Abstract
Purpose
Rising melanoma incidences lead to an increasing need for individual therapy strategies in old patients. Talimogene laherparepvec (T-VEC) is a modified herpes simplex virus, approved for the local treatment of unresectable metastatic melanoma. Since data on the efficacy and safety of geriatric patients are sparse, this study was conducted to gain further real-world experience in the treatment of old and oldest-old patients with T-VEC and to obtain data on therapy costs in this population in Germany.
Patients and Methods
We performed a retrospective analysis, including all patients with a minimum age of 75 years who were treated with T-VEC from August 2016 to September 2020 in the Skin Cancer Center of the University Hospital Frankfurt, Germany. Patient clinicopathological data, treatment responses, toxicities, treatment-specific data and therapy costs were assessed.
Results
Twelve patients with a median age of 83 years (75–89 years) at the start of treatment were identified. By the end of the study, three (25%) patients experienced complete remission (CR), four (33%) experienced partial response (PR), two patients (17%) remained at stable disease (SD) and three (25%) patients suffered from progressive disease (PD). Overall response rate was 58.3%, and durable response rate was 41.7%. There were no treatment-related adverse events grade 3 or higher. The median duration of treatment was seventeen weeks (3–57 weeks). Median medication costs in the patients who had completed treatment (n=10) were calculated to be 27,325 Euros in Germany.
Conclusion
This study provides further evidence for an effective use of T-VEC in old and oldest-old patients. The low rate of adverse events seems to be favorable compared to other systemic melanoma therapies. Furthermore, duration of treatment was short and therapy costs were lower than would have been expected from clinical trial data. Altogether, these data encourage the use of T-VEC in this special patient cohort.
Keywords: intralesional therapy, immunotherapy, oncolytic virus, advanced melanoma, cutaneous oncology, elderly patients
Introduction
Incidences of melanoma have increased over the last decades, worldwide.1 In this connection, it has to be noted that age-standardized melanoma incidence rises steadily and reaches its peak in the seventh and eighth decades of life.1 Furthermore there is evidence to suggest that older age is associated with higher melanoma mortality.2 Some data point to the fact that age is an unfavorable prognostic variable, independent of tumor thickness, mitotic rate, ulceration or tumor stage.3 These circumstances result in an increasing number of old patients in advanced tumor stages, which might require a pharmaceutical tumor therapy.
It has to be taken into account that the therapeutic needs of old patients differ from those of younger patients, as older patients are less able to compensate for severe and potentially irreversible side effects. Long-time hospitalization due to adverse events may contribute to increasing frailty, from which old patients have difficulties in recovering.
In the last decade, several new effective therapies have been approved for metastatic malignant melanoma. The immune-checkpoint inhibitors (ICI) targeting the programmed cell death protein 1 (PD-1) and the cytotoxic T-lymphocyte associated protein 4 (CTLA4), as well as the targeted therapies, namely BRAF-MEK inhibitors, have significantly improved progression-free and overall survival.4–8 However, these therapies are associated with considerable grade 3–4 side effects, which are in part irreversible and account for a significant hospitalization rate.9,10 In a randomized clinical trial with the ICI inhibitors nivolumab, ipilimumab and the combination therapy of both drugs, treatment-related grade 3–4 adverse events occurred in about 20–30% of the ICI monotherapies and in more than 50% of the combination therapy.4 For BRAF-MEK inhibitors, grade 3–4 adverse events have been observed in more than 50% of the patients treated with different drug combinations in clinical trials.11
Old and oldest-old patients have been underrepresented in clinical trials, and only limited data are available, mostly from retrospective real-world studies. In summary, these data indicate that the efficacy of ICI and BRAF-MEK inhibitors seems to be comparable to that in younger patients.12 However, with regard to the occurrence of adverse events, there is some evidence that toxicities might be higher in old and oldest-old patients compared to those reported in clinical trials.13–16 Considering that side effects and hospitalization strongly influence the quality of life and the cognitive abilities of old patients, a great need for effective therapies with a low incidence of adverse events remains.17,18
In 2015 and 2016 T-VEC, a genetically modified herpes simplex virus, was approved in the USA and in Europe for the intratumoral treatment of unresectable cutaneous, subcutaneous and nodal lesions in patients with melanoma. T-VEC selectively replicates in tumor cells due to the functional deletion of the infectious cell proteins (ICP) 34.5 and 47. It has a two-dimensional mechanism of action based on direct oncolytic effects and based on immune-mediated effects, which are supported by the overexpression of the human granulocyte-macrophage colony-stimulating factor (GM-CSF).19,20 In the phase III randomized controlled “OPTiM” trial, subgroup analysis revealed a highly significant survival improvement for American Joint Committee on Cancer (AJCC) stage IIIB-IVM1a patients (46.8 months vs 21.5 months; p=0.0008), but no significant benefit for AJCC stages IVM1b/c patients.21 In this study T-VEC had a tolerable safety profile with a low rate of treatment-related grade 3–4 adverse events (AE), which occurred in 11.3%.21 The most common AEs were fatigue, chills, pyrexia, nausea and influenza-like illness.21
