Abstract
Purpose
To determine the utility, diagnostic accuracy, sensitivity, specificity, and negative predictive value of the laboratory based Covid-19 antigen detection test (Coris Bio- Concept, Gembloux, Belgium) for the diagnosis of SARS-CoV-2 in a tertiary care hospital among symptomatic and asymptomatic patients.
Methods
The nasopharyngeal swab samples were collected from the symptomatic patients and their contacts. The diagnostic accuracy of this antigen kit was determined in comparison to SARS-CoV-2 real-time reverse transcriptase (RT-PCR).
Results
A total of 825 patients fulfilling the inclusion criteria were included in the study; RT-PCR and antigen detection was performed simultaneously for 484 samples to determine the sensitivity and specificity of the test. The overall specificity and sensitivity was 99.32% and 71.96% respectively. Also, 3.7% of the asymptomatic patients who were negative by RAT were detected positive by RT-PCR.
Conclusion
This rapid antigen test (RAT) was sensitive in the symptomatic patients presenting during the initial phase of the illness. Since, majority of the SARS-CoV-2 patients are asymptomatic and considering the huge population, the testing strategy formulated by Indian Council of Medical Research (ICMR) at the national level was cost effective. Thus, Ag-RDTs could play a pivotal role in early diagnosis, policy making and surveillance of the SARS-CoV-2.
Keywords: SARS-CoV-2, Rapid antigen detection, Point-of-care
Abbreviation list
- RAT
Rapid antigen tests
- SARS- CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- ICMR
Indian Council of Medical Research
Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China, on December 31, 2019 and was declared pandemic by World Health Organization (WHO) on March 11, 2020 [1,2]. As updated on April 27, 2021, the WHO reports 147, 539, 302 confirmed cases with 3,116,444 deaths spanning across the globe [3].
The Real-time Reverse Trancriptase PCR (RT-PCR) is the main modality for diagnosis, and nasopharyngeal (NP) swab is recommended sample for SARS-CoV-2. Although RT-PCR is sensitive, it requires skilled laboratory personnel and dedicated molecular laboratory set-up. The turn-around-time for RT-PCR process including nucleic-acid extraction is long (6–7 h). which demands development of rapid and easy-to-perform diagnostic methods. The major advantage of RAT is the availability of quick results, easy interpretation and suitability for use in field settings. The RAT has been used for viral illnesses like Hepatitis B, Human Immunodeficiency Virus, and dengue (NS1 antigen) [4]. The influenza RAT provides moderate sensitivity (50–70%) with high specificity. They are not routinely used as they do not provide information about the circulating types. Recently various RAT for SARS-CoV-2 diagnosis are available. These can detect viral antigen from the sample by the immobilized SARS-CoV-2 antibody on the device [5,6]. Although these tests are quite specific (80–100%) their sensitivity (29–93.9%) varies from kit to kit [7]. Due to this concern, the present study was planned to evaluate its utility in tertiary care hospital.
Currently, two types of RAT are commercially available for SARS-CoV-2 detection in nasopharyngeal samples; one can be performed bed-side as the samples are collected in lysis buffer while other is laboratory-based and has to be performed in biosafety level - II facility and the samples are collected in viral transport media. The present study evaluated the commercially available laboratory-based Coris Bio concept COVID-19 ag Respi-strip test, (Coris Bio-Concept, Gembloux, Belgium) for SARS-CoV-2 detection in nasopharyngeal samples. According to ICMR guidelines and testing algorithm, it has been recommended that results of antigen detection should be taken as confirmatory if the patient is asymptomatic while in case of symptomatic patients, the testing by RT-PCR is advised even if the RAT is negative [8]. However, in an institute set up, an asymptomatic patient missed by RAT could be a potential super spreader. Hence, we attempted to study the utility of rapid antigen detection tests in a tertiary care hospital in North India in both symptomatic subjects as well as their asymptomatic contacts.
Materials and methods
Study design and site
This was a prospective cross-sectional study conducted at the communicable disease ward of a tertiary care North Indian Hospital. Nasopharyngeal swab samples were collected from patients between August 22, 2020 to September 18, 2020.
