Editor—COVID-19 can progress rapidly to hypoxaemic respiratory failure and acute respiratory distress syndrome (ARDS) requiring invasive mechanical ventilation. Current guidelines recommend treating these patients with lung-protective ventilation, which has a proven survival benefit in ARDS.1 Additional strategies used frequently in cases of severe hypoxaemia are pharmacological paralysis and prone positioning.2 These interventions all require heavy sedation, which in turn necessitates circulatory support with vasopressor medications.
Vasopressin, a vasopressin-receptor agonist, is one such medication. Vasopressin infusion reduces total norepinephrine-equivalent dose requirements and may be renal function sparing.3 Literature also suggests that the vasoconstrictive effects of vasopressin spare the pulmonary vasculature.4 , 5 This is in contrast to catecholamine alternatives, such as norepinephrine, which increase both systemic and pulmonary vascular resistance.5 Patients with COVID-19 respiratory failure are thought to experience significant hypoxic pulmonary vasoconstriction, which in turn increases right ventricular afterload. Some propose vasopressin produces less right heart strain than catecholamine alternatives. For these reasons, vasopressin is commonly used in COVID-19 respiratory failure.
However, recent preclinical data show that molecular complexes form between the SARS-CoV-2 spike protein, soluble angiotensin-converting enzyme-2 (sACE-2), and vasopressin.6 These complexes facilitate cellular infection through vasopressin receptor-1b-mediated endocytosis.6 We previously showed that persistent viraemia was a feature of COVID-19 critical illness and associated with illness severity and worse outcomes.7 Therefore, we tested the hypothesis that vasopressin treatment could promote cellular infection and systemic viral dissemination.
We conducted an Institutional Review Board-approved post hoc analysis of a prospective observational cohort of patients (described previously by Yeung and colleagues6) who presented to Massachusetts General Hospital from March 24 to April 30, 2020. We analysed 52 patients intubated on the day of hospital presentation who had a blood sample drawn for proteomic analysis and viral mRNA measurement at least twice amongst hospital Days 0, 3, and 7. Plasma sACE-2 levels were measured by Olink® proximity (Olink Proteomics, Uppsala, Sweden) extension assay.6 Viral mRNA, measured as described,7 is a semi-quantitative measurement. The assay reports continuous quantifiable values above 2.00 log10 copies ml−1, detectable but unquantifiable levels below 2.00 log10 copies ml−1, or undetectable levels. In this study, we encoded detectable viral mRNA levels below the quantification limit as ‘1.00’ and undetectable mRNA as ‘0’ log10 copies ml−1.
The primary response variable was Day 3 viral mRNA level, adjusted for Day 0 mRNA level in multivariable linear regression. We also compared Day 7 mRNA levels in this manner. We compared patients who received >1 h of vasopressin before Day 3 vs those who did not. Standard practice was to administer vasopressin at only one rate (0.04 units min−1).
Additional outcomes were sACE-2 levels and the number of patients with viral ‘clearance’ between vasopressin-treated and untreated groups. Clearance was defined as undetectable viraemia by Day 7. Previously viraemic patients who died on or before Day 7 and before a subsequent viral mRNA measurement were considered to not have viral clearance. Sensitivity analysis confirmed that recoding patients who died before Day 7 as having clearance did not change results. Viral clearance was analysed with Wald 95% confidence intervals and Fisher's exact test. To assess the association between the total duration of vasopressin exposure with viral mRNA and sACE-2, we applied multivariable linear regression as done previously.
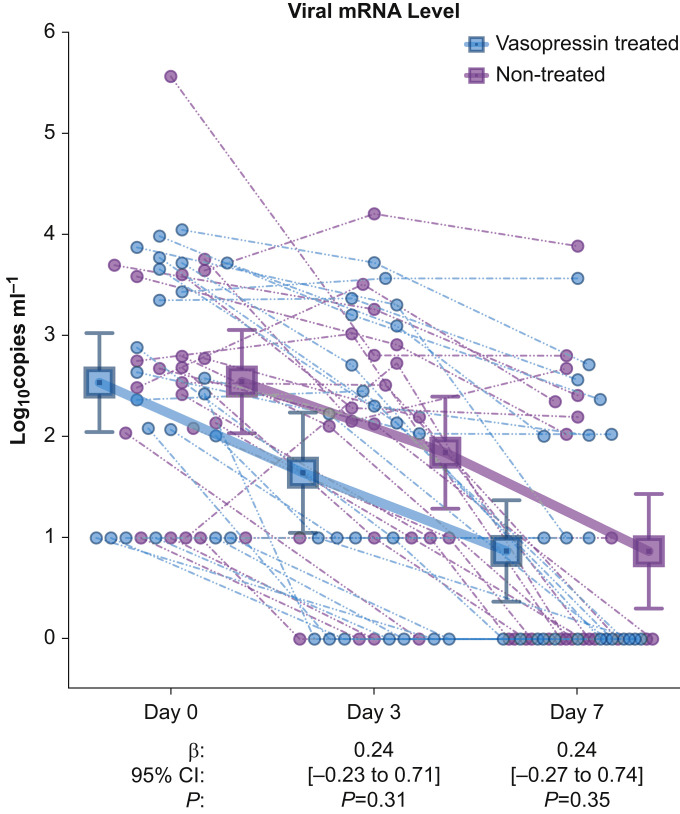
All patients (n=52) had blood collection at baseline (Day 0), and all were viraemic (Supplementary Table S1). Of these patients, 24 (46%) received vasopressin i.v. infusion before Day 3 with median treatment duration of 33 h (inter-quartile range: 12–64). Vasopressin treatment was not associated with higher viral mRNA level vs non-treatment at Day 3 (difference=0.24 log10 copies ml−1 [95% confidence interval: –0.23 to 0.71]; P=0.31) or Day 7 (difference=0.24 log10 copies ml−1 [–0.27 to 0.74]; P=0.35) (Fig. 1 ). The mean change in viral mRNA was –0.70 (standard deviation [sd] 1.02) log10 copies ml−1 in vasopressin-treated and –0.94 (sd 0.67) log10 copies ml−1 in untreated patients (difference in difference: –0.24 [–0.73 to 0.26]; P=0.34) (Supplementary Table S2).