These data indicate that T-VEC could be a good therapy option, especially in old and oldest-old patients. However, compared to younger patients, old patients have an altered immune system with a decreased number of dendritic cells, a decreased production of T-lymphocytes and a reduced T-lymphocyte receptor diversity.22 Considering these changes in the immune system of old people, and the fact that only 26.5% of patients investigated in the clinical trial have been older than 65 years, the question arises whether these data can be transferred in old patients in a real-world setting.21
In addition to these aspects of efficacy and safety, it becomes inevitable to discuss melanoma therapy costs, especially in old patients, from a socioeconomic point of view. The financial burden of malignant melanoma therapy on health care has been dramatically increasing in recent years, and it has been shown that ICI and targeted therapy medication costs have a major part in this.23,24 Cost-effectiveness analysis of ICI and BRAF-MEK inhibitors tends to conclude that most of these therapies are not cost-effective, and that only PD-1 monotherapies can be seen as cost-effective therapies, considering the usually accepted thresholds in oncology.25
To the best of our knowledge, there are no clinical trials or real-world studies that have analyzed therapy costs of T-VEC alone or compared to ICI or targeted therapies. For Germany, annual therapy costs of T-VEC, ICI and BRAF-MEK inhibitors have been evaluated in 2016 by the Joint Federal Committee in the initial benefit assessment of T-VEC. Since the T-VEC volume used per treatment can vary between one and four milliliters depending on the tumor volume, the annual medication costs have been calculated to range from 36,144 Euros to 289,151 Euros at the time. Compared to these, annual therapy costs for ICI monotherapy of different manufacturers ranged from 73,998 Euros to 144,057 Euros, and BRAF-MEK combination therapy ranged from 180,953 Euros to 196,130 Euros.26 Considering the broad range of annual therapy costs for T-VEC, there is a need for further evidence of therapy costs in a real-world setting, which has not been addressed so far.
The aim of this project was to investigate the efficacy and safety of T-VEC in old and oldest-old patients under real-world conditions in a single institution in Germany. It was hypothesized that despite the known alterations in the immune system of old people, T-VEC might be a good therapy option in this patient collective, especially because of its favorable toxicity profile. Furthermore, to date, there is very little evidence on therapy costs and cost-effectiveness of T-VEC, and therefore this study will provide further data on this.
Patients and Methods
We performed a retrospective single-institution analysis, including all patients with a minimum age of 75 years who received their first doses of T-VEC as standard of care therapy from August 2016 to September 2020, in an outpatient clinical setting at the Skin Cancer Center of the University Hospital Frankfurt. Follow-up, defined as the period between the last T-VEC injection and the last visit in our center (or the date of death), was conducted until February 2021.
T-VEC treatments were performed according to the manufacturer recommendations (Amgen, Applied Molecular Genetics, Thousand Oaks, CA, USA).27
For the initial treatments a maximum of 4 mL T-VEC were injected at a concentration of 106 plaque-forming units (PFU) per milliliter, followed by a three-week therapy-free interval. All subsequent injections were administered with a maximum of 4 mL at a concentration of 108 PFU/mL every two weeks. Nodal or subcutaneous metastases were injected by ultrasound assistance.
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the institutional Review Boards of the University Cancer Center (UCT) and the Ethical Committee at the University Hospital Frankfurt (project-number: SDO-01-2019). Written informed consent was obtained from all patients.
Patient baseline demographics and clinicopathological data, including age, sex, comorbidities, concomitant medication, performance status defined by the Eastern Cooperative Oncology Group (ECOG), lactate dehydrogenase (LDH) serum blood levels, BRAF mutational status, melanoma disease stage according to the AJCC classification (eighth edition, 2017) and prior treatments were obtained. Additional information regarding the treatment quantity and the injected volumes of T-VEC were assessed.
Adverse events were extracted from the clinical notes and graded based on Common Terminology Criteria for Adverse Events (CTCAE) guidelines Version 5.0 of the National Cancer Institute.28
Therapy costs were estimated based on direct drug costs. For Germany the direct drug costs for T-VEC, nivolumab, pembrolizumab, dabrafenib, trametinib, vemurafenib and cobimetinib were extracted as pharmacy selling prices from the AiD-Klinik Database V 4.7.0 2021/Dosing GmbH (Heidelberg, Germany).29 Annual therapy cost calculation was based on standard dosages as specified in the manufacturer’s product information.
The tumor responses (best overall response) were evaluated based on the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1).30 Changes in tumor burden were evaluated clinically based on tumor information in the medical records and using imaging methods (ultrasound, computed tomography and magnetic resonance imaging). Imaging was conducted before start of treatment and every twelve weeks during therapy and thereafter. Complete remission (CR) was defined as disappearance of all lesions, and the absence of new lesions confirmed with the next staging (at least four weeks after initial response) or by pathological confirmed complete response. Partial response (PR) was defined as decrease of at least 30% of target lesions compared to the baseline without any evidence of progression in non-target lesions or the appearance of new lesions. Progressive disease (PD) was defined as an increase of at least 20% in the longest diameter of target lesions or progression of non-target lesions or the appearance of a new lesion. All other cases were defined as stable disease (SD). Overall response rate (ORR) was defined as the proportion of patients who had a PR or CR, and disease control rate (DCR) was defined as the proportion of patients who had PR, CR or SD.