Sample collection and processing
The nasopharyngeal swab samples were collected by trained medical personnel using appropriate biosafety measures. The samples were put in a viral transport medium (VTM) and transported in a cold chain to the Department of Virology. The testing was carried out within the BSL class II facility according to the ICMR guidelines. The real-time PCR targeting E (envelope), ORF (Open-reading frame), and RdRp (RNA dependent RNA polymerase) genes were considered as a gold standard for comparing the results of the RAT. Among the confirmatory genes, the Ct value of ORF was used for comparison with the antigen positivity as it correlates best with the viral load/antigen load [9].
Patient inclusion criteria
Adult patients with the following criteria were included in study i) patients with influenza-like illness (ILI) which was defined as an acute respiratory infection with the measured fever of ≥38 C°, cough, and with onset within the last 10 days. ii) asymptomatic contacts of a known SARS-CoV-2 positive case at least at day 5 of contact with lab confirmed case.
Exclusion criteria
Pediatric patients with symptoms suggestive of SARS-CoV-2 were excluded from the study as this study was conducted in the adult communicable disease ward of the institute.
Sample size
A total of 825 patients fulfilling the above inclusion criteria were included in the study; RT-PCR and antigen detection was performed simultaneously for 484 samples to determine the sensitivity and specificity of the test. The Coris bioconcept COVID-19 ag respi-strip test (Coris Bio-Concept, Gembloux, Belgium) was used for rapid antigen detection according to the manufacturer's protocol. The manufacturers of this kit have claimed sensitivity of 57.6% and specificity of 99.5% [10]. Although the kit was already approved by the Indian Council of Medical Research (ICMR) and sensitivity and specificity were documented in the kit literature, we wanted to evaluate the efficacy of the kit in the tertiary care-setting of our institute for further policy making. For carrying out real-time PCR, the nucleic acid of the samples was extracted using the Qiagen viral mini kit (Qiagen, Germany) according to the manufacturer's protocol. The detection of SARS-CoV-2 by Real-Time PCR was performed by NIV-Single tube probe-based commercial kit (NIV-Single tube kit) in the Applied Biosystem 7500 real-time PCR machine (ABI, USA). Institutional ethical clearance and project approval was taken (NK/6744/study/521). Informed consent was taken from all the patients.
Data analysis
The data were entered using Jamovi software (version 1.6.7, https://www.jamovi.org). To compare relevant clinical data, Pearson's chi-squared and Fisher's exact test, (where appropriate) were used for comparisons of proportions. The receiver operator curve (ROC) was made by Excel spreadsheet (version 2019 16.0.6742.2048) and a cut-off value of maximum sensitivity and specificity was derived. A p-value less than or equal to 0.05 was considered statistically significant.
Results
Initially, RT-PCR and antigen detection was performed simultaneously for 484 samples to determine the sensitivity and specificity of the test. The demographic analysis included 484 nasopharyngeal samples for SARS-CoV-2 by RT-PCR and COVID-19 Ag Respi-Strip CORIS. The median age of the study population was 32 years (range: 13–90). The male population dominated in the positive groups of both antigen detection and RT-PCR detection (Table 1 ). Based on the results of the interim analysis of 484 patients wherein 100% concordance was observed between both the tests, it was decided to further perform only antigen testing in symptomatic patients as per ICMR guidelines. Thus, 341 patients were tested with RAT and if positive they were labeled as SARS-CoV-2 positive. RT-PCR was done in case where RAT was negative.
Table 1.
Demographic features of 484 patients for which both RT-PCR and RAT was done.
| COVID-19 Result by Real-Time PCR |
COVID-19 result by Ag Respi-Strip CORIS |
|||
|---|---|---|---|---|
| Positive (n) | Negative(n) | |||
| Gender | Male | Positive | 84 | 28 |
| Negative | 1 | 148 | ||
| Female | Positive (n) | 52 | 25 | |
| Negative (n) | 1 | 145 | ||
| Age | Mean | 35.5 | ||
| Median | 32 | |||
Since, on total 825 patients were tested using antigen kits, their clinical characteristics have been described in Table 2 . The mean duration of onset of symptoms in patients positive for SARS-CoV-2 antigen was 2 days. The most significant symptoms associated with the positivity of antigen detection were fever at evaluation, cough, body ache, and vomiting (p < 0.05).
Table 2.