Fig 1.
Viral mRNA levels over time in vasopressin treated vs. non-treated patients. Blue circles represent individual vasopressin-treated patients and purple circles represent individual non-treated patients. Dashed lines connect measurements within the same patient. Squares show the group mean at the indicated time point and error bars indicate the 95% confidence intervals. β indicates the regression coefficient for vasopressin treatment association with viral mRNA level at the indicated time point, adjusted for the Day 0 level.
Vasopressin treatment was not associated with sACE-2 at Day 3 (difference=0.04 normalised expression units [–0.33 to 0.41]; P=0.83) or Day 7 (difference=0.02 normalised expression units [–0.57 to 0.61]; P=0.95). Fourteen (58.3%) vasopressin-treated patients achieved viral clearance vs 18 (64.3%) untreated patients (difference=6.0% [–20.6% to 32.5%]; P=0.77).
The duration of vasopressin treatment before Day 3 plasma measurement was not associated with Day 3 viral mRNA (difference=0.00 log10 copies ml−1 [24 h]−1 of vasopressin exposure [–0.23 to 0.22]; P=0.96) or sACE-2 levels (difference=0.02 normalised expression units [24 h]−1 of vasopressin exposure [–0.21 to 0.25]; P=0.85). At Day 7, vasopressin treatment duration was again not associated with viral mRNA (difference=0.04 [–0.16 to 0.24]; P=0.71) or sACE-2 levels (difference=0.12 [–0.13 to 0.36]; P=0.35).
In this series of 52 critically ill patients with COVID-19 in hypoxaemic respiratory failure, vasopressin infusion was not associated with increased viral mRNA levels or decreased frequency of viral clearance. Vasopressin treatment was also not associated with an increase in sACE-2. Whilst the study is too small to exclude a meaningful effect of vasopressin treatment on viral clearance, the results suggest that the in vitro effects of vasopressin on the SARS-CoV-2 life cycle do not translate to clinically relevant increases in viraemia.
This small, single-centre, retrospective study was conducted on samples collected before widespread adoption of corticosteroid treatment for severe COVID-19.8 Immunosuppression could potentiate pro-viral effects of vasopressin to a clinically relevant degree. Corroborating our null findings in patients treated with multimodal immunosuppression is a worthwhile next step. Independent of an effect of vasopressin on SARS-CoV-2 viraemia, our study does not address whether SARS-CoV-2 infection alters the physiological response to vasopressin. Such effect could be present, given that uptake of virus–ACE-2–vasopressin complexes occur via endocytosis, which could decrease vasopressin receptor-1b availability for physiological signalling.
There are no randomised data and no currently registered trials addressing vasopressin treatment in COVID-19. In COVID-19 critical illness, selection of vasopressors to treat hypotension, much of which is likely induced by sedation, will remain without a solid evidence base at least for the short-term.
In conclusion, despite preclinical data raising the possibility that vasopressin facilitates SARS-CoV-2 dissemination in vivo, we did not observe evidence of a clinically relevant effect of vasopressin infusion on viral mRNA level in a pilot cohort of critically ill patients with COVID-19 who were not treated with corticosteroids or interleukin-6 antagonists.
Declarations of interest
All authors declare no conflicts of interest.
Funding
American Lung Association (COVID-19 Action Initiative) to MBG; Executive Committee on Research at Massachusetts General Hospital to MBG.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2021.07.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guérin C., Reignier J., Richard J.C., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 3.Russell J.A., Walley K.R., Singer J., et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877–887. doi: 10.1056/NEJMoa067373. [DOI] [PubMed] [Google Scholar]
- 4.Currigan D.A., Hughes R.J., Wright C.E., Angus J.A., Soeding P.F. Vasoconstrictor responses to vasopressor agents in human pulmonary and radial arteries: an in vitro study. Anesthesiology. 2014;121:930–936. doi: 10.1097/ALN.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar J., Golden P.J., Kajiura L.N., Murata L.A., Uyehara C.F. Vasopressin decreases pulmonary-to-systemic vascular resistance ratio in a porcine model of severe hemorrhagic shock. Shock. 2015;43:475–482. doi: 10.1097/SHK.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 6.Yeung M.L., Teng J.L.L., Jia L., et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell. 2021;184 doi: 10.1016/j.cell.2021.02.053. 2212–2228.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y., Schneider A.M., Mehta A., et al. SARS-CoV-2 viremia is associated with distinct proteomic pathways and predicts COVID-19 outcomes. J Clin Invest. 2021;131 doi: 10.1172/JCI148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterne J.A.C., Murthy S., Diaz J.V., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.