Durable response, defined as partial or complete response lasting for six months at any time after the date of best response, was evaluated.
Progression-free survival (PFS) was defined as the time from the start of treatment with T-VEC to the date of progression, death or of the last follow-up. Analogous overall survival (OS) was defined as the time from the start of treatment until death or of the last follow-up. PFS and OS probability were calculated by Kaplan–Meier survival analysis. Confidence intervals (Cl 95%) were provided if indicated.
Analyses were performed using Microsoft Excel 2016 and Systat SigmaPlot 11.
Results
Patient Demographics and Clinical Characteristics
Between August 2016 and September 2020 we identified twelve patients with a minimum age of 75 years who were treated with T-VEC in the University Hospital Frankfurt outside of clinical trials. Patients' characteristics are described in Table 1. The median age was 83 years (range 75–89 years) at the start of treatment and eight of twelve patients (67%) were older than 80 years. Consistent with the higher age, all twelve patients suffered from a slightly to moderately reduced ECOG performance status. Median number of independent comorbidities were four, and median number of prescribed co-medications were five. Most patients (n=10) were in AJCC stage III, and only two patients had distant cutaneous metastasis (IVM1a).
Table 1.
Patient Demographics and Clinical Characteristics
| Demographics and Characteristics | Patients N (%) |
|---|---|
| Total number of patients | 12 |
| Age | |
| Median age (years) | 83 |
| Range (years) | 75–89 |
| Gender | |
| Female | 6 (50) |
| Male | 6 (50) |
| ECOG | |
| 0 | 0 (0) |
| 1 | 9 (75) |
| 2 | 3 (25) |
| Comorbidities | |
| ≤2 | 1 (8) |
| 3–4 | 8 (67) |
| ≥5 | 3 (25) |
| Concomitant medication | |
| ≤2 | 1 (8) |
| 3–4 | 2 (17) |
| ≥5 | 9 (75) |
| Disease stage by AJCC 8th edition | |
| IIIB | 3 (25) |
| IIIC | 6 (50) |
| IIID | 1 (8) |
| IVM1a | 2 (17) |
| LDH | |
| ≤ ULN | 6 (50) |
| > ULN | 6 (50) |
| BRAF status | |
| Mutated | 3 (25) |
| Wild-type | 8 (67) |
| Unknown | 1 (8) |
| Location of treated lesions | |
| Head/neck | 1 (8) |
| Trunk | 0 (0) |
| Upper extremities | 1 (8) |
| Lower extremities | 10 (83) |
| Type of metastasis | |
| Cutaneous | 4 (33) |
| Sub-cutaneous | 4 (33) |
| Cutaneous and sub-cutaneous | 2 (17) |
| Nodal | 2 (17) |
The majority of the metastases were cutaneous or subcutaneous metastases, and most of them were located on the extremities (n=11), in ten cases on the lower extremities, and only one patient was treated in the head and neck area. Most (n=8) of the patients were BRAF wild-type.
Prior therapies are displayed in Table 2. Four patients received radical lymphadenectomy and three of them adjuvant radiotherapy. Seven patients had surgery of cutaneous or subcutaneous metastasis, and one patient received electrochemotherapy with bleomycin of local metastasis before T-VEC treatment. Two of the patients were treated with PD-1 ICI therapy, and two patients had received BRAF-MEK inhibitors prior to the T-VEC therapy.
Table 2.
Prior and Subsequent Therapies
| Prior Therapies | Patients N |
|---|---|
| Radical lymphadenectomy | 4 |
| Surgery of (sub-)cutaneous/nodal metastasis | 7 |
| Radiotherapy | 3 |
| Electrochemotherapy | 1 |
| Immune-checkpoint therapy | 2 |
| BRAF/MEK inhibitor treatment | 2 |
| Subsequent therapies | N |
| Surgery after relapse | 1 |
| Radiotherapy | 2 |
| ICI | 3 |
| Targeted therapies | 0 |
| Other therapies | 0 |
Response to Treatment
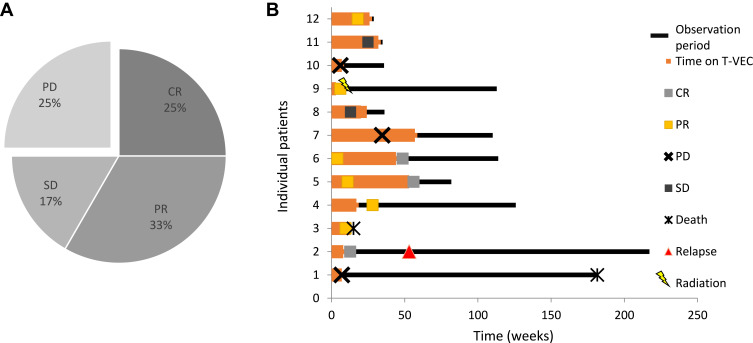
By the end of the study three (25%) patients experienced CR, four (33%) experienced PR, two patients (17%) remained at SD and the remaining three patients (25%) had PD. Therefore, ORR was 58.3% and DCR was 75.0% (Figure 1). The median follow-up was fourteen months (range 3–48 months). Durable response was observed in five of twelve patients (DRR 41.7%). Of the two patients who did not meet the criteria of durable response, one patient had died of a non-therapy-associated myocardial infarction, and another patient had not yet reached 6 months of follow-up at the time of the database lock. Median PFS was 20 months (Cl 95% 0–43.8), and median OS was 41 months (Cl 95% n.a.–n.a.).