Clinical Characteristics of the 825 Patients in relation to COVID-19 result by Ag Respi-Strip CORIS.
| COVID-19 result by Ag Respi-Strip CORIS |
||||
|---|---|---|---|---|
| Positive | Negative | p-value | ||
| SYMPTOMS AT PRESENTATION | Present | 115 | 518 | 0.04 |
| Absent | 23 | 169 | ||
| FEVER AT EVALUATION | Present | 93 | 268 | 0 |
| Absent | 45 | 419 | ||
| COUGH | Present | 55 | 198 | .01 |
| Absent | 83 | 489 | ||
| BREATHLESSNESS | Present | 3 | 17 | .83 |
| Absent | 135 | 670 | ||
| SORE THROAT | Present | 53 | 256 | .80 |
| Absent | 85 | 431 | ||
| DIARRHOEA | Present | 1 | 15 | .25 |
| Absent | 137 | 672 | ||
| CHEST PAIN | Present | 1 | 3 | .65 |
| Absent | 137 | 684 | ||
| VOMITING | Present | 9 | 18 | .01 |
| Absent | 129 | 669 | ||
| HAEMOPTYSIS | Present | 2 | 2 | .07 |
| Absent | 136 | 685 | ||
| NASAL DISCHARGE | Present | 7 | 25 | .42 |
| Absent | 131 | 662 | ||
| BODY ACHE | Present | 29 | 49 | 0.0 |
| Absent | 109 | 638 | ||
| SPUTUM | Present | 0 | 56 | NA |
| Absent | 138 | 631 | ||
| MALAISE | Present | 0 | 1 | NA |
| Absent | 139 | 685 | ||
Antigen detection and real-time PCR
Table 3 shows the sensitivity and specificity of the RAT test considering RT-PCR as the gold standard in 484 samples. The SARS-CoV-2 antigen was positive in 28% of subjects while RT-PCR was positive in 39% of subjects. However, RT-PCR could detect an additional 11% of subjects who were reported negative by antigen test resulting in a sensitivity of 71.96% (95 CI: 64.98%–78.24%). The 293 samples with a negative result with the RT-PCR technique were also negative with the rapid test, giving an overall specificity of 99.32% (95 CI: 97.57%–99.92%). The accuracy of the test was 88.64% (95 CI: 85.47%–91.32%).
Table 3.
Sensitivity, specificity, positive, and negative predictive values of COVID-19 Ag respi-strip CORIS for detection of SARS-CoV-2 in nasopharyngeal samples.
| COVID-19 Ag Respi-Strip CORIS | Positive Samples on RT-PCR (N = 189) | Negative Samples on RT-PCR (N = 295) | Total Samples (N = 484) |
Positive Predictive Value |
Negative Predictive Value |
Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|---|
| Positive | 136 | 2 | 138 | 98.55% | 71.96% | 88.64% | ||
| Negative | 53 | 293 | 346 | 84.68% | 99.32% |
Relation of antigen positivity with Ct value
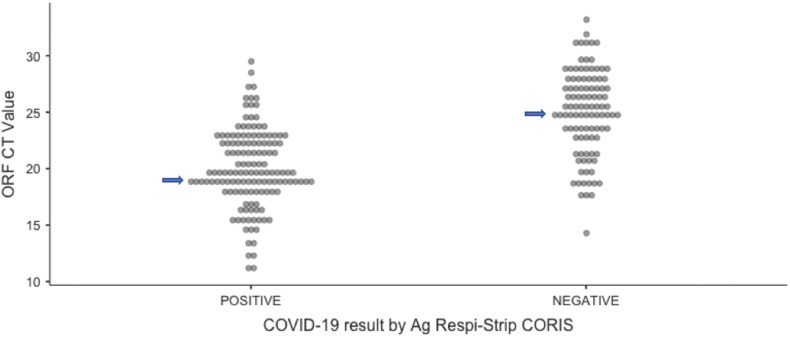
The 136 concordant positive samples (both antigen and RT PCR positive) had a median Ct value of ORF gene 19.6, whereas the median Ct value of the 94 discordant (positive RT-PCR with negative rapid test) samples was 25.6 (P < 0.0001 (95% CI:4.8503 to 7.1497)). (Fig. 1 ). The mean Ct value of the Open Reading Frame (ORF) of the antigen-positive samples (19.9 vs 25.4) was significantly lower than that of the antigen-negative samples (P < 0.0001 (95% CI: 4.3514 to 6.6486)).
Fig. 1.
