Scholar]