Figure 1.
Treatment evaluation. (A) Response to T-VEC therapy. Complete responses were observed in three of twelve patients, partial responses in four of twelve patients and stable disease was seen in two patients. Overall response rate was 58.3% (7/12 patients), and disease control rate was 75.0% (9/12 patients). (B) Individual therapy courses. Each lane of the swimmer plot represents the therapy course of one individual in this study over time. The red bars show the time on treatment; black bars represent the follow-up time. Events of response [complete response (CR): green box, partial response (PR): yellow box, stable disease (SD): grey box; progressive disease (PD): black cross], relapse (red triangle) and death (asterisk) are marked.
Looking further at the patients who had PD, all three patients were treated with T-VEC for cutaneous metastasis of the lower legs. Two patients developed distant metastases. One patient developed regional lymph-node metastasis and was treated beyond progressions with T-VEC. Of note, all three patients who suffered from PD with distant metastasis in the course of the T-VEC treatment experienced complete local tumor control of the T-VEC-treated lesions.
Considering all therapies applied after the end of T-VEC treatment, one patient had surgical excision of a cutaneous metastasis after relapse. One patient received radiotherapy after partial remission, two patients with PD received ICI therapy and one patient with PD received a combination of ICI therapy and local radiotherapy of inguinal lymph-node metastasis (Table 2).
Toxicities
In general, the treatment with T-VEC was well tolerated. There were no treatment-related adverse events grade 3 or higher observed; nor were there permanent discontinuations because of side effects or treatment-related hospitalizations. The most commonly reported side effects were mild pyrexia, influenza-like illness and chills, followed by injection site pain and fatigue (Table 3).
Table 3.
Treatment-Related Adverse Events
| Adverse Event | Grade 1–2 N (%) | Grade 3–4 N (%) |
|---|---|---|
| Any | 9 (75.0) | 0 (0) |
| Pyrexia | 5 (41.7) | 0 (0) |
| Chills | 5 (41.7) | 0 (0) |
| Headache | 2 (16.7) | 0 (0) |
| Influenza-like illness | 3 (25.0) | 0 (0) |
| Fatigue | 2 (16.7) | 0 (0) |
| Peripheral edema | 1 (8.3) | 0 (0) |
| Ulceration | 1 (8.3) | 0 (0) |
| Injections site pain | 4 (33.3) | 0 (0) |
One patient had a worsening of a chronic lymphedema of the leg after treating metastases of the same. Another patient developed a superficial ulceration in the area of the injections, which required therapy interruption of several weeks.
One patient who had initial PR died from acute myocardial infarction within the treatment period. The death of the 80-year-old patient occurred during routine surgery for a pre-existing femoral head necrosis in an external hospital. The death was attributed to the numerous, cardiovascular comorbidities, although no autopsy was performed. The interval between the occurrence of the event and the last injection was greater than two weeks.
Duration of Treatment and Therapeutic Expenses
The median duration of treatment was seventeen weeks (range 3–57 weeks). In patients who had completed T-VEC treatment at the end of study (n=10) the median injected volume of T-VEC throughout the whole treatment period was 22 mL (range 2–68 mL). The median number of therapy cycles in patients who had completed T-VEC was eight (range 1–24).
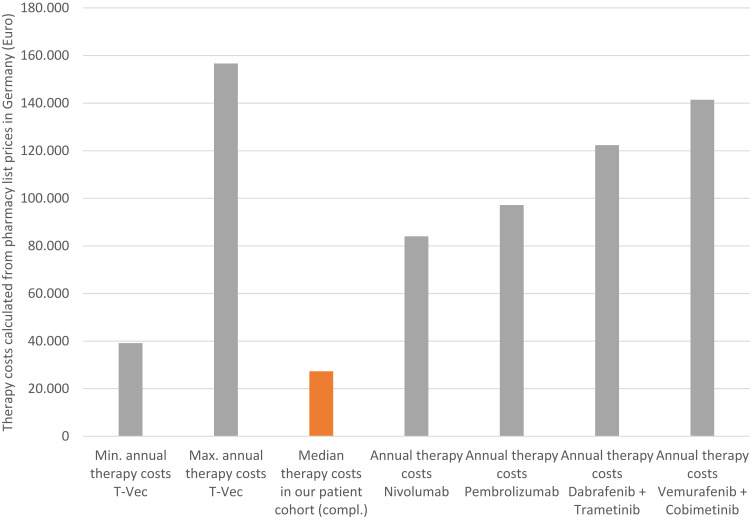
As the annual therapy costs of T-VEC depend on the volume that can be used per treatment (1–4 mL depending on the total diameter of all treatable lesions), one can calculate minimal annual therapy costs of 39,159 Euros and maximum annual costs of 156,636 Euros in Germany. In our study, the calculated median drug costs in the patients who had completed T-VEC treatment (n=10) were 27,325 Euros (range 3012–102,416 Euros).