Median Ct value of antigen positive and negative samples. (19.6 vs 25.6) (P < 0.0001 (95%CI:4.8503 to 7.1497)).
Among 484 samples tested by both methods, the antigen and RT-PCR was positive in 28.5% (138/484) and 39.0% (189/484) patients respectively. 60.5% (293/484) patients were negative by both methods. The RAT was not able to detect 3.7% of the asymptomatic patients who were positive by RT-PCR (Supplement Table 1). The median Ct values of the asymptomatic patients missed by RAT was 24.5.
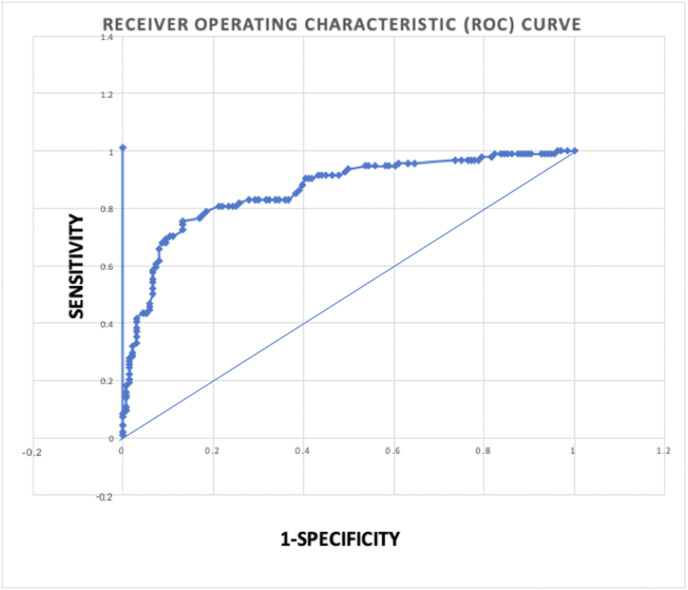
In order to determine the cut off of CT-value of ORF gene beyond which the RDT was not able to detect SARS-CoV-2 infection, ROC analysis was done. The Ag test achieved an area under the ROC curve (AUC) value of 0.835. This suggest that the RAT can accurately detect SARS-CoV-2 in nasopharyngeal samples (Fig. 2 ). The samples with Ct < 18,<20, <22; COVID-19 Ag Respi-Strip CORIS had a sensitivity of 96.81%, 91.49% and 82.98% respectively. In a few patients with low CT values, only the test line was visible and the control line was barely seen. This could be due to the presence of a high amount of virus antigen which binds to the antibody attached to the rapid antigen strip. (Supplement Figure 1).
Fig. 2.
Receiver operating characteristic (ROC) curve analysis. The rapid antigen test achieved an area under the ROC curve (AUC) value of 0.835.
Discussion
The rapid spread of SARS-CoV-2 demands the availability of a rapid, accurate, and affordable diagnostic test. Rapid diagnosis can limit the spread of the infection in the initial stages and can be very beneficial in asymptomatic contacts of symptomatic patients and super-spreaders. In a country with a huge population like India, SARS-CoV-2 testing by RT-PCR is a challenging task. Thus, RAT can be utilized for rapid SARS-CoV-2 detection as a point-of-care test. The nucleocapsid protein of the virus is present in abundance in the nasopharyngeal samples; thus it is a preferred target analyte for antigen detection.
Very few Ag-RDTs have undergone stringent regulatory review. Currently, 13 kits have received United States Food and Drug Administration (FDA) Emergency Use Authorization (EUA). Till now, only three companies have submitted documents toward WHO's Emergency Use Listing (EUL) procedure [5,6]. The Coris Bioconcept COVID-19 ag respi-strip test was recently approved by the Indian Council of Medical Research (ICMR) for in vitro diagnosis of SARS-CoV-2. Though this kit has been approved and validated by ICMR, to the best of our knowledge, this is the first report from India on its actual evaluation in a clinical set up among both symptomatic as well as asymptomatic patients. Compared to the RT-PCR test, this antigen test greatly reduces the turnaround time from 6 h to 30 min and can be used as a routine in a high-throughput setting, especially during a pandemic where infection control is the highest priority and there is an urgent need to isolate positive cases.