For comparison, annual therapy costs were calculated for the PD-1 inhibitors nivolumab and pembrolizumab and the BRAF-MEK inhibitor combinations dabrafenib plus trametinib and vemurafenib plus cobimetinib (Figure 2).
Figure 2.
Therapy costs of T-VEC in Germany. Median therapy costs of T-VEC were calculated to be 27,326 Euros based on the injected T-VEC volume and pharmacy selling prices. Annual therapy costs of ICI and BRAF-MEK inhibitors were calculated based on pharmacy selling prices and according to the manufacturer treatment recommendations.
Discussion
In this real-world dataset of old and oldest-old people, we analyzed 12 patients, most of them suffering from a reduced ECOG performance status, multimorbidity and polypharmacy, representing a realistic cross-section of geriatric patients who are generally underrepresented in clinical trials. In our study we observed high and durable response rates (ORR 58.3%, DRR 41.7%) with good survival outcomes (median PFS of 20 months (Cl 95% 0–43.8), median OS of 41 months (Cl 95% n.a.–n.a.), a low rate of adverse events without any treatment-related grade 3–4 adverse events, no melanoma-related hospitalizations and therapy costs which were lower than expected from clinical trial data (median therapy costs of 27,325 Euros).
Comparing the observed efficacy data with the final response analysis of the randomized phase III “OPTiM” trial where ORR was 46% and DRR was 28.8% in the corresponding subgroup, we find that the response rates and the duration of responses seem to be distinctly higher in old patients. These findings seem to be surprising, because real-world efficacy results are usually inferior to clinical trial data, especially since this retrospective analysis focused on an unfavorable collective of old and multimorbid patients. Searching for confirmation, we were able to identify subsets in two other real-world T-VEC studies in which patients of older age were included and in which ORR and DRR have been reported in a comparable AJCC stage IIIB-IVM1a (seventh AJCC classification).31,32 Interestingly, the ORRs in both studies (56.5%, 70%) and the DRR, which could be extracted from one dataset (50%) were also higher than in the initial phase III study.31,32 However, none of these studies have analyzed older patients separately, and so results could be influenced by younger patients in the relatively small patient collectives. The only real-world study that has evaluated age as a prognostic factor concluded that old patients had comparable PFS and OS compared to younger patients.33 However, it has to be taken into account that this study included only patients in AJCC stage IV, 15% of them in AJCC stage IVM1b, which is not representative for the use of T-VEC worldwide. In conclusion, the efficacy results in old and oldest-old patients are in line with previous reported data and substantiate a favorable outcome compared to clinical trial data.
To the present date, no clinical trial or retrospective study has compared the efficacy of T-VEC to ICI or BRAF-MEK inhibitors directly. Since T-VEC approval in Europe is limited to AJCC stage IIIB-IVM1a patients, patient characteristics and tumor burden differ significantly between T-VEC datasets, BRAF-MEK and ICI trials. Therefore, conclusions concerning therapy efficacy of these therapies in relation to each other are limited.
Comparing toxicities of our patients and real-world data of old patients treated with ICI, it becomes evident that ICI-related toxicities observed in the real world occur frequently.34 Archibald et al observed 20.6% severe immune-related adverse events and 3.8% treatment-related deaths in patients 75 years and older who were treated with PD-1 inhibitors for cutaneous malignancies in a real-world setting.35 Ibrahim et al observed grade 3–4 drug-related adverse events in 24.2% of melanoma patients treated with pembrolizumab and a high frequency of treatment-related discontinuations (42.5%).13 For BRAF-MEK inhibitors, real-world data in old patients are sparse, but it is known from clinical trials that older patients are more likely to experience serious adverse events and adverse events that lead to permanent discontinuation, dose reduction or interruption of treatment than are patients younger than 65 years.36
Compared to these observations in old patients, T-VEC was well tolerated in old patients with no treatment-related grade 3–4 adverse effects, which is in accordance to the real-world literature (Table 4), even though it has to be taken into account that adverse event reporting might be lower due to the retrospective design of most real-world studies.
Table 4.
Extracted Data of Published Real-Word Studies of T-VEC in Melanoma Patients
| Publication | Year | Patients Total N (Patients Evaluated for Response) | Patients IIIB-IVM1a Evaluated for Response | Median Age of All Patients | ORR Total/ORR (IIIB-IVM1a) | DRR Total/DRR IIIB-IVM1a | Survival | AJCC Version | Duration of Treatment | Grad 3–4 AEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Perez et al31 | 2018 | 27 (23) | 23 | 75 | 56.5%/56.5% | n.a. | Median OS not reached 1 Year OS 80% |
7 | n.a. | No CTCAE grading available (Majority with no significant reported AEs) |
| Louie et al49 | 2019 | 80 (79) | 63 (IIIB-D) | 69 | 44.3%/60.3% (IIIB-D) | n.a. | n.a. | 8 | n.a. (Median of 5 cycles) | No CTCAE grading available |
| Masoud et al50 | 2019 | 27 | 23 | 67 | 40.7%/n.a. | 37%/n.a. | Median OS was not reached | 8 | n.a. (Median of 4 cycles) | 3 (11%) |
| Franke et al51 | 2019 | 26 | 26 (no IVM1a) | 74 | 88.5%/88.5 (no IVM1a) | n.a. | n.a. | 7 | n.a. | 1 (3.8%) |
| Zhou et al33 | 2019 | 40 | 34 (IVM1a) | 73 | 47.5%/n.a. | 40%/n.a. | Median OS was not reached | 7 | n.a. | 3 (7.5%) |
| Fröhlich et al32 | 2020 | 14 | 10 | 73 | 64.3%/70% | 36%/50% | n.a. | 7 | Median of 8 cycles | None |
Abbreviations: T-VEC, talimogene laherparepvec; PD-1, programmed cell death protein 1; CTLA4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune-checkpoint inhibitor; BRAF, v-raf murine sarcoma viral oncogene homolog B1; MEK, mitogen-activated protein kinase; ICP, infectious cell protein; GM-CSF, granulocyte-macrophage colony-stimulating factor; AJCC, American Joint Committee on Cancer; ORR, overall response rate; AE, adverse event; PFU, plaque-forming units; UCT, university cancer center Frankfurt; LDH, lactate dehydrogenase; ECOG, Eastern Cooperative Oncology Group; CTCAE, Common Terminology Criteria of Adverse Events; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; DRR, durable response rate; WAC, wholesale acquisition cost; CYP450, cytochrome P450;.
In contrast to others, our study focused on geriatric oncological patients. Besides the high age (with a median age of 83 years and 42% of the patients being older than 85 years), our study was able to describe further relevant geriatric parameters. Thus, most of our patients had a reduced ECOG performance status, suffered in the median average from four concomitant diseases and used regularly five different prescribed drugs, which is meeting the definition of polypharmacy.37 Polypharmacy has been identified to be a negative prognostic factor, associated with reduced survival in patients treated with ICI.38 Furthermore, it plays a major role in BRAF-MEK inhibitor therapy, which interacts with cytochrome P450 (CYP450) enzymes.39 In contrast to this there are no known drug–drug interactions expected of T-VEC therapy.
In addition to polypharmacy as a negative prognostic factor, it is also known that the observed elevated ECOG performance status and multimorbidity are associated with an increased risk of death and inferior outcome of ICI in malignant melanoma.40–42 In conclusion, it becomes evident that T-VEC responses in our patient collective exceed expectation from clinical trial data, despite all these supposedly unfavorable factors.
Furthermore, the median duration of treatment with T-VEC was short in our study (17 weeks), which is in accordance to other published real-world and clinical-trial data (Table 4). As we know that the duration of therapy and the number of clinical visits (for therapy, additional blood samples, imaging and side effect management) cause patients to deviate from their normal life activities, one can imagine that the load of appointments might be stressful and challenging especially for older people.43 In comparison with ICI and BRAF-MEK inhibitors, it is important to know that both therapies are approved for a long-term use.44,45 In clinical trials, median durations of treatment vary from six months to one year for ICI and BRAF-MEK inhibitors.4,5,46 Concerning older patients, no major differences to clinical trial data were observed in patients treated with the ICI pembrolizumab.47 In conclusion, therapy with T-VEC in old and oldest-old patients seems to be advantageous particularly due to the short treatment phase and the limited number of clinical visits.
Another aspect of our study was focusing on therapy costs of T-VEC in Germany. In the more and more important debate on the distribution of financial resources in healthcare systems worldwide, the costs of oncological therapies are controversially discussed. Recently, several cost-effectiveness analyses on ICI and BRAF-MEK inhibitors have been published.25 In summary, PD-1 inhibitors have been concluded to be cost-effective, whereas all other medications failed to show cost-effectiveness, considering the usually accepted thresholds in oncology.25 Publications on therapy costs of T-VEC are extremely sparse. A cost-effectiveness estimation, done by the manufacturer (AMGEN®), found that T-VEC is more cost-effective than the ICI ipilimumab. However, the statistical model used for this comparison was criticized heavily and evaluated to be unsuitable.48 Thus, there are no published data on therapy costs of T-VEC in a real-world setting. The observed median therapy costs of 27,325 Euros in our collective have been lower than expected compared to the calculated minimum and maximum annual therapy costs for T-VEC.26 Also compared to the calculated annual therapy costs of ICI and BRAF-MEK inhibitors, therapy costs seem to be lower.
It has to be taken into account that our study has a relatively small sample size, a patient collective with a relatively high percentage of first-line therapies and a high number of patients with cutaneous and subcutaneous metastasis with a moderate tumor burden.21 Nevertheless, the low costs in this population seem to be interesting and point to the need for further evaluations of therapy costs of T-VEC.
Conclusion
In conclusion, this study provides further real-world evidence for a good tumor response of T-VEC in old and oldest-old patients. In this highly selective patient collective, tumor responses were higher than expected from final phase III study analysis. T-VEC was well tolerated with no related grade 3–4 adverse events. Furthermore, duration of treatment was short, and the treatment was feasible from a health economic point of view. All these data support the use of T-VEC in old and oldest-old patients.