In our scenario, the sensitivity of RAT was found to be 71.96% which is higher than Scohy et al. where low sensitivity of 30.2% was observed [11] and Niclot et al. where only 50.0% sensitivity was observed [12]. Scohy et al. performed tests on randomly selected samples and while Niclot et al. collected samples randomly from the general population. This could be the reason for the low sensitivity in their studies as in the current study symptomatic patients and their known contacts were tested. These Ag-RDTs are most likely to perform well in pre-symptomatic and early symptomatic phases of the patient when the viral load is high (Ct values ≤ 25 or >106 genomic virus copies/mL) [13,14] The manufacturers performed a retrospective study from symptomatic patients suspected of SARS-CoV-2 infections and derived the sensitivity of 57.6% for samples positive with a Ct value under 22. In the current study, based on the ROC analysis, the sensitivity of 82.98% was achieved at Ct value under 22 [10]. Thus, this test is more sensitive in patients with high viral loads and hence could be used for patients within initial days of presentation, offering the chance for early diagnosis of SARS-CoV-2 cases. This will aid in breaking the transmission of cases and their close contacts. The patients presenting in the later part of disease i.e. after 6–7 days onset of symptoms are more likely to have low level of viral load. Hence, the probability of false-negative results is higher with Ag-RDTs [15].
The current Indian guidelines [8] and the advisory bodies have recommended the initial testing by rapid antigen detection test alone in asymptomatic patients and results may be considered as true positive or true negative. In the present study only 3.7% of the asymptomatic patients who were negative by RAT were detected positive by RT-PCR. Since, majority of the SARS-CoV-2 patients are asymptomatic and considering the huge population, testing strategy at national level was formulated which is cost effective and can break the transmission chain by rapid diagnosis of those who are at high risk of transmission. However, among the symptomatic cases, RT-PCR has been advised in cases with negative RAT results.
Based on experience with antigen-based RATs for other respiratory diseases (influenza), in which affected patients have comparable concentrations of influenza virus in respiratory samples as seen in SARS-CoV-2, the sensitivity of these tests might be expected to vary from 34% to 80% [16]. In the case of the SARS-CoV-2 diagnosis, the RAT offers similar sensitivity but is more useful in the current pandemic situation. The R0 of the disease varies from 1.45 to 3.5 and person-to-person transmission is high. The RAT are advantageous in triage and emergency areas demanding prompt diagnosis. Currently, RATs are not recommended by the WHO for patient management. However, due to their potential of rapid diagnosis, its utility is highly encouraged in the clinical setting [17]. They are cost-effective and can be performed with minimal training. Also, in this kit, one nasopharyngeal sample is taken, which can be utilized for both antigen detection and RT-PCR. It is unlike other kits where different samples are required thus causing discomfort to the patient. The cost per test is approximately $7(INR 509) in comparison to the $20(INR 1450) of RT-PCR (RT-PCR cost during study period Aug2021).
There are few limitations as Coris RAT is not point-of care and has to be performed in BSL-II laboratory facilities thus limiting its utility in field settings. In comparison to RT-PCR, it has low sensitivity. The RT-PCR can amplify low viral load by the process of amplification. However, RAT accurately detected SARS-CoV-2 in all samples with Ct values < 22 while the sensitivity decreased in samples with high CT value. Thus, testing with RAT in patients suspected of SARS-CoV-2 infection can produce false-negative results. There were two false-positive samples for which RT-PCR could not be repeated due to lack of sample adequacy. In a highly viscous samples false-positive can occur although the degree of viscosity cannot be accurately defined [18]. The risks of cross-contamination when testing multiple patient specimens remains high and cannot be ruled out, although samples were tested with recommended precautions. Another limitation of the study is that we didn't compare the viral load with CT values.
Conclusion
We could not assess for cross-reactions with other endemic coronaviruses (OC43, NL63, 229E, and HKU1) and respiratory viruses. However, the manufacturers claim no cross-reactions with circulating other coronavirus infections [9]. These tests can't be used as a stand-alone test for the diagnosis. Despite these limitations, RAT has ability to play significant role in guiding patient management, surveillance and policy aking decision for public health in this pandemic. However, further prospective studies are required for its implementation in the best clinical setting.
Conflict of interest
None.
Funding
None.
Declarations of interest
None.