Disclosure
JK and MM report personal fees from Amgen for consulting and lecture outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Matthews NH, Li WQ, Qureshi AA. Epidemiology of melanoma. In: Ward WH, Farma JM, editors. Cutaneous Melanoma: Etiology and Therapy. Brisbane (AU): Codon Publications; December 21, 2017;5–11. [Google Scholar]
- 2.Cavanaugh-Hussey MW, Mu EW, Kang S, Balch CM, Wang T. Older age is associated with a higher incidence of melanoma death but a lower incidence of sentinel lymph node metastasis in the SEER databases (2003–2011). Ann Surg Oncol. 2015;22(7):2120–2126. doi: 10.1245/s10434-015-4538-8 [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622 [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535–1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 5.Hamid O, Robert C, Daud A, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi: 10.1093/annonc/mdz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2016;17(9):1248–1260. doi: 10.1016/S1470-2045(16)30122-X [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–636. doi: 10.1056/NEJMoa1904059 [DOI] [PubMed] [Google Scholar]
- 8.Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–1327. doi: 10.1016/S1470-2045(18)30497-2 [DOI] [PubMed] [Google Scholar]
- 9.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi: 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klink AJ, Chmielowski B, Feinberg B, Ahsan S, Nero D, Liu FX. Health care resource utilization and costs in first-line treatments for patients with metastatic melanoma in the United States. J Manag Care Spec Pharm. 2019;25(8):869–877. doi: 10.18553/jmcp.2019.18442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzerling L, Eigentler TK, Fluck M, et al. Tolerability of BRAF/MEK inhibitor combinations: adverse event evaluation and management. ESMO Open. 2019;4(3):e000491. doi: 10.1136/esmoopen-2019-000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: a systematic review and meta-analysis. Cancer Treat Rev. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim T, Mateus C, Baz M, Robert C. Older melanoma patients aged 75 and above retain responsiveness to anti-PD1 therapy: results of a retrospective single-institution cohort study. Cancer Immunol Immunother. 2018;67(10):1571–1578. doi: 10.1007/s00262-018-2219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAF(V600) mutated metastatic melanoma: an open-label, multicentre, safety study. Lancet Oncol. 2014;15(4):436–444. doi: 10.1016/S1470-2045(14)70051-8 [DOI] [PubMed] [Google Scholar]
- 15.García-Castaño A, González-Barrallo I, Rubio VEC, et al. 1118P retrospective analysis of safety in elderly BRAF V600 mutation-positive advanced melanoma patients treated with dabrafenib (D) and trametinib (T) and correlation with non-elderly patients. Ann Oncol. 2020;31:S753. doi: 10.1016/j.annonc.2020.08.1241 [DOI] [Google Scholar]
- 16.Chanal J, Kramkimel N, Ratour C, Aractingi S, Guegan S, Avril MF. Pembrolizumab for unresectable or metastatic melanoma in patients older than 85 years of age. Dermatology. 2019;235(3):219–224. doi: 10.1159/000492467 [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Hebert LE, Scherr PA, Dong X, Leurgens SE, Evans DA. Cognitive decline after hospitalization in a community population of older persons. Neurology. 2012;78(13):950–956. doi: 10.1212/WNL.0b013e31824d5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meira D, Lavoura P, Ferreira D, et al. Impact of hospitalization in the functionality and quality of life of adults and elderlies. 2015:PA3547.
- 19.Fountzilas C, Patel S, Mahalingam D. Review: oncolytic virotherapy, updates and future directions. Oncotarget. 2017;8(60):102617–102639. doi: 10.18632/oncotarget.18309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu JCC, Coffin RS, Davis CJ, et al. A Phase I Study of OncoVEX GM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12(22):6737–6747. doi: 10.1158/1078-0432.CCR-06-0759 [DOI] [PubMed] [Google Scholar]
- 21.Andtbacka RHI, Collichio F, Harrington KJ, et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in unresectable stage III-IV melanoma. J Immunother Cancer. 2019;7(1):145. doi: 10.1186/s40425-019-0623-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elias R, Karantanos T, Sira E, Hartshorn KL. Immunotherapy comes of age: immune aging & checkpoint inhibitors. J Geriatr Oncol. 2017;8(3):229–235. doi: 10.1016/j.jgo.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 23.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kandel M, Allayous C, Dalle S, et al. Update of survival and cost of metastatic melanoma with new drugs: estimations from the MelBase cohort. Eur J Cancer. 2018;105:33–40. doi: 10.1016/j.ejca.2018.09.026 [DOI] [PubMed] [Google Scholar]
- 25.Gorry C, McCullagh L, Barry M. Economic evaluation of systemic treatments for advanced melanoma: a systematic review. Value Health. 2020;23(1):52–60. doi: 10.1016/j.jval.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 26.Talimogen laherparepvec (Melanom) – nutzenbewertung gemäß § 35a SGB V. 2016. Available from: https://www.g-ba.de/downloads/92-975-1521/2016-06-15_Nutzenbewertung-IQWiG_Talimogen-laherparepvec_D-237.pdf. Accessed February15, 2021.
- 27.Imlygic EPAR product information. 2016. Available from: https://www.ema.europa.eu/en/documents/product-information/imlygic-epar-product-information_en.pdf. Accessed February15, 2021.
- 28.Common Terminology Criteria for Adverse Events (CTCAE v5.0). 2019. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed February10, 2021.
- 29.AidKlinik Database 4.9.0. 2021. Accessed February15, 2021.
- 30.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 31.Perez MC, Miura JT, Naqvi SMH, et al. Talimogene laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol. 2018;25(13):3960–3965. doi: 10.1245/s10434-018-6803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frohlich A, Niebel D, Fietz S, et al. Talimogene laherparepvec treatment to overcome loco-regional acquired resistance to immune checkpoint blockade in tumor stage IIIB-IV M1c melanoma patients. Cancer Immunol Immunother. 2020. doi: 10.1007/s00262-020-02487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou AY, Wang DY, McKee S, et al. Correlates of response and outcomes with talimogene laherperpvec. J Surg Oncol. 2019;120(3):558–564. doi: 10.1002/jso.25601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastiaannet E, Battisti N, Loh KP, et al. Immunotherapy and targeted therapies in older patients with advanced melanoma; young International Society of Geriatric Oncology review paper. J Geriatr Oncol. 2019;10(3):389–397. doi: 10.1016/j.jgo.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archibald WJ, Victor AI, Strawderman MS, Maggiore RJ. Immune checkpoint inhibitors in older adults with melanoma or cutaneous malignancies: the Wilmot Cancer Institute experience. J Geriatr Oncol. 2020;11(3):496–502. doi: 10.1016/j.jgo.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 36.Tafinlar: EPAR Product information. Available from: https://www.ema.europa.eu/en/documents/product-information/tafinlar-epar-product-information_en.pdf. Accessed February15, 2021.
- 37.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hakozaki T, Hosomi Y, Shimizu A, Kitadai R, Mirokuji K, Okuma Y. Polypharmacy as a prognostic factor in older patients with advanced non-small-cell lung cancer treated with anti-PD-1/PD-L1 antibody-based immunotherapy. J Cancer Res Clin Oncol. 2020;146(10):2659–2668. doi: 10.1007/s00432-020-03252-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazze G. Combination therapy for metastatic melanoma: a pharmacist’s role, drug interactions & complementary alternative therapies. Melanoma Manag. 2018;5(2):MMT07. doi: 10.2217/mmt-2017-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diem S, Kasenda B, Martin-Liberal J, et al. Prognostic score for patients with advanced melanoma treated with ipilimumab. Eur J Cancer. 2015;51(18):2785–2791. doi: 10.1016/j.ejca.2015.09.007 [DOI] [PubMed] [Google Scholar]
- 41.Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18(22):3782–3793. doi: 10.1200/JCO.2000.18.22.3782 [DOI] [PubMed] [Google Scholar]
- 42.Grann AF, Froslev T, Olesen AB, Schmidt H, Lash TL. The impact of comorbidity and stage on prognosis of Danish melanoma patients, 1987–2009: a registry-based cohort study. Br J Cancer. 2013;109(1):265–271. doi: 10.1038/bjc.2013.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fortner BV, Tauer K, Zhu L, et al. Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer. 2004;4(1):22. doi: 10.1186/1471-2407-4-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansen YJL, Rozeman EA, Mason R, et al. Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: clinical outcomes in advanced melanoma. Ann Oncol. 2019;30(7):1154–1161. doi: 10.1093/annonc/mdz110 [DOI] [PubMed] [Google Scholar]
- 45.Warburton L, Meniawy TM, Calapre L, et al. Stopping targeted therapy for complete responders in advanced BRAF mutant melanoma. Sci Rep. 2020;10(1):18878. doi: 10.1038/s41598-020-75837-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28(7):1631–1639. doi: 10.1093/annonc/mdx176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dutriaux C, Saiag P, Meyer N, et al. Outcomes of elderly treated with pembrolizumab for metastatic melanoma comparing with younger patients. J Clin Oncol. 2018;36(15_suppl):e21508–e21508. doi: 10.1200/JCO.2018.36.15_suppl.e21508 [DOI] [Google Scholar]
- 48.Fleeman N, Bagust A, Boland A, et al. Talimogene laherparepvec for treating metastatic melanoma: an Evidence Review Group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2017;35(10):1035–1046. doi: 10.1007/s40273-017-0504-6 [DOI] [PubMed] [Google Scholar]
- 49.Louie RJ, Perez MC, Jajja MR, et al. Real-world outcomes of talimogene laherparepvec therapy: a multi-institutional experience. J Am Coll Surg. 2019;228(4):644–649. doi: 10.1016/j.jamcollsurg.2018.12.027 [DOI] [PubMed] [Google Scholar]
- 50.Masoud SJ, Hu JB, Beasley GM, Stewart J, Mosca PJ. Efficacy of talimogene laherparepvec (T-VEC) therapy in patients with in-transit melanoma metastasis decreases with increasing lesion size. Ann Surg Oncol. 2019;26(13):4633–4641. doi: 10.1245/s10434-019-07691-3 [DOI] [PubMed] [Google Scholar]
- 51.Franke V, Berger DMS, Klop WMC, et al. High response rates for T-VEC in early metastatic melanoma (stage IIIB/C-IVM1a). Int J Cancer. 2019;145(4):974–978. doi: 10.1002/ijc.32172 [DOI] [PubMed] [Google Scholar]