CRediT authorship contribution statement
Rimjhim Kanaujia: Design of diagnostic strategy, development of definitions, writing of manuscript. Arnab Ghosh: Design of diagnostic strategy, development of definitions, writing of manuscript. Ritin Mohindra: Implementation of diagnostic strategy. Vidhi Singla: Implementation of diagnostic strategy. Kapil Goyal: Design of diagnostic strategy, development of definitions, writing of manuscript. Rajendra Gudisa: Data curation, Data retrieval, Writing – review & editing, review of manuscript. Vikrant Sharma: Implementation of diagnostic strategy. Lalit Mohan: Implementation of diagnostic strategy. Navpreet Kaur: Implementation of diagnostic strategy. Gursimran Kaur Mohi: Data curation, Data retrieval, Writing – review & editing, review of manuscript. Ishani Bora: Data curation, Data retrieval, Writing – review & editing, review of manuscript. Radha Kanta Ratho: Data curation, Data retrieval, Writing – review & editing, review of manuscript. Roop Kishor Soni: Implementation of diagnostic strategy. Ashish Bhalla: Data curation, Data retrieval, Writing – review & editing, review of manuscript. Mini P. Singh: Design of diagnostic strategy, development of definitions, writing of manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijmmb.2021.07.003.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization W. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. WHO Dir Gen Speeches 2020:4. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed March 12, 2020)..
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard n.d. https://covid19.who.int/(accessed October 14, 2020).
- 4.Singh M.P., Majumdar M., Singh G., Goyal K., Preet K., Sarwal A., et al. NS1 antigen as an early diagnostic marker in dengue: report from India. Diagn Microbiol Infect Dis. 2010;68:50–54. doi: 10.1016/j.diagmicrobio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.U. S. Food and Drug Administration In vitro diagnostics EUAs. Cent Drug Eval Res. 2020 accessed on march 12, 2021. [Google Scholar]
- 6.PMDA's Efforts to Combat COVID-19 | Pharmaceuticals and Medical Devices Agency n.d. https://www.pmda.go.jp/english/about-pmda/0002.html (accessed October 28, 2020).
- 7.Walle V.K.B.E. MedRxiv; 2020. Meta-analysis of the clinical performance of commercial SARS-CoV-2 nucleic acid, antigen and antibody tests up to 22 August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Advisory on Strategy for COVID-19 Testing in India 2020:1–4. https://www.mohfw.gov.in/pdf/AdvisoryonstrategyforCOVID19TestinginIndia.pdf (accessed January 27, 2021).
- 9.Alagarasu K., Choudhary M., Lole K., Abraham P., Potdar V. Evaluation of RdRp & ORF-1b-nsp14-based real-time RT-PCR assays for confirmation of SARS-CoV-2 infection: an observational study. Indian J Med Res. 2020;151(5):483–485. doi: 10.4103/ijmr.IJMR_1256_20. 151:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens P., De Vos N., Martiny D., Jassoy C., Mirazimi A., Cuypers L., et al. Development and potential usefulness of the COVID-19 Ag respi-strip diagnostic assay in a pandemic context. Front Med. 2020;7 doi: 10.3389/fmed.2020.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129:104455. doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert-Niclot S., Cuffel A., Le Pape S., Vauloup-Fellous C., Morand-Joubert L., Roque-Afonso A.-M., et al. Evaluation of a rapid diagnostic assay for detection of SARS-CoV-2 antigen in nasopharyngeal swabs. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00977-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss A., Jellingsø M., Sommer M.O.A. Spatial and temporal dynamics of SARS-CoV-2 in COVID-19 patients: a systematic review and meta-analysis. EBioMedicine. 2020;58:102916. doi: 10.1016/j.ebiom.2020.102916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020;8:CD013705. doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious severe acute respiratory Syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruning A.H.L., Leeflang M.M.G., Vos J.M.B.W., Spijker R., de Jong M.D., Wolthers K.C., et al. Rapid tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1026–1032. doi: 10.1093/cid/cix461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Who . 2020. Advice on the use of point-of-care immunodiagnostic tests for COVID-19; pp. 1–6. [Google Scholar]
- 18.Itoh K., Kawamitsu T., Osaka Y., Sato K., Suzuki Y., Kiriba C., et al. False positive results in severe acute respiratory coronavirus 2 (SARS-CoV-2) rapid antigen tests for inpatients. J Infect Chemother. 2021;27(7):1089–1091. doi: 10.1016/j.jiac.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.